Abstract
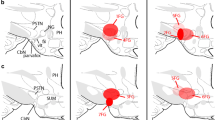
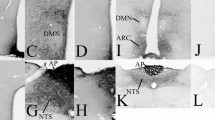
The pars tuberalis (PT) is the only pituitary region in close contact with the medial-basal hypothalamus and bathed by cerebrospinal fluid (CSF). Although PT has long been recognized as an endocrine gland, certain aspects of its structure remain obscure. The present investigation has been designed to gain information concerning (1) the cellular organization of PT, (2) the PT/median eminence spatial relationship and (3) the exposure of various cell compartments of PT to CSF. Non-endocrine cells (S100-reactive) appear as the organizer of the PT architecture. The apical poles of these cells line large cistern-like cavities and the processes of these cells establish a close spatial relationship with PT-specific secretory cells, portal capillaries and tanycytes. The cisterns are also endowed with clusters of ciliated cells and with a highly electron-dense and PAS-reactive content. The unique spatial organization of endocrine and non-endocrine cells of the PT supports a functional relationship between both cell populations. PT endocrine cells display a hallmark of PT-specific cells, namely, the paranuclear spot, which is a complex structure involving the Golgi apparatus, a large pool of immature secretory granules and a centriole from which originates a single 9+0 cilium projecting to the intercellular channels. Horseradish peroxidase (HRP) injected into the CSF readily reaches the intercellular channels of PT and the inner channel of the single cilium and is incorporated by the endocytic machinery of the secretory cells. The PT endocrine cells, through their single 9+0 cilium, may act as sensors of the CSF. HRP also reaches the lumen of the cisterns, indicating that this PT compartment is also exposed to CSF. PT endocrine cells establish direct cell-to-cell contacts with hypothalamic β1 tanycytes, suggesting a second means of brain-PT communication.









Similar content being viewed by others
References
Aguado LI, Schoebitz K, Rodríguez EM (1981) Intercellular channels in the pars tuberalis and their relationship to the subarachnoid space. Cell Tissue Res 218:345–354
Arvan P, Castle D (1998) Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J 332:593–610
Barret P, Messager S, Schuster C, Moar KM, Mercer JG, Morgan PJ (2002) Pituitary adenylate cyclase-activating polypeptide acts as a paracrine regulator of melatonin-responsive cells of the ovine pars tuberalis. Endocrinology 143:2366–2375
Bergmann M, Wittkowski W, Hoffmann K (1989) Ultrastructural localization of the thyrotropin (TSH)-like immunoreactivity in specific secretory cells of the hypophyseal pars tuberalis of the Djungarian hamster, Phodopus sungorus. Cell Tissue Res 256:649–652
Blázquez JL, Guerra M, Pastor F, Peruzzo B, Amat P, Rodríguez EM (2002) Antibodies obtained by xenotransplantation of organcultured median eminence specifically recognize hypothalamic tanycytes. Cell Tissue Res 308:241–253
Bloom GS, Brashear TA (1989) A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem 264:16083–16092
Bockers TM, Sourgens H, Wittkowski W, Jekat A, Pera F (1990) Changes in TSH-immunoreactivity in the pars tuberalis and pars distalis of the fetal rat hypophysis following maternal administration of propylthiouracil and thyroxin. Cell Tissue Res 260:403–410
Bockmann J, Bockers TM, Vennemann B, Niklowitz P, Muller J, Wittkowski W, Sabel B, Kreutz MR (1996) Short photoperiod-dependent down-regulation of thyrotropin-alpha and -beta in hamster pars tuberalis-specific cells is prevented by pinealectomy. Endocrinology 137:1804–1813
Bockmann J, Böckers TM, Winter C, Wittkowski W, Winterhoff H, Deufel TH, Kreuts R (1997) Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3, 5, 3″-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology 138:1019–1928
Borgonovo B, Ouwendijk J, Solimena M (2006) Biogenesis of secretory granules. Curr Opin Cell Biol 18:365–370
Cameron E, Foster CL (1972) Some light and electron microscopical observations on the pars tuberalis of the pituitary gland of the rabbit. J Endocrinol 54:505–511
Dardente H, Klosen P, Pévet P, Masson-Pévet M (2003) MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: effect of photoperiod. J Neuroendocrinol 15:778–786
Dellmann HD, Stoeckel ME, Hindelang-Gertner C, Porte A, Stutinsky F (1974) A comparative ultrastructural study of the pars tuberalis of various mammals, the chicken and the newt. Cell Tissue Res 148:313–329
Denef C (2008) Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol 20:1–70
Dupré SM, Burt DW, Talbot R, Downing A, Mouzaki D, Waddington D, Malpaux B, Davis JR, Lincoln GA, Loudon AS (2008) Identification of melatonin-regulated genes in the ovine pituitary pars tuberalis, a target site for seasonal hormone control. Endocrinology 149:5527–5539
