Abstract
Circadian behavioral patterns in mosquitoes can be observed through their locomotor activity, which includes fundamental behaviors such as foraging, mating, and oviposition. These habits, which are fundamental to the life cycle of Anopheles mosquitoes, are closely related to pathogen transmission to humans. While rhythmic cycles of locomotor activity have been described in Anopheles species, no studies have been conducted on Anopheles darlingi species, the main malaria vector in the Amazon region. The aim of this study was to investigate how insemination status, blood meal, and Plasmodium vivax infection affect the locomotor activity of An. darlingi. The experiments were performed with 3- to 10-day-old An. darlingi females, which had been fed with 15% honey solution. These mosquitoes were obtained from the Malaria Vector Production and Infection Platform (PIVEM)/FIOCRUZ–RO. The experimental groups were divided into four categories: virgin vs. inseminated, unfed virgin vs. blood-fed virgin, unfed inseminated vs. blood-fed inseminated, and infected blood vs. uninfected blood. Locomotor activity was monitored using the Flybox equipment, capturing images that were subsequently converted into video to measure the insect activity, using PySoLo software. The periodicity and rhythmicity of mosquito locomotor activity were analyzed using MatLab® software. The locomotor activity of An. darlingi females showed a nocturnal and bimodal pattern under LD conditions. When comparing the insemination states and blood meal, there was a reduction in the locomotor activity in inseminated and blood-fed females. However, the P. vivax+ infection did not increase locomotor activity of An. darlingi species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Locomotor activity of insects, mosquitoes included, follows a circadian rhythm that is regulated by molecular clocks and can be modulated by extrinsic factors, such as light and temperature (Meireles-Filho and Kyriacou 2013). At the beginning of mosquito locomotor activity studies, investigations were carried out using an acoustic flight recording equipment created by Jones (1964) and adapted in the following years (Jones et al. 1967; Charlwood and Jones 1979; Peterson 1980; Rowley et al. 1987). This approach was followed by a commonly used system known as locomotor activity monitor (LAM–TrikNetics) model, which functions on break-beam detection through infrared light sensors (Eaton 1980).
In more recent studies, real-time detection and recording of insect movement have been explored using high-resolution cameras (Fry et al. 2000; Araujo et al. 2020; Ziegler et al. 2022). Guo et al. (2017) developed a device called FlyBox, which was designed to study the locomotor activity of Drosophila. Subsequently, Araujo et al. (2020) applied FlyBox to monitor the locomotor activity of male and female mosquitoes, specifically the vector species Anopheles gambiae and Aedes aegypti. Additionally, Wolkoff et al. (2023) used FlyBox to verify the effects of light pollution on the activity rhythms of the mosquito vector Culex pipiens.
The advancement of technologies and equipment capable of automatically tracking locomotor patterns of insects has allowed researchers to study different aspects related to this behavior in laboratory settings (Spitzen and Takken 2018). As a result, it has been confirmed that the locomotor activity of several mosquito species can be affected by physiological factors, such as their insemination state (Jones and Gubbins 1978; Jones 1981; Rowland 1989; Gentile et al. 2013; Lima-Camara et al. 2014; Araujo et al. 2020; Traoré et al. 2021), level of nutrition (Gentile et al. 2013; Lima-Camara et al. 2014; Araujo et al. 2020; Traoré et al. 2021) or infection state (Lima-Camara et al. 2011; Tallon et al. 2020; Padilha et al. 2018). However, no studies to date have been conducted on locomotor activity of the major malaria vector in the Amazon region, Anopheles darlingi.
Anopheles darlingi is the predominant malaria vector in most areas of the Amazon region (Tadei and Thatcher 2000; Gil et al. 2003) and exhibits nocturnal habits, with crepuscular activity peaks, highly anthropophilic behavior and susceptibility to human malaria parasites (Consoli and Oliveira 1994; Gil et al. 2015; Carlos et al. 2019). The available biological information about periodicity, time of activity and feeding behavior of An. darlingi was obtained through field works (Gil et al. 2007, 2015; Gama et al. 2009; Andrade et al. 2021).
