Abstract
With the opening of the Suez Canal in 1869, many changes have occurred in the Mediterranean Sea ecosystem so became a home to many invasive Lessepsian marine species that have migrated from the Red Sea. About 500 marine species including pufferfish have immigrated and rapidly established a population in the Mediterranean Sea causing significant impact on its ecosystem and fisheries sector. The parasitic fauna of these pufferfish has scarcely been studied in the Mediterranean Sea and also in their native habitat. During this surveillance study on the invasive pufferfish species from the Egyptian Mediterranean coast, the female cymothoid isopod Elthusa raynaudii was detected from the branchial cavity and also in the buccal cavity of 23.9% of the examined Lagocephalus sceleratus. The isolated isopod species was firstly identified and described through electron microscopy and molecular phylogeny based on the sequences of mitochondrial 16S rRNA gene. Additionally, the description of eggs, embryonic stage, and manca of E. raynaudii was firstly provided. The pathological impact on the infested fish tissues was investigated and revealed curling and loss of secondary gill lamellae in addition to mucous exudates in between the gill filaments and granuloma formation in the gill arch. The study provided the first report of L. sceleratus as a new host for the isopod E. raynaudii collected from the Egyptian Mediterranean coast as a new locality record. The role of the Lessepsian invasive pufferfish in transmitting parasites to the native fish species was discussed.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breaking of natural aquatic barriers results in the spreading of some species into the ecosystems where they are alien. This dispersion can affect the native species and also the equilibrium of the aquatic invaded ecosystems (Golani 1998). Decline in the number of native species and introduction of pathogens are the negative impacts of this invasion (Oral 2010). The Mediterranean Sea became a home to many invasive marine species that have entered through many routs including the Suez Canal which is considered the major way of migrating the Lessepsian species from the Red Sea to the Mediterranean and vice versa (Bariche et al. 2004). Also, Gibraltar Strait and ship’s ballast water are important sources for the alien species introduction into the Mediterranean Sea. The silver-cheeked toadfish Lagocephalus sceleratus is one of the Lessepsian species which migrated through the Suez Canal and rapidly established a population in the Eastern Mediterranean (Yaglioglu, 2011). This Lessepsian species is considered to be among the worst invasive species in the Mediterranean Sea due to its significant impact on the aquatic ecosystem and fisheries sector (Streftaris and Zenetos 2006; Ozturk 2010). Migration of Lessepsian species has been increased on the Egyptian Mediterranean coast year after one and many cases of human death after consumption of pufferfish containing Tetrodotoxin poison were reported (Farrag et al. 2016) [7]. Cymothoidae (Crustacea, Isopoda) is among the most diverse parasitic isopods infesting various fish hosts. They are attached to the host body surface, buccal, or gill cavities (Mahmoud et al. 2014a, b; Trilles and Justine 2010) and occasionally burrowing in the musculature of their hosts, causing sever tissue damage and fish mortalities (Rhode 2005; Smit et al. 2014). In Egypt, interest on the Mediterranean isopods has increased in the last decade; it was reported as the main source of isopod infestation problems occurred in marine fish farms and the inland lakes. In 2013, different species of cymothoid isopod were transported to Qarun Lake with the infected wild mugiliid fry from the Mediterranean Sea resulting in complete destruction of the lake fish stock (Mahmoud et al. 2019a and 2023). Research on the pufferfish was restricted and mostly focused on its habitat (Kalogirou et al. 2012) and impacts on fish and fisheries (Kalogirou 2013), reproduction (Rousou et al. 2014) and consumption safety issues (Deeds et al. 2008; Katikou et al. 2009). Four studies were conducted on pufferfish parasitism from the Suez Canal and Mediterranean Sea with no record of isopod infestation (El-Lamie and Abdel-Mawla 2012; Ozak et al. 2012; Bakopoulos et al. 2017; Gabel et al. 2022). As data concerning parasitic isopod on pufferfishes is scarce in the eastern Mediterranean, the objective of this study was to identify the parasitic isopod of migrant L. sceleratus and estimate its role in transmitting parasitic isopod species to the invaded Egyptian Mediterranean sea coast. Additionally, the pathological impact of the identified isopod species on the affected fish tissues was illustrated and discussed.
Materials and methods
Sample collection and study area
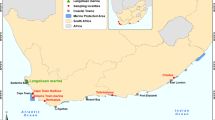
A total of 134 specimens of freshly dead Lagocephalus sceleratus (Gmelin,1789) were collected from local fishermen along the Egyptian coast of Mediterranean Sea, Alexandria (31° 00′ and 31° 36′ N and 29° 18′ and 30° 05′ E) (Fig. 1) from December to May 2022. Fish samples (15–47 mm in length) (Fig. 2C, D) were collected using commercial trawlers at a depth of 20–40 m and at a distance of about 2–3 miles from the coast. Fish samples were macroscopically inspected on spot, and each sample was placed in a bag with ice and transported to the laboratory of parasitology, Faculty of Veterinary Medicine, Cairo University, for further examination. Interviews with the local fishermen along the study area revealed that this fish species is being caught as by-catch in significant numbers and attacks fishes captured in the nets and also damages the fishing gears using its strong teeth.
