Abstract
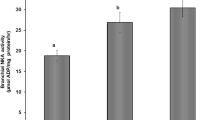
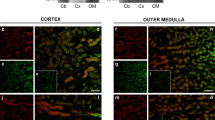
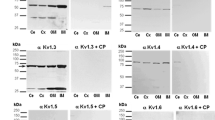
The amiloride-sensitive epithelial sodium channel (ENaC) has previously been shown to be involved in the maintenance of body fluid volume and in Na+ absorption across the skin and urinary bladder in amphibians. However, the function and distribution of ENaC have not been clearly described in amphibian kidney. We therefore cloned the ENaC α-subunit cDNA from kidney of the marine toad, Bufo marinus. The ENaC mRNA and protein were abundantly expressed in the kidney and in the urinary bladder and ventral pelvic skin. In an immunohistochemical study, the ENaC α-subunit protein was specifically localized to the apical membrane of the principal cells but not the intercalated cells from the late distal tubule to the collecting duct in the kidney or in the apical area of cells of urinary bladder epithelia. When toads were acclimated to dry and hyper-saline environments, the levels of ENaC mRNA expression in the kidney and urinary bladder decreased under hyper-saline acclimation, but not under dry conditions. Immunohistochemical observations indicated that the levels of ENaC protein expression were much lower in the apical area of renal distal tubules and urinary bladder epithelia of hyper-saline acclimated toad compared with controls. The present study suggests that Bufo ENaC is significantly expressed and functions during Na+ reabsorption in the apical membrane domain in the distal nephron of normal and desiccated toads. Natriuresis may be caused by decreases in ENaC expression and its trafficking to the cell surface in the distal nephron, a response to prevent excessive Na+ reabsorption in hyper-saline-acclimated toads.







Similar content being viewed by others
References
Alvarez de la Rosa D, Li H, Canessa CM (2002) Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells. J Gen Physiol 119:427–442
Bakker HR, Bradshaw SD (1977) Effect of hypothalamic lesions on water metabolism of the toad Bufo marinus. J Endocrinol 75:161–172
Bentley PJ (2002) The Amphibia. In: Bentley, PJ (ed) Endocrines and osmoregulation. A comparative account in vertebrates, vol 39. Springer, Heidelberg, pp 155–186
Biber TU, Curran PF (1970) Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol 56:83–99
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown D, Gluck S, Hartwig J (1987) Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ ATPase. J Cell Biol 105:1637–1648
Chew MM, Eliott AB, Wong HY (1972) Permeability of urinary bladder of Rana cancrivora to urea in the presence of oxytocin. J Physiol (Lond) 223:757–772
Fuchs W, Larsen EH, Lindemann B (1977) Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol (Lond) 267:137–166
Garty H, Palmer LG (1997) Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77:359–396
Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frokiaer J, Knepper MA, Nielsen S (2001) Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol 280:F1093–F1106
Hayashi M, Yamada H, Mitamura T, Horii T, Yamamoto A, Moriyama Y (2000) Vacuolar H+-ATPase localized in plasma membranes of malaria parasite cells, Plasmodium falciparum, is involved in regional acidification of parasitized erythrocytes. J Biol Chem 275:34353–34358
Hillyard SD (1999) Behavioral, molecular and integrative mechanisms of amphibian osmoregulation. J Exp Zool 283:662–674
Hillyard SD, Zeiske W, Van Driessche W (1982) A fluctuation analysis study of the development of amiloride-sensitive Na+ transport in the skin of larval bullfrogs (Rana catesbeiana). Biochim Biophys Acta 692:455–461
Jensik P, Holbird D, Cox T (2002) Cloned bullfrog skin sodium (fENaC) and xENaC subunits hybridize to form functional sodium channels. J Comp Physiol [B] 172:569–576
Katz U (1986) Volume regulation in salt-acclimated toad (Bufo viridis): the role of urea and urinary bladder. Comp Biochem Physiol [A] 84:505–509
Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82:735–767
Kleyman TR, Smith PR, Benos DJ (1994) Characterization and localization of epithelial Na+ channels in toad urinary bladder. Am J Physiol 266:C1105–C1111
Koefoed-Johnsen V, Ussing HH (1958) On the nature of the frog skin potential. Acta Physiol Scand 42:298–308