The establishment of an An. darlingi colony in the Western Brazilian Amazon region in 2018 (Araujo et al. 2019) allowed us to develop studies under controlled environment, including investigations of the locomotor activity of this mosquito vector. Studying whether the behavior of An. darlingi is modulated by different physiological changes remains an important step towards the understanding of its biology and the development and/or evaluation of vector control tools in the field.
The importance of the vector An. darlingi in malaria transmission and the gap of biological information about its behavior has led us to study the effects of the main physiological aspects of anopheline life, blood feeding, insemination, and Plasmodium infection. In the present work, we evaluated the pattern of locomotor activity of the malaria vector An. darlingi under different physiological states at laboratory conditions. The results showed here will serve as a basis for future investigations into the molecular aspects involved in the endogenous regulation of the circadian rhythm of the An. darlingi species.
Methods
Mosquito rearing
All experiments were carried out with female mosquitoes from a colony of An. darlingi maintained in PIVEM/FIOCRUZ-RO (Araujo et al. 2019). Mosquitoes were reared from larvae to adults under 12 h of light and 12 h of darkness (LD), at a temperature of 26 ± 1 °C and relative humidity of 70% ± 10%. Larvae were fed daily with TetraMin® Marine fish food and adults were fed with 15% honey solution ad libitum. Three- to 10-day-old females were used in the experiments.
Insemination and blood-feeding experiment
To assess the effect of insemination on the locomotor activity of An. darlingi, virgin, and inseminated female mosquitoes were used. To assure that newly emerged females would not mate (virgin group), the cage with pupae was checked every hour to remove males. In order to obtain inseminated females, we allowed that emerged adult males and females mated freely in the cage for 6 days. The insemination was confirmed by dissection of female spermathecae and visual inspection under microscopy for the presence of sperm.
Experiments to assess the effect of blood feeding on the locomotor activity of females were initiated 30 min after blood feeding, which was performed using an artificial feeding membrane (Hemotek®) after the previous fasting period (≥ 12 h).
Therefore, there were four distinct groups of An. darlingi females regarding locomotor activity analysis: unfed virgin, blood-fed virgin, unfed inseminated, and blood fed inseminated. Experiments in Flybox were performed with 12 individuals per group and were repeated five times.
Artificial infection experiment
Blood samples from malaria vivax patients were prepared for membrane-feeding assays. Each blood sample, collected in heparinizes vacutainer, was centrifuged at 1500 rpm for 10 min, and the plasma was replaced for an equal volume of inactivated human AB+ serum to reduce potential effects of human immunity and hence maximize the number of successfully infected mosquitoes. An aliquot of this blood sample was incubated at 42 °C for 30 min (Sangare et al. 2013).
Cages containing 60 female mosquitoes being 3 to 5 days old were placed under Hemotek feeders to allow blood feeding for 30 min. After that period, only fully fed mosquitoes were kept in the experimental cages. 15% honey solution on cotton wool pads were offered daily to the mosquitoes until midguts were dissected on the 7th day post-blood meal (pbm), then on the 10th day pbm locomotor activity assays started. After this procedure, there were two distinct groups of An. darlingi females to assess the P. vivax effect on locomotor activity: the mosquitoes fed with infected blood (P. vivax+) and those fed with uninfected blood (P. vivax−).
Successful experimental infection and heat inactivation
At day 7 pbm, ten mosquitoes were dissected to confirm that infection and heat inactivation were successful. Midguts were dissected in PBS 1X, stained with 0.2% commercial mercurochrome (SIGMA) and examined for the presence of oocysts using microscopy (× 10). The rest of the mosquitoes remained in the original cage until the locomotor activity assays, which started at 10-day pbm and concluded in the 14-day pbm. After locomotor activity assays, salivary glands of all mosquitoes were dissected in RPMI to confirm sporozoite infection.
