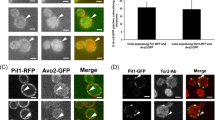
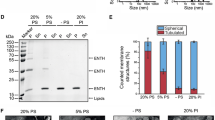
Abstract
Membrane compartmentalization allows the spatial segregation of different functions, such as signal transduction and protein trafficking, and ensures their fidelity and efficiency. Eisosomes constitute nanoscale furrow-like invaginations of the plasma membrane where proteins and lipids segregate. The intense interest elicited by eisosomes over the last few years has led to the identification and molecular characterization of their key constituents. This review addresses eisosome structure, functions and its implications for the mechanistic understanding of curvature-induced membrane nanodomains formation and signaling compartmentalization in living cells.


Similar content being viewed by others
References
Aguilar PS, Frohlich F, Rehman M, Shales M, Ulitsky I, Olivera-Couto A, Braberg H, Shamir R, Walter P, Mann M, Ejsing CS, Krogan NJ, Walther TC (2010) A plasma-membrane E-MAP reveals links of the eisosome with sphingolipid metabolism and endosomal trafficking. Nat Struct Mol Biol 17:901–908
Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB (2008) The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell 19:5214–5225
Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T (2008) Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab 7:148–158
Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD (2004) Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J 23:3747–3757
Bagnat M, Simons K (2002) Cell surface polarization during yeast mating. Proc Natl Acad Sci USA 99:14183–14188
Bagnat M, Keranen S, Shevchenko A, Simons K (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA 97:3254–3259
Bastiani M, Parton RG (2010) Caveolae at a glance. J Cell Sci 123:3831–3836
Berchtold D, Walther TC (2009) TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell 20:1565–1575
Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R (2012) Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 14:542–547
Bernardo SM, Lee SA (2010) Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol 10:133
Brach T, Specht T, Kaksonen M (2011) Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J Cell Sci 124:328–337
Chen L, Davis NG (2000) Recycling of the yeast a-factor receptor. J Cell Biol 151:731–738
de Bony J, Lopez A, Gilleron M, Welby M, Laneelle G, Rousseau B, Beaucourt JP, Tocanne JF (1989) Transverse and lateral distribution of phospholipids and glycolipids in the membrane of the bacterium Micrococcus luteus. Biochemistry 28:3728–3737
Demel RA, Jansen JW, van Dijck PW, van Deenen LL (1977) The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta 465:1–10
Deng C, Xiong X, Krutchinsky AN (2009) Unifying fluorescence microscopy and mass spectrometry for studying protein complexes in cells. Mol Cell Proteomics 8:1413–1423
Douglas LM, Wang HX, Keppler-Ross S, Dean N, Konopka JB (2012) Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. MBio 3(1). doi:10.1128/mBio.00254-11
Douglass AD, Vale RD (2005) Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell 121:937–950
Fiedler D, Braberg H, Mehta M, Chechik G, Cagney G, Mukherjee P, Silva AC, Shales M, Collins SR, van Wageningen S, Kemmeren P, Holstege FC, Weissman JS, Keogh MC, Koller D, Shokat KM, Krogan NJ (2009) Functional organization of the S. cerevisiae phosphorylation network. Cell 136:952–963
Fishov I, Woldringh CL (1999) Visualization of membrane domains in Escherichia coli. Mol Microbiol 32:1166–1172
Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H (2001) Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J 20:6783–6792
Frohlich F, Moreira K, Aguilar PS, Hubner NC, Mann M, Walter P, Walther TC (2009) A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol 185:1227–1242
Galindo A, Hervas-Aguilar A, Rodriguez-Galan O, Vincent O, Arst HN Jr, Tilburn J, Penalva MA (2007) PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32. Traffic 8:1346–1364
Galindo A, Calcagno-Pizarelli AM, Arst HN Jr, Penalva MA (2012) An ordered pathway for the assembly of ESCRT-containing fungal ambient pH signalling complexes at the plasma membrane. J Cell Sci 125:1784–1795. doi:10.1242/jcs.098897
Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK (2011) Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic 12:1341–1355
Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS (2003) Global analysis of protein expression in yeast. Nature 425:737–741
Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W (2007) Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 26:1–8
Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer WB, Opekarova M, Tanner W (2008) Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol 183:1075–1088
Guan XL, Souza CM, Pichler H, Dewhurst G, Schaad O, Kajiwara K, Wakabayashi H, Ivanova T, Castillon GA, Piccolis M, Abe F, Loewith R, Funato K, Wenk MR, Riezman H (2009) Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol Biol Cell 20:2083–2095
Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7:456–462
Hansen CG, Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20:177–186
Hayer A, Stoeber M, Bissig C, Helenius A (2010) Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11:361–382
Herrador A, Herranz S, Lara D, Vincent O (2010) Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol 30:897–907
Hosiner D, Sponder G, Graschopf A, Reipert S, Schweyen RJ, Schuller C, Aleschko M (2011) Pun1p is a metal ion-inducible, calcineurin/Crz1p-regulated plasma membrane protein required for cell wall integrity. Biochim Biophys Acta 1808:1108–1119
Jin H, McCaffery JM, Grote E (2008) Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J Cell Biol 180:813–826
Johannes L, Mayor S (2010) Induced domain formation in endocytic invagination, lipid sorting, and scission. Cell 142:507–510
Kabeche R, Baldissard S, Hammond J, Howard L, Moseley JB (2011) The filament-forming protein Pil1 assembles linear eisosomes in fission yeast. Mol Biol Cell 22:4059–4067
Kaiser HJ, Orlowski A, Rog T, Nyholm TK, Chai W, Feizi T, Lingwood D, Vattulainen I, Simons K (2011) Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc Natl Acad Sci USA 108:16628–16633
Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7:404–414
Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y (2005) Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol 25:7239–7248
Kamble C, Jain S, Murphy E, Kim K (2011) Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae. J Biosci 36:79–96
Karotki L, Huiskonen JT, Stefan CJ, Ziolkowska NE, Roth R, Surma MA, Krogan NJ, Emr SD, Heuser J, Grunewald K, Walther TC (2011) Eisosome proteins assemble into a membrane scaffold. J Cell Biol 195:889–902
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34:351–378
Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK (2011) Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci 36:604–615
Lagorce A, Hauser NC, Labourdette D, Rodriguez C, Martin-Yken H, Arroyo J, Hoheisel JD, Francois J (2003) Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J Biol Chem 278:20345–20357
Lauwers E, Andre B (2006) Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic 7:1045–1059
London E, Brown DA (2000) Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta 1508:182–195
Lopez D, Kolter R (2010) Functional microdomains in bacterial membranes. Genes Dev 24:1893–1902
Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC (2008) The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J Biol Chem 283:10433–10444
Malinska K, Malinsky J, Opekarova M, Tanner W (2003) Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 14:4427–4436
Malinska K, Malinsky J, Opekarova M, Tanner W (2004) Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci 117:6031–6041
Markin VS (1981) Lateral organization of membranes and cell shapes. Biophys J 36:1–19
Matsumoto K, Kusaka J, Nishibori A, Hara H (2006) Lipid domains in bacterial membranes. Mol Microbiol 61:1110–1117
McLaughlin S, Murray D (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438:605–611
Moor H, Muhlethaler K (1963) Fine structure in frozen-etched yeast cells. J Cell Biol 17:609–628
Moreira KE, Walther TC, Aguilar PS, Walter P (2009) Pil1 controls eisosome biogenesis. Mol Biol Cell 20:809–818
Mouritsen OG, Bloom M (1984) Mattress model of lipid-protein interactions in membranes. Biophys J 46:141–153
Mueller NS, Wedlich-Soldner R, Spira F (2012) From mosaic to patchwork: Matching lipids and proteins in membrane organization. Mol Membr Biol. doi:10.3109/09687688.2012.687461
Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D (1994) Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol 125:381–391
Munro S (2003) Lipid rafts: elusive or illusive? Cell 115:377–388
Murphy ER, Boxberger J, Colvin R, Lee SJ, Zahn G, Loor F, Kim K (2011) Pil1, an eisosome organizer, plays an important role in the recruitment of synaptojanins and amphiphysins to facilitate receptor-mediated endocytosis in yeast. Eur J Cell Biol 90:825–833
Nakano K, Yamamoto T, Kishimoto T, Noji T, Tanaka K (2008) Protein kinases Fpk1p and Fpk2p are novel regulators of phospholipid asymmetry. Mol Biol Cell 19:1783–1797
Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T (2012) Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci USA 109:1536–1541