Fauquier T, Lacampagne A, Travo P, Bauer K, Mollard P (2002) Hidden face of the anterior pituitary. Trends Endocrinol Metab 13:304–309
Ferrara N, Schweigerer L, Neufeld G, Mitchell R, Gospodarowicz D (1987) Pituitary follicular cells produce basic fibroblast growth factor. Proc Natl Acad Sci USA 84:5773–5777
Fitzgerald KT (1979) The structure and function of the pars tuberalis of the vertebrate adenohypophysis. Gen Comp Endocrinol 37:383–399
Gall C von, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH (2002) Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci 5:234–238
Gall C von, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW (2005) Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci 1040:508–511
Gao YS, Alvarez C, Nelson DS, Sztul E (1998) Molecular cloning, characterization, and dynamics of rat formiminotransferase cyclodeaminase, a Golgi-associated 58-kDa protein. J Biol Chem 273:33825–33834
Gospodarowicz D, Lau K (1989) Pituitary follicular cells secrete both vascular endothelial growth factor and follistatin. Biochem Biophys Res Commun 165:292–298
Graham ES, Webster CA, Hazlerigg DG, Morgan PJ (2002) Evidence for the biosynthesis of a prolactin-releasing factor from the ovine pars tuberalis, which is distinct from thyrotropin-releasing hormone. J Neuroendocrinol 14:945–954
Gross DS (1984) The mammalian hypophyseal pars tuberalis: a comparative immunocytochemical study. Gen Comp Endocrinol 56:283–298
Guerra M, Rodríguez EM (2001) Identification, cellular and subcellular distribution of 21 and 72 kDa proteins (tuberalins?) secreted by specific cells of the pars tuberalis. J Endocrinol 168:363–379
Guerra M, Rodríguez EM (2009) Expression of tuberalin II, α-subunit (GSU) and β-TSH in the pars tuberalis of the rat. Immunocytochemical evidence for PT-specific cell types. Neuroendocrinology (in press)
Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pévet M, Morgan PJ, Hazlerigg DG (2008) Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol 18:1147–1152
Hazlerigg DG, Hastings MH, Morgan PJ (1996a) Production of a prolactin releasing factor by the ovine pars tuberalis. J Neuroendocrinol 8:489–492
Hazlerigg DG, Barrett P, Hastings MH, Morgan PJ (1996b) Are nuclear receptors involved in pituitary responsiveness to melatonin? Mol Cell Endocrinol 123:53–59
Inoue K, Couch EF, Takano K, Ogawa S (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch Histol Cytol 62:205–218
Jilg A, Moek J, Weaver DR, Korf HW, Stehle JH, Gall C von (2005) Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J NeuroSci 22:2845–2854
Kameda Y (1990) Occurrence of colloid-containing follicles and ciliated cysts in the hypophysial pars tuberalis from guinea pigs of various ages. Am J Anat 188:185–198
Kameda Y, Miura M, Ohno S (2000) Expression of the common alpha-subunit mRNA of glycoprotein hormones during the chick pituitary organogenesis, with special reference to the pars tuberalis. Cell Tissue Res 299:71–80
Kanematsu N, Mori Y, Hayashi S, Hoshino K (1989) Presence of a distinct 24-hour melatonin rhythm in the ventricular cerebrospinal fluid of the goat. J Pineal Res 7:143–152
Kell CA, Stehle JH (2005) Just the two of us: melatonin and adenosine in rodent pituitary function. Ann Med 37:105–120
Klosen P, Bienvenu C, Demarteau O, Dardente H, Guerrero H, Pévet P, Masson-Pévet M (2002) The mt1 melatonin receptor and RORbeta receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J Histochem Cytochem 50:1647–1657
Knowles FRS, Kumar A (1969) Structural changes related to reproduction in the hypothalamus and in the pars tuberalis of the rhesus monkey. Part I: the hypothalamus. Part II: the pars tuberalis. Philos Trans R Soc Lond Biol 256:357–375
Kurotani R, Tahara S, Sanno N, Teramoto A, Mellon PL, Inoue K, Yoshimura S, Osamura RY (1999) Expression of Ptx1 in the adult rat pituitary glands and pituitary cell lines: hormone-secreting cells and folliculo-stellate cells. Cell Tissue Res 298:55–61
Lechan RM, Fekete C (2006) The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235
Morgan PJ, King TP, Lawson W, Davidson G (1991) Ultrastructure of melatonin-responsive cells in the ovine pars tuberalis. Cell Tissue Res 263:529–534
Morgan PJ, Barrett P, Davidson G, Lawson W, Hazlerigg D (1994) p72, a marker protein for melatonin action in ovine pars tuberalis cells: its regulation by protein kinase A and protein kinase C and differential secretion relative to prolactin. Neuroendocrinology 59:325–335
Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Ligo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T (2008) Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452:317–322