Parasitological examination
The fish body surface and all openings (skin, fins, gills, eyes, nostrils, anus, and buccal and branchial cavities) were macroscopically investigated. The detected isopods were gently removed from the infested fish. Eggs were obtained from the ovigerous females by cutting the marsupium using a dissecting needle and fine forceps (Hadfield and Smit 2020). Manca larvae released from some of the live ovigerous females were also collected. The embryonic stage was recovered from the eggs after cutting the egg envelope and removing the vitelline membrane. The parasite specimens were counted, accurately measured using an ocular micrometer, and stored in 70% ethyl alcohol. The investigated fish species was identified as Lagocephalus sceleratus according to Otero et al. (2013) and Farrag et al. (2016). Prevalence and intensity of infestation were calculated following Margolis et al. (1982).
Morphological examinations and taxonomy of the isolated isopods
The freshly dead and the preserved isopod specimens were light microscopy examined using a dissecting stereo-microscope (Olympus Japan SZ40) and photographed by a digital camera (Canon 12 megapixel). The morphological identification and taxonomic classification of the isolated isopods were performed according to the keys of Bruce (1990) and Van der Wal (2019).
Morph-metric ultrastructure examination
The isopod specimens were washed with 0.9% physiological saline solution, fixed in 2.5% glutaraldehyde (Colwell et al. 2007), dehydrated using an ethanol series (95% and 100%) for 10 min, then subjected to critical point drying in a CO2 drier (Autosamdri-815, Germany) (Lee 1992). The specimens were then fixed over SEM stubs, coated with gold (Spi-Module Sputter Coater, UK), and examined and photographed by SEM (JSM 5200, Electron Probe Microanalyzer, Jeol, Japan) in the Electron Microscope Unit at the Faculty of Agriculture, Cairo University.
Molecular identification
DNA extraction and amplification of 16S rRNA
DNA from the isolated isopod species were extracted using DNeasy Tissue Kit (Qiagen, Germany) according to the manufacturer’s instructions. The extracted DNAs were stored at − 20 °C till used. The primers, Fish-F1 (5′AGCC-CTGTTCAATGGGATTA-3′) and Fish-R1 (5′TCCCTGGGGTAGTTTCATCTT-3′), were used with the extracted DNAs to amplify a 532 bp fragment of the 16S ribosomal RNA gene (Thangara et al. 2014). PCR reaction was done according to Thangara et al. (2014) and Mahmoud et al. (2019b).
DNA sequencing and phylogenetic analysis
The positive specimens of PCR products were purified by using a QIA quick purification kit (Qiagen, Germany), then sequencing using Big Dye Terminator V3.1 kit in an ABI 3500 Genetic Analyzer (Applied Biosystems, USA). The detected sequences were compared with those available in the GenBank using a BLAST server on the NCBI website (Ali et al. 2021).
Nucleotide sequence
Partial sequences of the isopod species 16S rRNA gene were submitted to GenBank. Sequences were aligned against other sequences of the 16S rRNA gene recorded worldwide. The analysis was carried out using the Clustal W, BioEdit software (ver. 7.0.9). A Maximum Likelihood method and Tamura-Nei model were done for phylogenetic tree using Mega 6.06 software, and bootstrap analysis was obtained with 1000 replicates (Hegab et al. 2022a, b). Pairwise comparisons were constructed using K2P model distance within Elthusa raynaudii compared with the most similar reference sequences (GenBank) (Mega 6.06 software) and detect inter- and intra-species variations of genetic distance values of isolated isopods (Thangara et al. 2014).
Nucleotide sequence accession number
Partial sequences of Elthusa raynaudii (Milne-Edwards 1840) adult stages isolated from Lagocephalus sceleratus (Rabbit fish) in Egyptian Mediterranean Coast. 16S rRNA gene was submitted to GenBank, with accession numbers ON599340.1 and OK316895.1, respectively.
Histopathological examination
Infested gill tissues with the attached isopods were fixed in 10% neutral buffered formalin, dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. 3-µm-thick tissue sections were made using a microtome (Leica 2135, Germany) and stained with hematoxylin and eosin stain, Giemsa stain, and periodic acid stain (PAS) (Suvarna et al. 2012). A light microscope equipped with a digital camera was used for examination and capturing photographs.
Results
Prevalence of isopod infestation
Out of the examined 134 L. sceleratus samples, 32 were found infested with the isopod Elthusa raynaudii (23.9%). The total number of the detected parasites was 41 with a mean intensity of 1.29 ± 0.46.