Konno N, Hyodo S, Takei Y, Matsuda K, Uchiyama M (2005) Plasma aldosterone, angiotensin II, and arginine vasotocin concentrations in the toad, Bufo marinus, following osmotic treatments. Gen Comp Endocrinol 140:86–93
Konno N, Hyodo S, Matsuda K, Uchiyama M (2006) Effect of osmotic stress on expression of a putative facilitative urea transporter in the kidney and urinary bladder of the marine toad, Bufo marinus. J Exp Biol 209:1207–1216
Larsen EH, Christoffersen BC, Jensen LJ, Sørensen JB, Willumsen NJ (1996) Role of mitochondria-rich cells in epithelial chloride uptake. Exp Physiol 81:525–534
Leaf A, Anderson J, Page LB (1958) Active sodium transport by the isolated toad bladder. J Gen Physiol 41:657–668
Lim WC, Kim D, Park JB, Kim SH, Lee YJ (2004) Sodium chloride regulation of the alpha epithelial amiloride-sensitive sodium channel (αENaC) gene requires syntheses of new protein(s). J Steroid Biochem Mol Biol 88:305–310
Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B (2000) Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279:F252–F258
Loffing J, Summa V, Zecevic M, Verrey F (2001a) Mediators of aldosterone action in the renal tubule. Curr Opin Nephrol Hypertens 10:667–675
Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F (2001b) Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280:F675–F682
Møbjerg N, Larsen EH, Jespersen Å (1998) Morphology of the nephron in the mesonephros of Bufo bufo (Amphibia, Anura, Bufonidae). Acta Zool 79:31–51
Møbjerg N, Larsen EH, Novak I (2004) Ion transport mechanisms in the mesonephric collecting duct system of the toad Bufo bufo: microelectrode recordings from isolated and perfused tubules. Comp Biochem Physiol [A] Mol Integr Physiol 137:585–595
Palmer LG (1985) Interaction of amiloride and other blocking cations with the apical Na channel in the toad urinary bladder. J Membr Biol 87:191–199
Puoti A, May A, Canessa CM, Horisberger JD, Schild L, Rossier BC (1995) The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am J Physiol 269:C188–C197
Stetson DL, Steinmetz PR (1985) Alpha and beta types of carbonic anhydrase-rich cells in turtle bladder. Am J Physiol 249:F553–F565
Stoner LC, Engbretson BG, Viggiano SC, Benos DJ, Smith PR (1995) Amiloride-sensitive apical membrane sodium channels of everted Ambystoma collecting tubule. J Membr Biol 144:147–156
Takada M, Shimomura T, Hokari S, Jensik PJ, Cox TC (2006) Larval bullfrog skin expresses ENaC despite having no amiloride-blockable transepithelial Na+ transport. J Comp Physiol [B] 176:287–293
Tallini NY, Stoner LC (2002) Amiloride-sensitive sodium current in everted Ambystoma initial collecting tubule: short-term insulin effects. Am J Physiol Cell Physiol 283:C1171–C1181
Uchiyama M, Yoshizawa H (2002) Nephron structure and immunohistochemical localization of ion pumps and aquaporins in the kidney of frogs inhabiting different environments. Symp Soc Exp Biol 54:109–128
Uchiyama M, Murakami T, Yoshizawa H, Wakasugi C (1990) Structure of the kidney in the crab-eating frog, Rana cancrivora. J Morphol 204:147–156
Verrey F, Loffing J, Zecevic M, Heitzmann D, Staub O (2003) SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem 13:21–28
Acknowledgements
The authors thank Prof. H. Ota (Tropical Biosphere Research Center, University of the Ryukyu) and Dr. Y. Hokama (Agricultural Experiment Station of Okinawa Prefecture) for their kind help and suggestions with collecting animals. They are also grateful to Prof. T. Wakasugi (Department of Life and Environmental Science, University of Toyama) for his valuable advice regarding the molecular biological study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by a research grant (a priority project in Science Faculty) from Toyama University and by a Ground-based Research Announcement for Space Utilization promoted by the Japan Space Forum to M.U.; N.K. is grateful to the Japan Society of the Promotion of Science (JSPS) for a young scientist’s fellowship.
Rights and permissions
About this article
Cite this article
Konno, N., Hyodo, S., Yamada, T. et al. Immunolocalization and mRNA expression of the epithelial Na+ channel α-subunit in the kidney and urinary bladder of the marine toad, Bufo marinus, under hyperosmotic conditions. Cell Tissue Res 328, 583–594 (2007). https://doi.org/10.1007/s00441-007-0383-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0383-9