Abstract
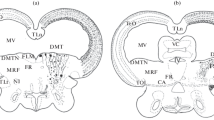
The subcommissural organ (SCO) is an ependymal differentiation located in the diencephalon under the posterior commissure (PC). SCO-spondin, a glycoprotein released by the SCO, belongs to the thrombospondin superfamily and shares molecular domains with axonal pathfinding molecules. Several lines of evidence suggest a relationship between the SCO and the development of the PC in the chick: (1) their close location to each other, (2) their differentiation at the same developmental stage in the chick, (3) the abnormal PC found in null mutants lacking an SCO and (4) the release by the SCO of SCO-spondin. By application of DiI crystals in the PC of chick embryos, we have identified the neurons that give rise to the PC. Labelling is confined to the magnocellular nucleus of the PC (MNPC). To gain insight into the role of the SCO in PC development, coculture experiments of explants of the MNPC region (MNPCr) from embryos at embryonic day 4 (E4) with SCO explants from E4 or E13 embryos have been performed and the neurite outgrowth from the MNPCr explants has been analysed. In the case of coculture of E4 MNPCr with E4 SCO, the number of neurites growing from the MNPCr is higher at the side facing the SCO. However, when E4 MNPCr and E13 SCO are cocultured, the neurites grow mostly at the side opposite to the SCO. These data suggest that, at early stages of development, the SCO releases some attractive or permissive molecule(s) for the growing of the PC, whereas at later stages, the SCO has a repulsive effect over neurites arising from MNPCr.






Similar content being viewed by others
References
Adams JC, Tucker RP (2000) The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn 218:280–299
Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233–248
Bovolenta P, Dodd J (1990) Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development 109:435–447
Burstyn-Cohen T, Tzarfaty V, Frumkin A, Feinstein Y, Stoeckli E, Klar A (1999) F-spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23:233–246
Cobos I, Shimamura K, Rubenstein JL, Martinez S, Puelles L (2001) Fate map of the avian anterior forebrain at the four-somite stage, based on the analysis of quail-chick chimeras. Dev Biol 239:46–67
Colamarino SA, Tessier-Lavigne M (1995) The role of the floor plate in axon guidance. Annu Rev Neurosci 18:497–529
Creveaux I, Gobron S, Meiniel R, Dastugue B, Meiniel A (1998) Complex expression pattern of the SCO-spondin gene in the bovine subcommissural organ: toward an explanation for Reissner’s fiber complexity? Brain Res Mol Brain Res 55:45–53
Chedotal A, Pourquie O, Sotelo C (1995) Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur J Neurosci 7:198–212
Danielian PS, McMahon AP (1996) Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature 383:332–334
Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298:1959–1964
Didier R, Meiniel R, Meiniel A (1992) Monoclonal antibodies as probes for the analysis of the secretory ependymal differentiation in the subcommissural organ of the chick embryo. Dev Neurosci 14:44–52
Didier R, Meiniel O, Meiniel A (2007) Molecular cloning and early expression of chick embryo SCO-spondin. Cell Tissue Res 327:111–119
El-Bitar F, Bamdad M, Dastugue B, Meiniel A (2001) Effects of SCO-spondin thrombospondin type 1 repeats (TSR) in comparison to Reissner’s fiber material on the differentiation of the B104 neuroblastoma cell line. Cell Tissue Res 304:361–369
Estivill-Torrus G, Vitalis T, Fernandez-Llebrez P, Price DJ (2001) The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mech Dev 109:215–224
Feinstein Y, Klar A (2004) The neuronal class 2 TSR proteins F-spondin and mindin: a small family with divergent biological activities. Int J Biochem Cell Biol 36:975–980
Fernandez-Llebrez P, Perez J, Nadales AE, Perez-Figares JM, Rodriguez EM (1987) Vascular and leptomeningeal projections of the subcommissural organ in reptiles. Lectin-histochemical, immunocytochemical, and ultrastructural studies. Histochemistry 87:607–614