Attachment mode
All the detected female Elthusa raynaudii were found in L. sceleratus branchial cavities, attached to the gills, and only one case showed female at the base of the buccal cavity (Fig. 2A, B).
Taxonomy and morphometric description of the isolated isopod
Taxonomy
The recovered isopod species was identified as Elthusa raynaudii (Milne-Edwards 1840) (order: Isopoda, suborder: Cymothoida, superfamily: Cymothoidea, family: Cymothoidae, genus: Elthusa, and species: Elthusa raynaudii).
Morphometric description of the isolated isopod (light stereo-microscopy)
Female Elthusa raynaudii (Milne-Edwards 1840)
The body is dark creamy in color and the dorsal surface is smooth and polished in appearance. It is ovate, measuring 23–28 (mean: 24.86 ± 1.52) mm long and 13.2–14.1 (mean: 13.60 ± 0.27) mm wide, and appears symmetrical or slightly twisted to one side in some specimens. The greatest width is at pereonite 5. The Cephalon is sub-truncate and partially immersed in pereonite 1 with blunt ventrally folded frontal margin. Eyes are oval with prominent margins. Lateral posterior margins of pereonites are moderately curved. The anterior border of pereonite 1 is straight medially and curved laterally with narrow rounded antero-lateral angle extends to the medial region of eyes. Pereonites 2–5 are subequal, and pereonites 6 and 7 are slightly narrower. Pereonite 7 has roundly pointed postero-lateral angles. Coxae 2 and 3 are wide with rounded postero-ventral angles. Coxal plates of pereonites 5–7 are conspicuous in dorsal view. Coxae 4–7 are not extended beyond the pereonite posterior margin. The pleon is short. Pleonites 1–4 posterior margins are concave while that of pleonite 5 is straight. Pleonite 1 is slightly narrower then pleonite 2 and largely concealed by pereonite 7. Pleonite 2 are partially overlapped by the postero-lateral margin of pereonite 7. Pleonites 3–5 are subequal in length, with postero-lateral angles narrowly rounded. Pleotelson is semiarc in shape with round posterior end. It is narrower than pereonite 7 and about 1.35 times as wide as long and 0.2 of the total length. Brood pouch is made up of 5 pairs of alternately overlapping oostegites arising from the coxae 1–4 and 6 (Figs. 3 and 4). Number of eggs per brood pouch ranged from 85 to 120 depending on the size of the ovigerous female.
Body parts of the isolated female Elthusa raynaudii. A Dorsal view of female. B Ventral view of ovigeorus female. C Ventral view of female Showed brood pouch of 5 pairs of alternately overlapping oostegites. Scale bar (S.b) = 10 mm. Pr: perionite; Ple: pleonite; Pt: pleotelson; Mar: marsupium; Os: oostigite; Bp: broad pouch
Eggs
Eggs are ovoid in shape, dark yellow in color, measuring 1.01–1.03 × 0.95–0.98 mm in diameter (Fig. 5A).
The embryonic stage
The body is between 1.18 and 1.23 mm in length and 0.62 to 0.79 mm in width (Fig. 5A).
The manca larva (just after spawning)
The manca body length is 1.25–1.36 mm. It has 7 pereonites and 6 pairs of pereopods and obvious large compound eyes. The cephalon is semi-circular in shape. The preon consists of 7 pereonites and the pleon is narrowed with 5 pleonites progressively decrease towards the posterior. Pleotelson posterior margin is rounded with numerous setae. Antennule composed of 6 articles and shorter than antenna which composed of 11 articles and both provided with spiny setae. All pereopods similar with acute dactyli increase slightly in length from pereopod 1 to 6, with spines or setae on pereopods 2–6. Pleopods are with lamellar rami and provided with long setae. Uropod rami apices are rounded and extend beyond the posterior of pleotelson. Rami are subequal in length with plumose setae on the posterior margin (Fig. 5B).
SEM examination of the female Elthusa raynaudii
Antennule is shorter than the antenna and composed of 8 articles. Articles are longer than wide. Article 2 is with 2 setae and a tuft of setae on the terminal article. Antenna is thinner than antennules, consists of 11 articles, and extends to about the middle of pereonite 1. It has 3 setae on both article 9 and 10 and a tuft of setae on the terminating article (Fig. 6A, B). Mandible has prominent molar. Article 2 of the mandibular palp has 5 setae, and article 3 has 2 setae. Maxillule is with 4 spines. Maxilla has 2 spines on each of medial and lateral lobes respectively. Maxilliped article 3 has 2 terminal spines (Fig. 6E, F). All pereopods are without setae. Pereopod 1 basis is 1.7 times as long as the greatest width; ischium is 0.6 times as long as basis, merus has no proximal bulbous protrusion, and carpus has rounded proximal margin; propodus 2 is times as long as wide, and dactylus is 0.8 times as long as propodus and not extended beyond the carpus. Pereopod 7 basis is with carina, ischium is 0.4 times as long as basis; merus 0.6 is times as long as ischium, carpus is 0.7 times as long as ischium, propodus is 0.9 times as long as ischium, and dactylus is 0.9 times as long as the basal width. The posterior margin of Sternite 7 has 2 submedian distal fleshy lobes (Fig. B–D). Pleopods 1–5 become markedly smaller. All pleopods are simple with lamellar rami and the exopod being larger than endopod (Fig. 7A, B, D). Uropod is broadly rounded and did not reach the posterior margin of pleotelson. Exopod is about as long as the peduncle and slightly longer than the endopod (Fig. 7C).