Locomotor activity assays
In the 24-well plate, a piece of cotton soaked in 15% honey solution was placed at the bottom of each well, and individual anesthetized mosquitoes were added to each well. Then the plate was covered with a transparent plastic film, with holes poked over each well to promote air circulation. FlyBox, which is an automated video recording system developed to detect activity and sleep patterns in adult Drosophila flies (Guo et al. 2017), was used to monitor the locomotor activity profile of An. darlingi. This system has also been used to assess the locomotor activity patterns of two important tropical disease vectors, An. gambiae and Ae. aegypti (Araujo et al. 2020).
The locomotor activity was monitored for up to 4 days using the Flybox system under a 12-h light/12-h dark photoperiod condition. Images of 24-well plate within the FlyBox were captured every 10 s and saved using the WebCan Image Save software (1.11). These images were then converted into video and the distance traveled by each mosquito was analyzed using PySoLo (version 1.1) (Gilestro and Cirelli 2009). The generated data were converted into a.txt file for analysis in the MatLab® software (version R2015b) (Donelson et al. 2012), which included locomotor activity quantification, rhythmicity, and periodicity, as described by (Araujo et al. 2020).
Data analysis
The study analyzed the average locomotor activity of mosquitoes over a period of 3 consecutive days. The first 12 h of activity under LD conditions were excluded from the analysis to mitigate effects of the anesthesia from the ice-cooling. The mean locomotor activity was calculated for the following time intervals: the completed 24-h cycle (ZT0 to ZT23), photophase (ZT0 to ZT11), scotophase (ZT12 to ZT23), principal peak of activity (ZT12), and secondary peak (ZT0). The normality of data was assessed using the Shapiro–Wilk test. The analyses comparing time intervals were performed using the T-Student and Mann–Whitney tests. Graphs were generated in Excel (2016) and the statistical analysis was performed using GraphPad Prism software version 9.1.
Results
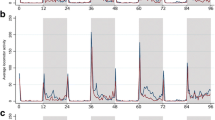
The graphs in Fig. 1 show the average locomotor activity profile of An. darlingi females under LD conditions for 3 consecutive days. In general, these mosquitoes exhibited nocturnal behavior with two distinct peaks, one during the scotophase (ZT12) and a second less intense peak at the beginning of photophase (ZT0).
Average profile of the locomotor activity of Anopheles darlingi females during 3 days of analysis for each physiological condition: A unfed virgin vs. unfed inseminated; B unfed virgin vs. blood-fed virgin; C unfed inseminated vs. blood-fed inseminated; D infected P. vivax+ vs. uninfected P. vivax.−. White column represents the photophase and gray column, the scothophase. Time is measured in Zeitgeber (or ZT) hour, ZT0 being the onset of lights on and ZT12 being the onset of lights off as a 12-h light, 12-h dark schedule inside the Flybox. The data correspond to five independent experiments. Asterisks indicate statistical significance: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
The analysis of the locomotor activity of females under different insemination conditions revealed that unfed virgin females exhibit greater activity than unfed inseminated ones (Fig. 1A), with statistical differences in the ZT12 of the first and second day, as well as during the photophase on the third day (see supplementary Table S1). Spermatheca dissection showed that 100% of females from the inseminated group were positive to sperm presence and 100% of virgin females were negative.
When virgin females were subjected to blood feeding and compared to unfed virgin ones, it could be noticed that the unfed virgin females had significantly higher activity levels in all time intervals analyzed on the first and second day, except by the photophase on the second day (Fig. 1B). On the third day, the higher activity levels of unfed virgin females were only significant at ZT0, although this group still had higher locomotor activity when compared to the blood-fed virgin group (Fig. 1B). A similar pattern was observed in the analysis of locomotor activity of inseminated females which were either blood-fed or blood-unfed (Fig. 1C). Unfed inseminated females exhibited greater locomotor activity in all time intervals of the first day and higher activity levels was observed at ZT0 and ZT12 on subsequent days (Fig. 1C; see supplementary Table S1).