Oh Y, Bi E (2011) Septin structure and function in yeast and beyond. Trends Cell Biol 21:141–148
Okuzaki D, Satake W, Hirata A, Nojima H (2003) Fission yeast meu14+ is required for proper nuclear division and accurate forespore membrane formation during meiosis II. J Cell Sci 116:2721–2735
Olivera-Couto A, Grana M, Harispe L, Aguilar PS (2011) The eisosome core is composed of BAR domain proteins. Mol Biol Cell 22:2360–2372
Penalva MA, Tilburn J, Bignell E, Arst HN Jr (2008) Ambient pH gene regulation in fungi: making connections. Trends Microbiol 16:291–300
Poveda JA, Fernandez AM, Encinar JA, Gonzalez-Ros JM (2008) Protein-promoted membrane domains. Biochim Biophys Acta 1778:1583–1590
Prosser DC, Drivas TG, Maldonado-Baez L, Wendland B (2011) Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J Cell Biol 195:657–671
Rankin BR, Moneron G, Wurm CA, Nelson JC, Walter A, Schwarzer D, Schroeder J, Colon-Ramos DA, Hell SW (2011) Nanoscopy in a living multicellular organism expressing GFP. Biophys J 100:L63–L65
Reijnst P, Walther A, Wendland J (2011) Dual-colour fluorescence microscopy using yEmCherry-/GFP-tagging of eisosome components Pil1 and Lsp1 in Candida albicans. Yeast 28:331–338
Robinson JM, Karnovsky MJ (1980) Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J Histochem Cytochem 28:161–168
Roelants FM, Torrance PD, Bezman N, Thorner J (2002) Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell 13:3005–3028
Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J (2010) A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci USA 107:34–39
Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B (2005) Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 24:1537–1545
Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA (1998) Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem 273:1150–1157
Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100:5795–5800
Seger S, Rischatsch R, Philippsen P (2011) Formation and stability of eisosomes in the filamentous fungus Ashbya gossypii. J Cell Sci 124:1629–1634
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3:a004697
Sivadon P, Peypouquet MF, Doignon F, Aigle M, Crouzet M (1997) Cloning of the multicopy suppressor gene SUR7: evidence for a functional relationship between the yeast actin-binding protein Rvs167 and a putative membranous protein. Yeast 13:747–761
Snaith HA, Thompson J, Yates JR 3rd, Sawin KE (2011) Characterization of Mug33 reveals complementary roles for actin cable-dependent transport and exocyst regulators in fission yeast exocytosis. J Cell Sci 124:2187–2199
Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, Bassereau P (2009) Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA 106:5622–5626
Spira F, Mueller NS, Beck G, von Olshausen P, Beig J, Wedlich-Soldner R (2012) Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat Cell Biol 14(6):640–648. doi:10.1038/ncb2487
Stradalova V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, Malinsky J (2009) Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci 122:2887–2894
Streiblova E (1968) Surface structure of yeast protoplasts. J Bacteriol 95:700–707
Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, Fujita T, Kawasaki T, Kozutsumi Y (2000) Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol 20:4411–4419
Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD (2006) The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol 26:5861–5875
Takeo K (1984) Lack of invaginations of the plasma membrane during budding and cell division of Saccharomyces cerevisiae and Schizosaccharomyces pombe. FEMS Microbiol Lett 22:97–100
Tanos B, Rodriguez-Boulan E (2008) The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene 27:6939–6957
Valdez-Taubas J, Pelham HR (2003) Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol 13:1636–1640
van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K (1987) Sorting of sphingolipids in epithelial (Madin–Darby canine kidney) cells. J Cell Biol 105:1623–1635
Vangelatos I, Roumelioti K, Gournas C, Suarez T, Scazzocchio C, Sophianopoulou V (2010) Eisosome organization in the filamentous ascomycete Aspergillus nidulans. Eukaryot Cell 9:1441–1454
Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P (2006) Eisosomes mark static sites of endocytosis. Nature 439:998–1003
Walther TC, Aguilar PS, Frohlich F, Chu F, Moreira K, Burlingame AL, Walter P (2007) Pkh-kinases control eisosome assembly and organization. EMBO J 26:4946–4955
Wang HX, Douglas LM, Aimanianda V, Latge JP, Konopka JB (2011) The Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryot Cell 10:72–80