A Cephalon of female Elthusa raynaudii dorsal view. B Cephalon lateral view. C Pereopod 1. D Pereopod 6 and 7. E, F Mouth parts; An: antenna; Au; antennule; Ce: cephaon; Ey: eye; Pr1: pereonite 1; Per1: pereopod 1; Per2: pereopod 2; Da: dactylus; Pp: propodus; Ca: carpus; Me: merus; Is: ischium; Ba: basis; Md: Mandible; Ar 1: Md article1; Ar 2: Md article2; Ar 3: Md article3; Max: Maxilla; Mxp: Maxilliped; Mxp3: Maxilliped article 3; Mxu: Maxillule
Genetic and phylogenetic analysis
The sequence analysis of Elthusa raynaudii adult stages explained that the samples were 96.35% and 95.96%, respectively, identical with Elthusa spp. (LC159454.1) previously isolated from Japan. This species of Elthusa raynaudii isolated from Lagocephalus sceleratus (Rabbit fish) in Mediterranean Coast, Egypt, was recorded in the GenBank on the NCBI with accession numbers ON599340.1 and OK316895.1, respectively.
In this study, the PCR assays revealed an amplified DNA fragment of Elthusa raynaudii approximately 526–532 bp. Additionally, phylogenetic analysis based on the 16S rRNA gene data reveals that this detected isopoda was closely affiliated to the genospecies of Elthusa sp. isolated from Japan (LC159453.1) and can be differentiated clearly from other isopods genospecies by Maximum Likelihood method. The Maximum Likelihood method tree having 24 clades with the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) (Fig. 8). The comparison between inter- and intra-species analysis of genetic distance among 24 isopod species revealed that the genetic identity of Elthusa raynaudii (ON599340.1 and OK316895.1) isolated from Mediterranean Coast in Egypt was verified with a high sequence homology with Elthusa sp. isolated from Japan (LC159453.1) (Table 1).
Phylogenetic relationships based on 16S ribosomal RNA (16S- rRNA) sequences of Elthusa raynaudii adult stages isolated from Lagocephalus sceleratus (Rabbit fish) in Egyptian Mediterranean Coast. The trees were constructed and analyzed using a Maximum Likelihood method and Tamura-Nei model. Phreaomerus latipes with accession number JQ612655 is considered outgroup
On the other hand, low level of genetic divergence (GD) of the isolated Elthusa raynaudii from Mediterranean Coast, Egypt, with the genospecies of Elthusa sp. isolated from Japan (LC159453.1) was 0.02 detected (Table 1).
Histopathological findings
The parasite caused a variety of histopathological alterations in the gills of infected fish. Parts of the parasite were observed between the gill filaments exerting pressure atrophy on them. The secondary gill lamellae suffered from severe curling, erosions, and epithelial desquamation and sometimes were completely lost. Edema was also observed in the primary gill lamellae. Mucous exudates with suspected bacterial aggregations as demonstrated by PAS stain and Giemsa stain were seen in between the gill filaments. Dark blue-stained cells with Giemsa stain between gill lamellae were recorded (Fig. 9). Microscopy of the gill arch revealed a granulome which is consisted of central necrosed area and leukocytes surrounded by a fine rim of fibroblasts. The lesion caused thickening of the gill arches (Fig. 10).