Regarding the locomotor activity of mosquitoes infected with P. vivax and uninfected ones, a statistical difference was registered in the photophase of the first and second day of analysis, the uninfected females showing greater locomotor activity (Fig. 1D; see supplementary Table S1).
Comparison of circadian periodicity was also performed under physiological conditions. As expected, all experimental groups exhibited an average circadian periodicity of approximately 24 h, and there was no significant difference observed between groups (Fig. 2). The rhythmicity of female mosquito groups (virgin and inseminated) was above 92% (Fig. 3A). For the groups designed to assess the impact of blood meal on the locomotor activity, the rhythmicity was above 81% for both virgin and inseminated females (Fig. 3B, C). The P. vivax+ mosquito groups exhibited rhythmicity above 73% (Fig. 3D). No statistical difference was observed in the rhythmicity comparisons between the groups tested.
Average duration of the period in hours of locomotor activity of Anopheles darlingi females for each physiological condition: A unfed virgin vs. unfed inseminated; B unfed virgin vs. blood-fed virgin; C unfed inseminated vs. blood-fed inseminated; D infected P. vivax+ vs. uninfected P. vivax−. The data correspond to five independent experiments
Quantification (%) of rhythmic (pink) and arrhythmic (gray) Anopheles darlingi females for each physiological condition: A unfed virgin vs. unfed inseminated; B unfed virgin vs. blood-fed virgin; C unfed inseminated vs. blood-fed inseminated; D infected P. vivax+ vs. uninfected P. vivax−. No statistically significant differences were observed among all groups. The data correspond to five independent experiments
Supplementary Table S2 presents the total number of mosquitoes analyzed for locomotor activity, periodicity, and rhythmic and arrhythmic cycles for each experimental group. Mortality was less than 35% for all physiological conditions in each experimental group (see supplementary Table S2).
Discussion
This is the first report that evaluated the locomotor activity, rhythm, and the influence of different physiological states of An. darlingi, the main malaria vector in the Amazon region, under laboratory conditions. Similar studies have been described for other anopheline species (Jones and Gubbins 1978; Rowland 1989; Araujo et al. 2020; Traoré et al. 2021), as well as other mosquito vectors, including those from the genus Culex (Jones and Gubbins 1979; Chiba et al. 1990, 1992; Newman et al. 2016) and Aedes (Lima-Camara et al. 2011, 2013, 2014; Feitoza et al. 2020). The reason why there had been no circadian research on An. darlingi species is that colonization of this species had only been achieved in the last decade (Moreno et al. 2014; Villarreal-Treviño et al. 2015; Araujo et al. 2019; Puchot et al. 2022). Thus, the availability of An. darlingi colonies will improve the development of studies and provide further knowledge about the circadian rhythm and the biology of this vector.
In this study, An. darlingi exhibited a bimodal and predominantly nocturnal activity profile under LD conditions. The duration of activity period was found to be around 24 h, which was consistent with other anopheline species under 12-h light and dark cycles (LD 12-h photoperiod) (Jones et al. 1967, 1974; Sampaio et al. 2017; Duffield et al. 2019; Araujo et al. 2020; Traoré et al. 2021;). This bimodal pattern and nocturnal activity are recorded in field studies; however, the peaks of activity could vary depending on local geographical characteristics, climate, and human presence (Charlwood and Jones 1979; Forattini 1987; Tadei et al. 1998; Gil et al. 2007; Gama et al. 2009; Andrade et al. 2021).