Xu T, Shively CA, Jin R, Eckwahl MJ, Dobry CJ, Song Q, Kumar A (2010) A profile of differentially abundant proteins at the yeast cell periphery during pseudohyphal growth. J Biol Chem 285:15476–15488
Young ME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, Wieland FT, Cooper JA (2002) The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol Cell Biol 22:927–934
Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA (2004) Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell 13:677–688
Zhang X, Lester RL, Dickson RC (2004) Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J Biol Chem 279:22030–22038
Zimmerberg J, Kozlov MM (2006) How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 7:9–19
Ziolkowska NE, Karotki L, Rehman M, Huiskonen JT, Walther TC (2011) Eisosome-driven plasma membrane organization is mediated by BAR domains. Nat Struct Mol Biol 18:854–856
Acknowledgments
We thank Héctor Yuyo Romero for helping with phylogenetic reconstructions and analysis. We also thank Arlinet Kierbel and Gustavo Pesce for collaborating with stimulating discussions and critical reading of the manuscript. This work was supported by the Agencia Nacional de Investigación e Innovación (INNOVA URUGUAY-DCI-ALA/2007/19.040 URU-UE, P.S.A.; Sistema Nacional de Becas, A.O.-C.; and Sistema Nacional de Investiga-dores, P.S.A.) and the Programa de Desarrollo de Ciencias Básicas (A.O.-C. and P.S.A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Box 1: Eisosome conservation and organization in other fungi
There is currently no evidence for eisosome presence in organisms outside the fungi kingdom. However, structurally comparable domains called caveolae are found in the plasma membrane of mammalian cells. Caveolae are 60–80 nm flask-shaped stable invaginations formed by scaffolding of the integral membrane proteins caveolins and the membrane-associated proteins cavins (Hansen and Nichols 2010). Like eisosomes, caveolae concentrate sterols and phosphoinositides and have been implicated in plasma membrane stress-mediated signaling and regulation of lipid homeostasis (Bastiani and Parton 2010; Hansen and Nichols 2010). Besides these appealing similarities, the repertoire of known core components and mechanism of assembly of both domains are quite dissimilar (Moreira et al. 2009; Hayer et al. 2010). Eisosomes and caveolae might, therefore, represent a case of convergent evolution.
Conservation of different eisosomal components across all fungi is disparate. Most eisosomal components are present in ascomycetes, Pil1 and Lsp1 and few others are found in basidiomycetes and no eisosomal proteins were found in zygomycetes or chytridiomycetes (Olivera-Couto et al. 2011). A phylogenetic analysis across the ascomycota phylum shows that most eisosomal proteins share a history filled with multiple events of gene duplication and gene loss, a common feature in this phylum (see Online Resource 1). In addition to extensive work in budding yeast, eisosomes are being actively characterized in four other ascomycetes, annotation of eisosomal proteins and their phylogenetic relationships are summarized in Table 2 and Online Resource 1:
Aspergillus nidulans: In ungerminated conidia of this filamentous fungus, eisosome organization is similar to budding yeast: Pil1/Lsp1 orthologs (PilA and PilB) and Sur7 ortholog (SurG) all colocalize at the spore periphery forming a dense net of foci (Vangelatos et al. 2010). In contrast, in actively growing hyphae eisosome organization is markedly different: PilA forms foci, whereas PilB is cytoplasmic and SurG shows vacuolar and endosomal localization. PilA is required for organization of SurG peripheral foci but not of PilB foci. On the other hand, SURG deletion leads to loss of PilB (but not PilA) peripheral foci (Vangelatos et al. 2010).
Ashbya gossypii: Eisosomes in this filamentous fungus share several common features with their S. cerevisiae counterparts: Pil1 and Lsp1 form static and stable foci that are assembled de novo at active sites of cellular growth (Seger et al. 2011). Also, eisosomes organization depends on the presence of Pil1 (but not of Lsp1). Unlike S. cerevisiae, deletion of NCE102 in A. gossypii does not affect eisosome organization, suggesting that there is no connection between Nce102 and Pkh kinases signaling. In addition to loss of eisosome organization, PIL1 deletion in A. gossypii leads to severe reduction of polar surface expansion and formation of abnormal bulged hyphae (Seger et al. 2011). The ortholog of YMR086w/YKL105c, SEG1, is needed to maintain eisosome stability (Seger et al. 2011).