Micrographs of rabbitfish infested with isopod in the gill chamber. (a) Part of the parasite presents between gill lamellae (star) causing curling and pressure on secondary gill lamellae (× 40). (b) Occurrence of exudates between gill lamellae with complete loss of secondary gill lamellae (× 100). (c) Desquamated cells between gill lamellae (arrow) with curling of secondary gill lamellae (× 100). Hematoxylin and eosin stain. (d) PAS-positive mucous exudates in between the gill lamellae (periodic acid Schiff × 100), (e) dark blue-stained cells with Giemsa stain between gill lamellae (Giemsa stain × 100). (f) Purplish-blue organisms in the mucous exudates (Giemsa stain × 200)
Discussion
In this study, the female cymothoid isopod Elthusa raynaudii was recorded for the first time from the invasive pufferfish L. sceleratus collected from the Egyptian Mediterranean Sea coast. The infestation rate was 23.9% found markedly higher than that reported for the isopod Gnathia sp. praniza larva by Bakopoulos et al. 2017 (2.4%) from the same fish species caught from northern and southern locations of the eastern Aegean Sea. Morphologically, Elthusa raynaudii is characterized by the cephalon with a narrow truncate rostrum; the pereonite 1 with straight anterior margin; the subequal pleonites; and the broad rounded uropod apices which extend to more than half the pleotelson length. The morphological traits and morphometric measures of the detected E. raynaudii were consistent with the description of Bruce (1990) and also Van der Wal et al. (2019) who collected E. raynaudii specimens from the west coast of South Africa in 2010 without mentioning the host Bruce (1990) and Van der Wal et al. (2019). The current study is considered the 1st provided the molecular characterization of L. raynaudii and recorded this isopod species in the GenBank. All the specimens of E. raynaudii in this study were isolated from the branchial cavity attached to the gills, but a non-ovigerous female was oddly detected in the mouth of L. sceleratus specimens with its cephalon directed up-side-down. The isopod in this odd location probably was either introduced to the mouth of L. sceleratus while preying on its natural host or the parasite migrated from the gill cavity to the mouth after death of the captured L. sceleratus. The same odd cases for this isopod species were reported by Hurley (1961) (in New Zealand) and Williams et al. (2010) (in Taiwan) from the stomach and the roof of the mouth of shark, respectively, and they described the cases as being accidental. The isopod L. raynaudii was originally described from the Cape of Good Hope in South Africa (Milne-Edwards 1840) from unknown host and also recorded several times from a wide range of localities within the Indo-Pacific region and sub-Saharan Africa (Van der Wal et al. 2019). Regarding the establishment of L. sceleratus in this investigated area of the Mediterranean Sea coast, it might be attributed to the high rate of water pollution recorded through our previous investigation on the water quality of this area revealing severe water deterioration and concluded a strong positive relationship between isopod prevalence and water pollution. The same conclusion was provided by Ashmawy et al. (2018) and Chapman et al. (2015). In the present study, the record of isopod infestation among the invasive pufferfish L. sceleratus from the Egyptian Mediterranean Sea coast indicated the role of this invasive fish in transmitting parasites such as isopods and perhaps other types of pathogens to the Egyptian marine water resources and consequently to the native fish species (Mahmoud et al. 2023). This explain the spreading of isopod fauna to the inland fish farms and lakes as occurred in Qarun Lake where many isopod species were transported with the fry from the Mediterranean Sea causing great problems (Fahmy et al. 2021). Histopathological alteration of the gills due to isopod infestation was reported in several studies (Ravichandran et al. 2010a; Kumar et al. 2012). In the current study, The recorded histopathological changes could be attributed to the pressure of the large sized isopod L. raynaudii inhabits the branchial cavity. The imprint of the parasite parts on the gill filament was observed associated with severe curling, erosions, and complete loss of secondary gill lamellae in severe cases. Granulome formation in the gill arches recorded in this study is similar to the report of Rameshkumar and Ravichandran (2014) and Panakkool-Thamban et al. (2016) who mentioned that the large size of the parasitic isopods causes a variety of histopathological alterations to gills including erosions, thickening of gill filaments in addition to granulomas formation. They also reported that granulomes are formed of macrophages and epithelioid cells which sometimes enclosed by a fine capsule. They also observed a lipofibrosis in which acidophilic plasmocytes, lymphocytes, and granulocytes were seen. This tissue reaction was proposed to be due to the continuous irritation of the parasite’s body and appendages and also to the blood feeding habit of the parasite (Rameshkumar and Ravichandran 2014; and Panakkool-Thamba et al., 2016). The detected bacterial aggregations in the gill lamellae of a cymothoid isopod infested host are possibly due to lesions and contamination with the respiratory water. This reduces the respiration and nitrogenous waste excretion of the host (Trilles 1994; Ravichandran et al. 2009 and Ravichandran et al. 2011). The parasite also increases the susceptibility to secondary infections with bacteria and fungi due to tissue damage, anemia, and stress (Rameshkumar and Ravichandran 2014; Purivirojkul and Songsuk 2020). Hyperplastic and hypertrophic changes in the gill lamellae were not observed in the present study unlike to previous studies which showed that gill hyperplasia and hypertrophy are the main changes observed in the gills due to isopod infestation (Ravichandran et al. 2010b). This could be related to the species of isopod and infested fish. The current study provides that L. sceleratus in the eastern Mediterranean should be considered an important host for the low host specificity isopod parasites. The extent to which the isopod L. raynaudii infestation could have a negative impact on L. sceleratus populations needs more further investigation. On the other hand, the recorded isopod species can accumulate in the invasive L. sceleratus populations over time as the opportunities for the parasite to adapt to the host increase, the case which can result in Poulin and Mouillot 2003; Cornell and Hawkins 1993.
