Abstract.
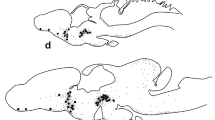
The expression of the long form of prolactin receptor (PRL-R) mRNAs was studied in reproductive organs from both male and female Typhlonectes compressicauda, an amphibian, by quantitative in situ hybridization. These PRL-R mRNAs were expressed in all the organs studied. In ovarian tissue, mRNAs coding for the long form of PRL-R were strongly expressed in ovaries with compact and vascularized corpora lutea, i.e., during the middle and the end of pregnancy. During the other periods of cycle, sexual rest, vitellogenesis and beginning of pregnancy, expression of these mRNAs was lower than in other periods. In the testis, mRNAs coding for the long form of PRL-R were expressed in germ cells as well as in Leydig and Sertoli cells. In müllerian glands, important variations of expression in these mRNAs have been observed with a large increase during the breeding period and a decrease during sexual rest. Variations of gene expression of the long form of PRL-R for all tissues studied are correlated to synthesis of PRL in the pituitary in both male and female Typhlonectes compressicauda.



Similar content being viewed by others
References
Anjubault E, Exbrayat JM (1998) Yearly cycle of Leydig-like cells in testes of Typhlonectes compressicaudus (Amphibia, Gymnophiona). In: Miaud C, Guyetant R (eds) Current studies in herpetology. SEH, Le Bourget du Lac, France, pp 53–58
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268
Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA (1988) Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin gene family. Cell 53:69–77
Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA (1989) Identification of cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol 3:1455–1461
Bowen JM, Telleria CM, Towns R, Keyes PL (2000) Down regulation of long-form prolactin receptor mRNA during prolactin-induced luteal regression. Eur J Endocrinol 143:285–292
Clarke DL, Arey BJ, Linzer DI (1993) Prolactin receptor messenger ribonucleic acid expression in the ovary during the rat estrous cycle. Endocrinology 133:2594–2603
Clarke LA, Wathes DC, Jabbour HN (1997) Expression and localization of prolactin receptor messenger ribonucleic acid in red deer ovary during the estrous cycle and pregnancy. Biol Reprod 57:865–872
Exbrayat JM (1983) Premières observations sur le cycle annuel de l'ovaire de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Batracien Apode vivipare. C R Acad Sci Paris 296:493–498
Exbrayat JM (1985) Cycle des canaux de Müller chez le mâle adulte de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Apode. C R Acad Sci Paris 301:507–512
Exbrayat JM (1986) Quelques aspects de la biologie de la reproduction chez Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Apode. Doctorat ès Sciences Naturelles, Université Paris VI
Exbrayat JM (1988a) Variations pondérales des organes de réserve (corps adipeux et foie) chez Typhlonectes compressicaudus, Amphibien Apode vivipare au cours des alternances saisonnières et des cycles de reproduction. Ann Sci Nat Zool Paris 13éme sér 9:45–53
Exbrayat JM (1988b) Croissance et cycle des voies génitales femelles chez Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Apode vivipare. Amphibia-Reptilia 9:117–137
Exbrayat JM (1989) The cytological modifications of the distal lobe of the hypophysis in Typhlonectes compressicaudus (Duméril and Bibron, 1841), Amphibia Gymnophiona, during the cycles of seasonal activity. I—In adult males. Biol Struct Morphol 2:117–123
Exbrayat JM (1992) Reproduction et organes endocrines chez les femelles d'un Amphibien Gymnophione vivipare, Typhlonectes compressicaudus. Bull Soc Herp Fr 64:37–50
Exbrayat JM (1996) Croissance et cycle du cloaque chez Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Gymnophione. Bull Soc Herp Fr 121:99–104