Candida albicans: Studies in this human pathogen, which exhibits both budding yeast and hyphae morphologies, uncovered novel functional roles for Sur7. Like in S. cerevisiae, in C. albicans Pil1, Lsp1, Sur7 and Fmp45 form static foci in budding cells and also in hyphae (Alvarez et al. 2008; Reijnst et al. 2011; Wang et al. 2011). C. albicans eisosomes are absent at the tips of growing hyphae or buds, suggesting that de novo assembly is also restricted to areas of active growth (Reijnst et al. 2011). In contrast to S. cerevisiae, deletion of C. albicans SUR7 ortholog leads to several phenotypes, including lack of septin localization at bud necks, defective growth polarization, ectopic growth of cell wall, defective biofilm formation and, more importantly, decreased virulence in a mouse model of infection (Alvarez et al. 2008; Bernardo and Lee 2010; Wang et al. 2011; Douglas et al. 2012). Since septins control the correct positioning of actin patches and cell wall-synthesizing enzymes, it has been proposed that many of the phenotypes observed in sur7Δ cells are due to defects in septin organization (Alvarez et al. 2008). How Sur7 is implicated in septin organization remains to be elucidated. Notably, SUR7 deletion does not affect Lsp1 foci organization (Alvarez et al. 2008). Currently, there is no published evidence about the phenotypes caused by deletion of PIL1 or LSP1 in C. albicans. Thus, it is currently unknown whether C. albicans eisosomes mediate plasma membrane organization and if they are needed to sustain Sur7 functions.
Schizzosaccharomyces pombe: Both frozen-etch EM and fluorescent microscopy data showed that fission yeast has 1–2 μm long eisosomes, much larger than those present in S. cerevisiae (Moor and Muhlethaler 1963; Streiblova 1968; Takeo 1984; Kabeche et al. 2011). Like S. cerevisiae, eisosomes are formed de novo behind the active sites of growth. Eisosomes are also stable and static (Kabeche et al. 2011). However, during cell division eisosomes are actively removed from the future zone of septation. This clearance includes breakage, disassembly and even directional movement of eisosomes away from the cell division zone (Kabeche et al. 2011). Gene swapping experiments suggest that there is a Pil1-independent mechanism that regulates eisosome assembly in both fission and budding yeasts. In an S. pombe pil1Δ pil2Δ strain, expression of S. cerevisiae PIL1-GFP leads to formation of 1–2 μm long filaments that are indistinguishable from those observed in wild-type fission yeast cells. Conversely, expression of S. pombe Pil1-GFP in a pil1Δ S. cerevisiae strain renders Pil1-GFP puncta that colocalize with endogenous Lsp1 (Kabeche et al. 2011).
Like Nce102 in S. cerevisiae, S. pombe Fhn1 presents a dual localization being dispersed along the plasma membrane and concentrated at Pil1 filaments. Similarly, in pil1Δ cells Fhn1 disperses homogeneously in the plasma membrane and Pil1 filaments are less numerous and shorter in fhn1Δ cells (Kabeche et al. 2011). Surprisingly, S. pombe Slm1 and Sur7 orthologues do not colocalize with Pil1 or depend on Pil1 for their localization stressing the functional divergence that exists in budding and fission yeast’s Pil1 (Kabeche et al. 2011). There is a third, more distantly related Pil1/Lsp1 ortholog, Meu14, which is required for maturation of the forespore membrane (FSM) during sporulation (Okuzaki et al. 2003). The FSM is a double-membrane envelope that extends and engulfs each of the haploid nuclei produced by meiosis during sporulation. The extending FSM adopts a cup-like shape and Meu14 localizes at the rim, which is called the leading edge. In Meu14-depleted cells, the leading edges are abnormally assembled and FSMs are morphologically aberrant (Okuzaki et al. 2003). A high sensitivity search for distant orthologs suggested that, like Pil1 and Lsp1, Meu14 is a BAR domain-containing protein (our unpublished results). Thus, it is tempting to speculate that the molecular function of Meu14 is to maintain the high curvature of the FSM leading edge.
Box 2: Outstanding questions
-
Is there a physiological or genetic context in which eisosome domain organization is essential for cell growth?
-
Are Pil1 and Lsp1 sufficient to segregate proteins and lipids?
-
What are the molecular features that distinguish eisosome targeting of H+ -symporters from proteins of the Sur7 and Nce102 families?
-
Which are the molecular functions of still uncharacterized eisosomal proteins?
-
Which are the Pil1 and Lsp1 phosphatases?
-
What keeps eisosomes static?
-
How is eisosome assembly controlled?
-
Which are the molecular features that distinguish Pil1 from Lsp1?
-
Is there an eisosome-dependent endocytic pathway?
-
What is the molecular mechanism that releases Slm1 from eisosomes upon plasma membrane stress?
Rights and permissions
About this article
Cite this article
Olivera-Couto, A., Aguilar, P.S. Eisosomes and plasma membrane organization. Mol Genet Genomics 287, 607–620 (2012). https://doi.org/10.1007/s00438-012-0706-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-012-0706-8