Fernandez-Llebrez P, Grondona JM, Perez J, Lopez-Aranda MF, Estivill-Torrus G, Llebrez-Zayas PF, Soriano E, Ramos C, Lallemand Y, Bach A, Robert B (2004) Msx1-deficient mice fail to form prosomere 1 derivatives, subcommissural organ, and posterior commissure and develop hydrocephalus. J Neuropathol Exp Neurol 63:574–586
Figdor MC, Stern CD (1993) Segmental organization of embryonic diencephalon. Nature 363:630–634
Fiore R, Puschel AW (2003) The function of semaphorins during nervous system development. Front Biosci 8:s484–s499
Funato H, Saito-Nakazato Y, Takahashi H (2000) Axonal growth from the habenular nucleus along the neuromere boundary region of the diencephalon is regulated by semaphorin 3F and netrin-1. Mol Cell Neurosci 16:206–220
Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A (1996) SCO-spondin: a new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109:1053–1061
Gobron S, Creveaux I, Meiniel R, Didier R, Dastugue B, Meiniel A (1999) SCO-spondin is evolutionarily conserved in the central nervous system of the chordate phylum. Neuroscience 88:655–664
Gobron S, Creveaux I, Meiniel R, Didier R, Herbet A, Bamdad M, El Bitar F, Dastugue B, Meiniel A (2000) Subcommissural organ/Reissner’s fiber complex: characterization of SCO-spondin, a glycoprotein with potent activity on neurite outgrowth. Glia 32:177–191
Guiñazú MF, Richter HG, Rodriguez EM (2002) Bovine floor plate explants secrete SCO-spondin. Cell Tissue Res 308:177–191
Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–91
Hauser R (1976) Distortion of body axis in young minnows (Phoxinus laevis) following destruction of the subcommissural organ. Rev Suisse Zool 83:898–903
Heffner CD, Lumsden AG, O’Leary DD (1990) Target control of collateral extension and directional axon growth in the mammalian brain. Science 247:217–220
Hoyo-Becerra C, Lopez-Avalos MD, Alcaide-Gavilan M, Gomez-Roldan MC, Perez J, Fernandez-Llebrez P, Grondona JM (2005) Reissner’s fiber formation depends on developmentally regulated factors extrinsic to the subcommissural organ. Cell Tissue Res 321:429–441
Hoyo-Becerra C, Lopez-Avalos MD, Perez J, Miranda E, Rojas-Rios P, Fernandez-Llebrez P, Grondona JM (2006) Continuous delivery of a monoclonal antibody against Reissner’s fiber into CSF reveals CSF-soluble material immunorelated to the subcommissural organ in early chick embryos. Cell Tissue Res 326:771–786
Huwiler KG, Vestling MM, Annis DS, Mosher DF (2002) Biophysical characterization, including disulfide bond assignments, of the anti-angiogenic type 1 domains of human thrombospondin-1. Biochemistry 41:14329–14339
Imondi R, Kaprielian Z (2001) Commissural axon pathfinding on the contralateral side of the floor plate: a role for B-class ephrins in specifying the dorsoventral position of longitudinally projecting commissural axons. Development 128:4859–4871
Irigoin C, Rodriguez EM, Heinrichs M, Frese K, Herzog S, Oksche A, Rott R (1990) Immunocytochemical study of the subcommissural organ of rats with induced postnatal hydrocephalus. Exp Brain Res 82:384–392
Kamata T, Katsube K, Michikawa M, Yamada M, Takada S, Mizusawa H (2004) R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta 1676:51–62
Kaprielian Z, Imondi R, Runko E (2000) Axon guidance at the midline of the developing CNS. Anat Rec 261:176–197
Kaprielian Z, Runko E, Imondi R (2001) Axon guidance at the midline choice point. Dev Dyn 221:154–181
Klar A, Baldassare M, Jessell TM (1992) F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell 69:95–110
Lehmann C, Naumann WW (2005) Axon pathfinding and the floor plate factor Reissner’s substance in wildtype, cyclops and one-eyed pinhead mutants of Danio rerio. Brain Res Dev Brain Res 154:1–14
Lehmann W, Naumann W, Wagner U (1993) Tissue culture of bovine subcommissural organ. Anat Embryol 187:505–514
Lichtenfeld J, Viehweg J, Schutzenmeister J, Naumann WW (1999) Reissner’s substance expressed as a transient pattern in vertebrate floor plate. Anat Embryol (Berl) 200:161–174
Losecke W, Naumann W, Sterba G (1986) Immuno-electron-microscopic analysis of the basal route of secretion in the subcommissural organ of the rabbit. Cell Tissue Res 244:449–456
Louvi A, Wassef M (2000) Ectopic engrailed 1 expression in the dorsal midline causes cell death, abnormal differentiation of circumventricular organs and errors in axonal pathfinding. Development 127:4061–4071