Exbrayat JM (2001) Genome visualization by classic methods in light microscopy. CRC, Boca Raton, USA
Exbrayat, JM Collenot G (1983) Quelques aspects de l'évolution de l'ovaire de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Batracien Apode vivipare. Etude quantitative et histochimique des corps jaunes. Reprod Nutr Dev 23:889–898
Exbrayat JM, Morel G (1990/1991) The cytological modifications of the distal lobe of the hypophysis of Typhlonectes compressicaudus (Duméril and Bibron, 1841), Amphibia Gymnophiona, during the cycles of seasonal activity. II—In adult males. Biol Struct Morphol 3:129–138
Exbrayat JM, Morel G (1995) Prolactin (PRL)-coding mRNA in Typhlonectes compressicaudus, a viviparous gymnophionan amphibian. An in situ hybridization study. Cell Tissue Res 280:133–138
Exbrayat JM, Ouhtit A, Morel G (1997) Visualization of gene expression of prolactin receptors (PRL-R) by in situ hybridization, in Typhlonectes compressicaudus, a Gymnophionan amphibian. Life Sci 61:1915–1928
Exbrayat JM, Pujol P, Leclercq B (1998) Quelques aspects des cycles sexuels et nycthéméraux chez les Amphibiens. Bull Soc Zool Fr 123:113–124
Garcia-Caballero T, Mertani HM, Lambert A, Gallego R, Fraga M, Pintos E, Forteza J, Chevallier M, Lobie PE, Vonderhaar BK, Beiras A, Morel G (2000) Increased expression of growth hormone and prolactin receptors in hepatocellular carcinomas. Endocrine 12:265–271
Gaytan F, Bellido C, Morales C, Sanchez-Criado JE (2001) Cyclic changes in the responsiveness of regressing corpora lutea to the luteolytic effects of prolactin in rats. Reproduction 122:411–417
Girod C (1983) Immunohistochemistry of the vertebrate adenohypophysis. In: Graumann W, Newmann K (eds) Handbuch der Histochemie, vol VIII, suppl, part 5. G Fisher, Stuttgart, pp 1–536
Guillaumot P, Tabone E, Benahmed M (1996) Sertoli cells as potential targets of prolactin action in the testis. Mol Cell Endocrinol 122:199–206
Higashimoto Y, Nakao N, Ohkubo T, Tanaka M, Nakashima K (2001) Structure and tissue distribution of prolactin receptor mRNA in Japanese flounder (Paralichtys olivaceus): conserved and preferential expression in osmoregulatory organs. Gen Comp Endocrinol 123:170–179
Hondo E, Kurohmaru M, Sakai S, Ogawa K, Hayashi Y (1995) Prolactin receptor expression in rat spermatogenic cells. Biol Reprod 52:1284–1290
Howell-Skalla LA, Bunick D, Nelson RA, Bahr JM (2000) Testicular recrudescence in the male black bear (Ursus americanus): changes in testicular luteinizing hormone-, follicle-stimulating hormone-, and prolactin-receptor ribonucleic acid abundance and dependency on prolactin. Biol Reprod 63:440–447
Jabbour HN, Clarke LA, McNeilly AS, Edery M, Kelly PA (1998) Is prolactin a gonadotrophic hormone in red deer (Cervus elaphus)? Pattern of expression of the prolactin receptor gene in the testis and epididymis. J Mol Endocrinol 20:175–182
Jahn GA, Edery M, Belair L, Kelly PA, Djiane J (1991) Prolactin receptor gene expression in rat mammary gland and liver during pregnancy and lactation. Endocrinology 128:2976–2984
Lesueur L, Edery M, Ali S, Paly J, Kelly PA, Djiane J (1991) Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A 88:824–828
Mazzi V, Vellano C, Toscano C (1967) Antigonadal effects of prolactin in adult male newt (Triturus cristatus carnifex Laur.). Gen Comp Endocrinol 8:320–324
Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G (1998) Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer 79:202–211
Morel G, Cavalier A (2000) In situ hybridisation in light microscopy. CRC, Boca Raton, USA
Nevalainen MT, Valve EM, Ingleton PM, Harkonen PL (1996) Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate. Endocrinology 137:3078–3088
Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL (1997) Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest 99:618–627
Nicoll CS, Bern HA (1972) On the actions of prolactin among the vertebrates: is there a common denominator? In: Wolstenholme GEW, Knight J (eds) Lactogenic hormones. Churchill Livingstone, London
Ohkubo T, Tanaka M, Nakashima K, Sharp PJ (1998) Relationship between prolactin receptor mRNA in the anterior pituitary gland and hypothalamus and reproductive state in male and female bantams (Gallus domesticus). Gen Comp Endocrinol 111:167–176
Ouhtit A, Morel G, Kelly PA (1993a) Visualization of gene expression of short and long forms of prolactin receptor in the rat. Endocrinology 133:135–144
Ouhtit A, Morel G, Kelly PA (1993b) Visualization of gene expression of short and long forms of prolactin receptor in rat reproductive tissues. Biol Reprod 49:528–536
Ouhtit A, Kelly PA, Morel G (1994) Visualization of gene expression of short and long forms of prolactin receptor in rat digestive tissues. Am J Physiol 266:G807–815
Peirce SK, Chen WY, Chen WY (2001) Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J Endocrinol 171:R1–4
Sandra O, Le Rouzic P, Cauty C, Edery M, Prunet P (2000) Expression of the prolactin receptor (tiPRL-R) gene in tilapia Oreochromis niloticus: tissue distribution and cellular localization in osmoregulatory organs. J Mol Endocrinol 24:215–224
Shirota M, Banville D, Ali S, Jolicoeur C, Boutin JM, Edery M, Djiane J, Kelly PA (1990) Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol 4:1136–1143
Taylor EH (1968) The Caecilians of the world. A taxonomic view. University of Kansas Press, Lawrence, KS
Vlaming VL de (1979) Actions of prolactin among the vertebrates. In: Barrington EJW (ed) Hormones and evolution, vol 2. Academic, New York, pp 561–642
Wake MH (1968) Evolutionary morphology of the caecilian urogenital system, part I: the gonads and fat bodies. J Morphol 126:291–332
Wake MH (1970a) Evolutionary morphology of the caecilian urogenital system, part II: the kidneys and urogenital ducts. Acta Anat 75:321–358
Wake MH (1970b) Evolutionary morphology of the caecilian urogenital system, part III: the bladder. Herpetologica 26:120–128
Wake MH (1972) Evolutionary morphology of the caecilian urogenital system, part IV: the cloaca. J Morphol 136:353–366
Wake MH (1977a) Fetal maintenance and its evolutionary significance in the Amphibia: Gymnophiona. J Herpetol 11:379–386
Wake MH (1977b) The reproductive biology of Caecilians. An evolutionary perspective. In: Taylor DH, Guttman SI (eds) The reproductive biology of Amphibians. Miami University, Oxford, OH, USA, pp 73–100
Wake MH (1980) Reproduction, growth and population structure of the central american Caecilian Dermophis mexicanus. Herpetologica 36:244–256
Wake MH (1981) Structure and function of the male mullerian gland in Caecilians (Amphibia: Gymnophiona), with comments on its evolutionary significance. J Herpetol 15:17–22
Wake MH, Exbrayat JM, Delsol M (1985) The development of the chondrocranium of Typhlonectes compressicaudus (Gymnophiona), with comparison to other species. J Herpetol 19:568–577
Zhang FP, Rannikko A, Toppari J, Bartke A, Huhtaniemi I (1995) Developmental expression of the prolactin receptor gene in rat gonads. J Endocrinol 147:497–505
Acknowledgements.
We are grateful to P. Kelly, INSERM U344, Hôpital Necker, Paris, for providing the probes and to M.T. Laurent for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Exbrayat, JM., Morel, G. Visualization of gene expression of prolactin-receptor (PRL-R) by in situ hybridization in reproductive organs of Typhlonectes compressicauda, a gymnophionan amphibian. Cell Tissue Res 312, 361–367 (2003). https://doi.org/10.1007/s00441-003-0730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0730-4