Τo our knowledge, this study constitutes the first report on parasites infesting L. sceleratus from the Egyptian Mediterranean Sea. Additionally, the study identified L. sceleratus as a new host for the isopod E. raynaudii and therefore was the first report for this isopod species in a tetraodontid fish.
Conclusions
This study revealed that L. sceleratus in the eastern Mediterranean is considered an important host for the low host specificity isopod parasites, providing the negative impact on the affected fish.
Recommendations
Further studies on the parasitic infestation of the invasive fish species are required and included different localities in the Mediterranean Sea to evaluate the prevalence and impact of the parasites in the long run. The governments of countries that suffer from the spread of the tetraodontid fish on their coasts should ban the fishing and selling these fishes and set laws that contribute the elimination of it and reduce their rates of reproduction.
Data availability
The datasets generated or analyzed during this current study are available in this research article and the GENE BANK repository (accession numbers: ON599340.1, OK316895.1).
References
Ali KM, Hassan EA, Abuowarda MM, Mahmoud MA, Torad FA (2021) Bilateral panophthalmia as a late sequel of leishmaniasis in dogs. Pak Vet J 41(1):13–18. https://doi.org/10.29261/pakvetj/2021.006
Ashmawy KI, Hiekal FA, Abo-Akadda SS, Nadia E, Laban NE (2018) The inter-relationship of water quality parameters and fish parasite occurrence. AJVS 59(1):97–106
Bakopoulos V, Karoubali I, Diakou A (2017) Parasites of the Lessepsian invasive fish Lagocephalus sceleratus (Gmelin 1789) in the eastern Mediterranean Sea. J Nat Hist 51(7–8):421–434
Bariche M, Letourneur Y, Harmelin-Vivien M (2004) Temporal fluctuations and settlement patterns of native and Lessepsian herbivorous fishes on the Lebanese coast (eastern Mediterranean). Environ Biol Fish 70:81–90. https://doi.org/10.1023/B:EBFI.0000022928.15148.75
Bruce NL (1990) The genera Catoessa, Elthusa, Enispa, Ichthyoxenus, Indusa, Livoneca and Norileca n. gen. (Isopoda, Cymothoidae), crustacean parasites of marine fishes, with descriptions of eastern Australian species. Rec Austr Mus 42:247–300
Chapman JM, Marcogliese DJ, Suski CD, Cooke SJ (2015) Variation in parasite communities and health indices of juvenile Lepomis gibbosus across a gradient of watershed land-use and habitat quality. Ecol Indic 57:564–572
Colwell MA, Hurley SJ, Hall JN, Dinsmore SJ (2007) Age-related survival and behavior of Snowy Plover chicks. The Condor 109(3):638–647
Cornell HV, Hawkins BA (1993) Accumulation of native parasitoid species on introduced herbivores: a comparison of hosts as natives and hosts as invaders. Am Nat 141(6):847–865
Deeds JR, White KD, Etheridge SM, Landsberg JH (2008) Concentrations of saxitoxin and tetrodotoxin in three species of puffers from the Indian River Lagoon, Florida, the location for multiple cases of saxitoxin puffer poisoning from 2002 to 2004. Trans Am Fish Soc 137(5):1317–1326
El-Lamie MM, Abdel-Mawla HI (2012) Investigation on common parasitic diseases in marine Puffer fish (Lagocephalus lunaris) in relation to heavy metal pollution in Lake Temsah. SCVMJ 17(2):199–211
Fahmy MM, Mahmoud NE, Mousa MR, Ismael E, Abuowarda M (2021) Influence of parasite infestation and water quality deterioration during mass fish mortality event in manzala lake and its corresponding fish farms. Adv Anim Vet Sci 10(5):955–966
Farrag M, El-Haweet AA, Moustafa MA (2016) Occurrence of puffer fishes (Tetraodontidae) in the eastern Mediterranean, Egyptian coast-filling in the gap. BioInvasions Rec 5(1):47–54. https://doi.org/10.3391/bir.2016.5.1.09
Gabel M, Unger P, Theisen S, Palm HW, Rothman SBS, Yitzhak N, Morov AR, Stern N (2022) Parasites of pufferfish, Lagocephalus spp. and Torquigener flavimaculosus of the Israeli Mediterranean: a new case of Lessepsian endoparasites. Int J Parasitol: Parasites Wildl 19:211–221
Golani D (1998) Impact of Red Sea fish migrants through the Suez Canal on the aquatic environment of the Eastern Mediterranean. Bulletin of Yale School Forest. Environ Studies 103: 375-387
Hadfield KA, Smit NJ (2020) Review of the global distribution and hosts of the economically important fish parasitic isopod genus Ceratothoa (Isopoda: Cymothoidae), including the description of Ceratothoa springbok n. sp. from South Africa. Int J Parasitol 50(10–11):899–919. https://doi.org/10.1016/j.ijpara.2020.07.001