Lumsden AG, Davies AM (1983) Earliest sensory nerve fibres are guided to peripheral targets by attractants other than nerve growth factor. Nature 306:786–788
Lustig M, Erskine L, Mason CA, Grumet M, Sakurai T (2001) Nr-CAM expression in the developing mouse nervous system: ventral midline structures, specific fiber tracts, and neuropilar regions. J Comp Neurol 434:13–28
Marin O, Blanco MJ, Nieto MA (2001) Differential expression of Eph receptors and ephrins correlates with the formation of topographic projections in primary and secondary visual circuits of the embryonic chick forebrain. Dev Biol 234:289–303
Martinez S (1987) Estudio experimental de la conectividad tectal en relación con la región pretectal y la comisura posterior. PhD thesis. University of Murcia, Murcia, Spain
Mastick GS, Easter SS Jr (1996) Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev Biol 173:79–94
Meiniel A (2001) SCO-spondin, a glycoprotein of the subcommissural organ/Reissner’s fiber complex: evidence of a potent activity on neuronal development in primary cell cultures. Microsc Res Tech 52:484–495
Meiniel O, Meiniel A (2007) The complex multidomain organization of SCO-spondin protein is highly conserved in mammals. Brain Res Rev 53:321–327
Meiniel A, Meiniel R, Goncalves-Mendes N, Creveaux I, Didier R, Dastugue B (2003) The thrombospondin type 1 repeat (TSR) and neuronal differentiation: roles of SCO-spondin oligopeptides on neuronal cell types and cell lines. Int Rev Cytol 230:1–39
Monnerie H, Boespflug-Tanguy O, Dastugue B, Meiniel A (1996) Soluble material from Reissner’s fiber displays anti-aggregative activity in primary cultures of chick cortical neurons. Brain Res Dev Brain Res 96:120–129
Monnerie H, Dastugue B, Meiniel A (1997) Reissner’s fibre promotes neuronal aggregation and influences neuritic outgrowth in vitro. Cell Tissue Res 287:285–295
Monnerie H, Dastugue B, Meiniel A (1998) Effect of synthetic peptides derived from SCO-spondin conserved domains on chick cortical and spinal-cord neurons in cell cultures. Cell Tissue Res 293:407–418
Murbach V, Hauser R (1974) Deranged axial growth of the Xenopus laevis larva following elimination of the subcommissural organ. Rev Suisse Zool 81:678–684
Naumann WW, Lehmann W, Debbage P (1993) The subcommissural organ and ontogenetic development of the brain. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 61–69
Nualart F, Hein S (2001) Biosynthesis and molecular biology of the secretory proteins of the subcommissural organ. Microsc Res Tech 52:468–483
Oksche A (1961) Vergleichende Untersuchungen über die sekretorische Aktivität der Subkommissuralorgans und den Gliacharakter seiner Zellen. Z Zellforsch Mikrosk Anat 54:549–612
Oksche A (1962) Histological, histochemical and experimental studies on the subcommissural organ of Anura (with reference to the epiphysial complex). Z Zellforsch Mikrosk Anat 57:240–326
Oksche A (1969) The subcommissural organ. J Neurovisc Relat Suppl 9:111–139
Peruzzo B, Perez J, Fernandez-Llebrez P, Perez-Figares JM, Rodriguez EM, Oksche A (1990) Ultrastructural immunocytochemistry and lectin histochemistry of the subcommissural organ in the snake Natrix maura with particular emphasis on its vascular and leptomeningeal projections. Histochemistry 93:269–277
Pombal MA, Puelles L (1999) Prosomeric map of the lamprey forebrain based on calretinin immunocytochemistry, Nissl stain, and ancillary markers. J Comp Neurol 414:391–422
Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z (2006) The development of cortical connections. Eur J Neurosci 23:910–920
Puelles L, Rubenstein JL (2003) Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26:469–476
Puelles L, Javier Milan F, Martinez-de-la-Torre M (1996) A segmental map of architectonic subdivisions in the diencephalon of the frog Rana perezi: acetylcholinesterase-histochemical observations. Brain Behav Evol 47:279–310
Ramos C, Fernandez-Llebrez P, Bach A, Robert B, Soriano E (2004) Msx1 disruption leads to diencephalon defects and hydrocephalus. Dev Dyn 230:446–460