Anopheles darlingi is considered eurigamous, like other anopheline species (Charlwood and Jones 1979; Consoli and Oliveira 1994; Diabate et al. 2003; Rund et al. 2012) whose females become refractory to further insemination after copulation (Baimai and Green 1987; Forattini 2002; Baldini et al. 2012; Baeshen 2022). The intense activity peak of virgin females observed at ZT12 in the present study coincides with the formation of mating swarms initiated by males in the dusk (Forattini 2002; Rund et al. 2012; Consoli e Oliveira 1994; Jones et al. 1967; Charlwood and Jones 1979). Such overlap in the locomotor activity of males and females of An. darlingi was observed in the experiment comparing both sexes under virgin condition (supplementary Figure S1). For inseminated females, this same peak at ZT12 may indicate an intense search for blood meal to proceed with the next stages of the gonotrophic cycle (Clements 1992; Baldini et al. 2012).
The reduction in locomotion of female mosquitoes after insemination corroborates previous findings for An. gambiae (Jones et al. 1967; Jones and Gubbins 1977, 1978; Araujo et al. 2020), An. stephensi (Rowland 1989), Ae. aegypti (Lima-Camara et al. 2014; Jones 1981), and mosquito vectors from the genus Culex (Jones and Gubbins 1979; Chiba et al. 1990, 1992). These mosquitoes showed several behavioral and physiological changes after mating due to the substances of the male accessory glands that had been transferred to females during copulation (Klowden 1999). The modulation of locomotor activity was already observed in virgin females that received a small dose of accessory gland solution by intrathoracic injection (Lima-Camara et al. 2013).
Overall, regarding physiological state (inseminated or virgin), the mosquitoes showed a significant decrease in locomotor activity after feeding on blood. This reduction in locomotor activity after blood meal is thought to be related to biological factors of blood digestion in mosquitoes and is consistent with previous studies (Jones and Gubbins 1978; Rowland 1989; Meireles-Filho et al. 2006). The timing of the reduction in locomotor activity (on the first and second days after feeding) suggests that the first 48 h of blood digestion may be more critic to An. darlingi (Consoli and Oliveira 1994; Forattini 2002). Inseminated females showed a similar decrease in locomotor activity after blood feeding, which is likely due to the energetic requirements of the oogenesis process, thus the mosquito would remain less active until oviposition, which generally occurs 72 h after blood meal (Jones and Gubbins 1978; Rowland 1989; Consoli and Oliveira 1994; Forattini 2002). The reduction on locomotor activity is related to a sensitivity reduction to human odors. Blood feeding can inhibit host search in female mosquitoes of the An. gambiae species (Takken et al. 2001) and is associated to genic regulation of chemosensory genes in Ae. aegypti (Hill et al. 2019) and Culex quinquefasciatus (Taparia et al. 2017).
Another important aspect to mention is that blood feeding is a behavior regulated by the circadian clock (Das and Dimopoulos 2008). Molecular evidence has shown changes in the expression of key circadian clock genes (per, tim, cyc, clk) in engorged vector insects that exhibit reduced locomotion (Meireles-Filho et al. 2006; Gentile et al. 2013). The authors suggest that this may be a response to oxidative stress during blood digestion. With respect to the An. gambiae vector, it has been described that some molecular regulations related to detoxification processes of products generated during blood digestion are rhythmic (Rund et al. 2011, 2016). However, it is not yet clear by which mechanisms oxidative stress affects endogenous circadian control.
The primary behavioral changes in anophelines resulting from infection occur during the infective stages of Plasmodium sporozoites (Rowland and Boersma 1988; Anderson et al. 1999; Cator et al. 2013; Thiévent et al. 2019). Many of these changes, such as increased blood meal frequency (Wekesa et al. 1992; Koella et al. 1998; Koella and Packer 1996; Ferguson and Read 2004) and heightened response to host odors (Smallegange et al. 2013; Cator et al. 2013, 2015), are closely tied to the locomotion of the anophelines. Experiments with the Ae. aegypti vector have revealed not only changes in feeding behavior (Maciel-De-Freitas et al. 2013; Sylvestre et al. 2013) but also modifications to their locomotor activity profile. Specifically, female mosquitoes infected with dengue virus serotypes 1 and 2 become more active (Lima-Camara et al. 2011; Tallon et al. 2020), whereas those infected with Zika virus show a reduction in the activity of females of this species (Padilha et al. 2018).