Hegab AA, Omar HM, Abuowarda M, Ghattas SG, Mahmoud NE, Fahmy MM (2022a) Screening and phylogenetic characterization of tick-borne pathogens in a population of dogs and associated ticks in Egypt. Parasit Vectors 15(1):222
Hegab AA, Fahmy MM, Omar HM, Ghattas SG, Mahmoud NE, Abuowarda M (2022b) Occurrence and genotyping of Theileria equi in dogs and associated ticks in Egypt. Med Vet Entomol 37(2):252–262
Hurley DE (1961) A checklist and key to the Crustacea Isopoda of New Zealand and Subantarctic Islands. Trans R Soc N Z (Zoology) 1:259–292
Kalogirou S (2013) Ecological characteristics of the invasive pufferfish Lagocephalus sceleratus (Gmelin, 1789) in Rhodes, Eastern Mediterranean Sea. A case study from Rhodes. Med Mar Sci 14:251–260
Kalogirou S, Wennhage H, Pihl L (2012) Nonindigenous species in Mediterranean fish assemblages: contrasting feeding guilds of Posidonia oceanica meadows and sandy habitats. Estuar Coast Shelf Sci 96:209–218
Katikou P, Georgantelis D, Sinouris N, Petsi A, Fotaras T (2009) First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 54(1):50–55
Kumar GR, Ravichandran S, Trilles JP (2012) Observation on an isopod parasitizing the edible fish Parastromateus niger in the Parangipettai coast of India. J Environ Biol 33(2):191–193
Lee PE (1992) Scanning Electron Microscopy and X-Ray Microanalysis. Prentice Hall, Englewood Cliffs, p 458
Mahmoud NE, Badawy MFM, Fahmy MM (2014a) Investigations on mass mortalities among Oreochromis niloticus at mariotteya stream, Egypt: parasitic infestation and environmental pollution impacts. J Aquacult Res Dev 5(2):1000219
Mahmoud NE, Fahmy MM, Badawy MF (2014b) Investigations on mass mortalities among Oreochromis niloticus at Mariotteya stream, Egypt: parasitic infestation and environmental pollution impacts. Fish Aquac J 5(2):1
Mahmoud NE, Fahmy MM, Abuowarda MM, Zaki MM, Ismael E, Ismail EM (2019a) Influence of water quality parameters on the prevalence of Livoneca redmanii (Isopoda; Cymothoidae) infestation of Mediterranean Sea fishes, Egypt. Int J Vet Sci 8:174–181
Mahmoud NE, Fahmy MM, Abuowarda MM, Zaki MM, Ismail EM, Ismael ES (2019b) Mediterranean Sea fry; a source of isopod infestation problem in egypt with reference to the effect of salinity and temperature on the survival of livoneca redmanii (isopoda: cymothoidae) juvenile stages. J Egypt Soc Parasitol 49(1):235–242
Mahmoud NE, Fahmy MM, Khatab MS, Abuowarda M (2023) Morphological, ultrastructural, and molecular characterization of Livoneca redmanii (Leach, 1818) (Isopoda: Cymothoidae) infestation among Solea. Parasitol Int 92:102696
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA (1982) The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J Parasitol 68:131–133
Milne-Edwards H (1840) Histoire naturelle des crustaces: comprenant l’anatomie, la physiologie et la classification de ces animaux. Atlas (Vol. 3). Roret, Rue Hautfeuille, No. 10 BIS
Oral M (2010) Alien fish species in the Mediterranean - Black Sea Basin. J Black Sea Mediterr Environ 16(1):87–132
Otero M, Cebrian E, Francour P, Galil B, Savini D (2013) Monitoring marine invasive species in Mediterranean marine protected areas (MPAs): a strategy and practical guide for managers. IUCN Centre for Mediterranean Cooperation, Malaga, Spain, pp 1–136
Ozak AA, Demirkale I, Yanar A (2012) First record of two species of parasitic copepods on immigrant pufferfishes (Tetraodontiformes: Tetraodontidae) caught in the Eastern Mediterranean Sea. Turk J Fish Aquat Sci 12:675–681
Ozturk B (2010) Draft document on the alien species in the Mediterranean and the Black Sea. General fisheries Comission for the Mediterranean, Scientific Advisory Committee, GFCM-SAC, p 12
Panakkool-Thamban A, Ameri Kottarathil H, Kappalli S (2016) Branchial cymothoids infesting the marine food fishes of Malabar coast. J Parasitic Dis 40(4):1270–1277
Poulin R, Mouillot D (2003) Host introductions and the geography of parasite taxonomic diversity. J Biogeogr 30:837–845
Purivirojkul W, Songsuk A (2020) New records of fish parasitic isopods (Crustacea: Isopoda) from the Gulf of Thailand. Animals 10(12):2298. https://doi.org/10.3390/ani10122298
Rameshkumar G, Ravichandran S (2014) Problems caused by isopod parasites in commercial fishes. J Parasit Dis 38:138–141. https://doi.org/10.1007/s12639-012-0210-4