Redies C, Arndt K, Ast M (1997) Expression of the cell adhesion molecule axonin-1 in neuromeres of the chicken diencephalon. J Comp Neurol 381:230–252
Richter HG, Munoz RI, Millan CS, Guinazu MF, Yulis CR, Rodriguez EM (2001) The floor plate cells from bovine express the mRNA encoding for SCO-spondin and its translation products. Brain Res Mol Brain Res 93:137–147
Rodriguez EM (1970) Ependymal specializations. II. Ultrastructural aspects of the apical secretion of the toad subcommissural organ. Z Zellforsch Mikrosk Anat 111:15–31
Rodriguez EM, Oksche A, Hein S, Rodriguez S, Yulis R (1984) Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res 237:427–441
Rodriguez EM, Oksche A, Hein S, Yulis CR (1992) Cell biology of the subcommissural organ. Int Rev Cytol 135:39–121
Rodriguez EM, Jara P, Richter H, Montecinos H, Flandez B, Wiegand R, Oksche A (1993) Evidence for the release of CSF-soluble secretory material from the subcommissural organ, with particular reference to the situation in the human. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 121–131
Rodriguez EM, Rodriguez S, Hein S (1998) The subcommissural organ. Microsc Res Tech 41:98–123
Rogers JH, Ciossek T, Ullrich A, West E, Hoare M, Muir EM (1999) Distribution of the receptor EphA7 and its ligands in development of the mouse nervous system. Brain Res Mol Brain Res 74:225–230
Rühle HJ (1971) Anomalien im Wachstum der Achsenorgane nach experimenteller Ausschaltung des Komplexes Subcommissuralorgan-Reissnerscher Faden. Untersuchungen am Rippenmolch (Pleurodeles waltii). Acta Zool 52:23–68
Schoebitz K, Garrido O, Heinrichs M, Speer L, Rodriguez EM (1986) Ontogenical development of the chick and duck subcommissural organ. An immunocytochemical study. Histochemistry 84:1–40
Schoebitz K, Rodriguez EM, Garrido O, Brio MA del (1993) Ontogenetic development of the subcommissural organ with reference to the flexural organ. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 41–49
Shu T, Richards LJ (2001) Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci 21:2749–2758
Shu T, Valentino KM, Seaman C, Cooper HM, Richards LJ (2000) Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J Comp Neurol 416:201–212
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Stoeckli ET, Sonderegger P, Pollerberg GE, Landmesser LT (1997) Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron 18:209–221
Tuttle R, Braisted JE, Richards LJ, O’Leary DD (1998) Retinal axon guidance by region-specific cues in diencephalon. Development 125:791–801
Vio K, Rodriguez S, Yulis CR, Oliver C, Rodriguez EM (2008) The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res 5:3
Williams SE, Mason CA, Herrera E (2004) The optic chiasm as a midline choice point. Curr Opin Neurobiol 14:51–60
Yoon MS, Puelles L, Redies C (2000) Formation of cadherin-expressing brain nuclei in diencephalic alar plate divisions. J Comp Neurol 421:461–480
Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M (2000) Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102:363–375
Acknowledgements
The authors are grateful to Dr. de la Rosa (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain) for providing the rabbit polyclonal antibody against Ng-CAM, to José Esteban Casares Mira and Pedro Jiménez Palomo for valuable technical assistance and to David Navas Fernández for his technical assistance in the confocal microscopy study.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Hoyo-Becerra and M. D. López-Ávalos contributed equally to this study and should be considered as joint first authors.
C. Hoyo-Becerra was a recipient of a predoctoral fellowship (PFPI) from the Ministerio de Ciencia y Tecnología (Spain). This work was supported by grants from the Ministerio de Ciencia y Tecnología (Spain) (BFU 2006-11754) and the Junta de Andalucía (P07-CVI-03079; SAS-S 0742).
Rights and permissions
About this article
Cite this article
Hoyo-Becerra, C., López-Ávalos, M.D., Cifuentes, M. et al. The subcommissural organ and the development of the posterior commissure in chick embryos. Cell Tissue Res 339, 383–395 (2010). https://doi.org/10.1007/s00441-009-0899-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0899-2