Nichols BJ, Lippincott-Schwartz J (2001) Endocytosis without clathrin coats. Trends Cell Biol 11:406–412
Ojeda SR, Lomniczi A, Sandau US (2008) Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol 20:732–742
Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T (2008) Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA 105:18238–18242
Oomizu S, Chaturvedi K, Sarkar DK (2004) Folliculostellate cells determine the susceptibility of lactotropes to estradiol’s mitogenic action. Endocrinology 145:1473–1480
Ozawa H, Kurosumi K (1989) Postnatal development of thyrotrophs in the rat anterior pituitary as studied by immunogold electron microscopy. Anat Embryol (Berl) 180:207–212
Pazour GJ, Agrin N, Leszyk J, Witman GB (2005) Proteomic analysis of a eukaryotic cilium. J Cell Biol 170:103–113
Peruzzo B, Pastor FE, Blázquez JL, Amat P, Rodríguez EM (2004) Polarized endocytosis and transcytosis in the hypothalamic tanycytes of the rat. Cell Tissue Res 317:147–164
Praetorius HA, Spring KR (2005) A physiological view of the primary cilium. Annu Rev Physiol 67:515–529
Reppert SM, Perlow MJ, Tamarkin L, Klein DC (1979) A diurnal melatonin rhythm in primate cerebrospinal fluid. Endocrinology 104:295–301
Rodríguez EM (1969) Fixation of the central nervous system by perfusion of the cerebral ventricles with a threefold aldehyde mixture. Brain Res 15:395–412
Rodríguez EM, González CB, Delannoy L (1979) Cellular organization of the lateral and postinfundibular regions of the median eminence in the rat. Cell Tissue Res 201:377–408
Rodríguez EM, Blázquez JL, Pastor F, Pelaez B, Peña P, Peruzzo B, Amat P (2005) Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164
Sakai T, Inoue K, Kurosumi K (1992) Light and electron microscopic immunocytochemistry of TSK-like cells occurring in the pars tuberalis of the adult male rat pituitary. Arch Histol Cytol 55:151–157
Sakai T, Sakamoto S, Ijima K, Matsubara K, Kato Y, Inoue K (1999) Characterization of TSH-positive cells in foetal rat pars tuberalis that fail to express Pit-1 factor and thyroid hormone beta2 receptors. J Neuroendocrinol 11:187–193
Satir P, Christensen S (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129:687–693
Schuster C (2007) Sites and mechanisms of action of melatonin in mammals: the MT1 and MT2 receptors. J Soc Biol 201:85–96
Skinner DC, Malpaux B (1999) High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology 140:4399–4405
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1970) The unlabelled antibody-enzyme method of immunohistochemistry: preparation and properties of a soluble antigen-antibody complex (horseradish-peroxidase-antiperoxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–345
Stoeckel ME, Hindelang C, Klein MJ, Poissonnier M, Felix JM (1994) Expression of the alpha-subunit of glycoprotein hormones in the pars tuberalis-specific glandular cells in rat, mouse and guinea pig. Cell Tissue Res 278:617–624
Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM (1997) Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138:3359–3368
Wagner GC, Johnston JD, Tournier BB, Ebling FJ, Hazlerigg DG (2007) Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur J Neurosci 25:485–490
Williams LM, Morgan PJ (1988) Demonstration of melatonin binding sites on the pars tuberalis of the rat. J Endocrinol 119:R1–R3
Wittkowski W, Bockmann J, Kreutz MR, Böckers T (1999) Cell and molecular biology of the pars tuberalis of the pituitary. Int Rev Cytol 185:157–194
Wong AO, Li WS, Lee EK, Leung MY, Tse LY, Chow BK, Lin HR, Chang JP (2000) Pituitary adenylate cyclase activating polypeptide as a novel hypophysiotropic factor in fish. Biochem Cell Biol 78:329–343
Yasuo S, Yoshimura T, Ebihara S, Korf HW (2007) Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology 148:4385–4392
Yokoyama T (2004) Motor or sensor: a new aspect of primary cilia function. Anat Sci Int 79:47–54
Yoshimura F, Nogami H, Yashiro T (1982) Fine structural criteria for pituitary thyrotrophs in immature and mature rats. Anat Rec 204:255–263
Acknowledgements
We are grateful to Genaro Alvial for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Francisco Pastor died after the present investigation had been completed. We wish to dedicate this publication to him.
This work was supported by grants from DID and MECESUP of Universidad Austral to M.G and a grant from Fondecyt no. 1070241, Chile, to E.M.R.
Rights and permissions
About this article
Cite this article
Guerra, M., Blázquez, J.L., Peruzzo, B. et al. Cell organization of the rat pars tuberalis. Evidence for open communication between pars tuberalis cells, cerebrospinal fluid and tanycytes. Cell Tissue Res 339, 359–381 (2010). https://doi.org/10.1007/s00441-009-0885-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0885-8