Ravichandran S, Rameshkumar G, Kumaravel K (2009) Variation in the morphological features of isopod fish parasites. World J Fish Mar Sci 1(2):137–140
Ravichandran S, Kumar TA, Ross PR, Muthulingam M (2010a) Histopathology of the infestation of parasitic isopod Joryma tartoor of the host fish Parastromateus niger. Res J Parasitol 5(4):303–306
Ravichandran S, Rameshkumar G, Balasubramanian T (2010b) Infestation of isopod parasites in commercial marine fishes. J Parasit Dis 34:97–98. https://doi.org/10.1007/s12639-010-0014-3
Ravichandran S, Rameshkumar G, Trilles JP (2011) New records of two parasitic cymothoids from Indian fishes. J Parasit Dis 35:232–234
Rhode K (2005) Marine parasitology. CABI. Aust Folia Parasitol 53:77–78
Rousou M, Ganias K, Kletou D, Loucaides A, Tsiganis M (2014) Maturity on the pufferfish Lagocephalus sceleratus in the southeastern Mediterranean Sea. Sex Early Dev Aquat Org 1:35–44
Smit NJ, Bruce NL, Hadfield KA (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol: Parasites Wildl 3:188–197
Streftaris N, Zenetos A (2006) Alien marine species in the Mediterranean - the 100 ‘Worst Invasives’ and their Impact. Mediterr Mar Sci 7(1):87–118
Suvarna SK, Layton C, Bancroft JD (2012) Bancroft’s theory and practice of histological techniques, 7th edn. Churchill Livingstone, New York
Thangaraj M, Saranya S, Divya S, Ramanadevi V, Subburaj J (2014) Molecular phylogenetic status of some marine Cymothoid isopods in southeast coast of India. Ind J Geo-Mar Sci 43(2):271–276
Trilles JP, Justine JL (2010) Elthusa epinepheli sp nov. (Crustacea, Isopoda, Cymothoidae) a branchial parasite of the grouper Epinephelus howlandi (Serranidae, Epinephelinae) from off New Caledonia. Acta Parasitol 55:177–187
Trilles JP (1994) Les Cymothoidae (Crustacea, Isopoda) du monde (Prodrome pour une faune). Stud Mar 21(22):1–288
Van der Wal S, Smit NJ, Hadfield KA (2019) Review of the fish parasitic genus Elthusa Schioedte & Meinert, 1884 (Crustacea, Isopoda, Cymothoidae) from South Africa, including the description of three new species. ZooKeys 841:1–37. https://doi.org/10.3897/zookeys.841.32364
Williams E, Bunkley-Williams L, Ebert D (2010) An accidental attachment of Elthusa raynaudii (Isopoda, Cymothoidae) in Etmopterus sp (Squaliformes, Etmopteridae). Acta Parasitologica 55(1):99–101. https://doi.org/10.2478/s11686-010-0006-6
Yaglioglu D, Turan C, Erguden D, Mevlut G (2011) Range expansion of Silverstripe Blaasop, L. sceleratus (Gmelin, 1789) to the NorthEastern Mediterranean Sea. Biharean Biologist 5(2):159–161
Acknowledgements
The authors are grateful to the fishermen that have allowed researchers to collect samples and data, so that this study could be conducted.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mahmoud Nisreen and Magdy Fahmy were the main contributors to the design of the study and supervised all work. All authors contributed to the analysis and interpretation of the data. Mahmoud Nisreen, Magdy Fahmy, and Abuowarda Mai performed parasitology examination, SEM, and molecular analysis. Khattab Marwa performed histopathological examination. All authors were major contributors in writing the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Conflict of interests
The authors declare no competing interests.
Animal handling and use
The work was done on freshly dead animals as the samples were collected alive by fishing net operation then normally died within 1–3 min and immediately investigated. No approval of research ethics committee was required.
Additional information
Handling Editor: Una Ryan
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Isolation of Elthusa raynaudii from the invasive pufferfish species on the Egyptian Mediterranean with 21.8% of the examined Lagocephalus sceleratus.
Identification and description of E. raynaudii using electron microscopy, the molecular phylogeny.
Investigated estimation of the pathological impacts of the detected isopod species on the affected fish tissues.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, N.E., Fahmy, M.M., Khattab, M.S. et al. Phylogeny and ultrastructure of Elthusa raynaudii (Isopoda, Cymothoidae) firstly recorded from the invasive silver cheeked toadfish (Lagocephalus sceleratus) (Gmelin 1789) in eastern Mediterranean Sea coast. Parasitol Res 123, 86 (2024). https://doi.org/10.1007/s00436-023-08100-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08100-1