From the results obtained in this study, we do not consider that the statistically significant difference recorded indicates biologically relevant changes in the locomotor activity of An. darlingi females in view of P. vivax infection. This is mainly because An. darlingi is a nocturnal mosquito species, and the data presented here showed a natural reduction in activity during a diurnal period, when it is typically less active (Gama et al. 2009; Gil et al. 2007; Andrade et al. 2021; Villarreal-Treviño et al. 2015). Among the studies about the effects of Plasmodium infection on mosquito locomotor activity, only one recorded a small reduction in locomotion of An. stephensi mosquitoes infected with the rodent malaria parasite P. yoelii, starting the monitoring on day 9 post-infection (Rowland and Boersma 1988). Competition for nutrients and tissue damage during parasite development are possible causes for the reduction in locomotion. In another study (Vantaux et al. 2015) which used long and short-range techniques (olfactometry and recording of locomotor activity), no significant differences were found in the group of An. coluzzi females infected by P. falciparum compared to the uninfected control group. Studies evaluating Plasmodium infection on the locomotor activity of anopheline mosquitoes are scarce, which limits our understanding of how the parasite affects this rhythm in the malaria vector. Therefore, in the future, it will be of interest to assess the locomotor activity of An. darlingi at different stages of P. vivax development to better understand the effects of the parasite on this important behavior in the malaria vector.
Conclusions
In conclusion, An. darlingi exhibits bimodal, mainly nocturnal locomotor activity. Insemination and blood feeding are among the physiological factors that influence this malaria vector’s locomotor activity. However, P. vivax infection does not significantly alter the locomotor activity of An. darlingi in a manner that may affect the disease transmission under experimental conditions. These data will be essential for further studies of locomotor activity and circadian rhythms of this vector, particularly at the molecular level.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- PIVEM :
-
Malaria Vector Production and Infection Platform
- LD :
-
Light and dark
- LAM :
-
Locomotor activity monitor
- pbm :
-
Post-blood meal
References
Anderson RA, Koella FJC, Hurd H (1999) The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc Royal Soc B 266:1729–1733. https://doi.org/10.1098/rspb.1999.0839
Andrade AO, Santos NACD, Castro RB, Araújo ISD, Bastos ADS, Moreno MDSR, Pereira DB, Medeiros JF, Araújo MS (2021) Description of malaria vectors (Diptera Culicidae) in two agricultural settlements in the Western Brazilian Amazon. Rev Inst Med Trop São Paulo 63:e60. https://doi.org/10.1590/S1678-9946202163060
Araujo MS, Andrade AO, Santos NAC, Pereira DB, Costa GS, De Paulo PFM, Rios CT, Moreno M, Pereira-da-Silva LH, Medeiros JF (2019) Brazil’s first free-mating laboratory colony of Nyssorhynchus darlingi. Rev Soc Bras Med Trop 52:10–12. https://doi.org/10.1590/0037-8682-0159-2019
Araujo MS, Fang G, Michael R (2020) Video recording can conveniently assay mosquito locomotor activity. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-61733-5
Baeshen R (2022) Swarming behavior in Anopheles gambiae (sensu lato): current knowledge and future outlook. J Med Entomol 59:56–66. https://doi.org/10.1093/jme/tjab157
Baimai V, Green CA (1987) Monandry (monogamy) in natural populations of anopheline mosquitoes. J Am Mosq Control Assoc 3(3):481–484
Baldini F, Gabrieli P, Rogers DW, Catteruccia F (2012) Function and composition of male accessory gland secretions in Anopheles gambiae: a comparison with other insect vectors of infectious diseases. Pathog Glob Health 106:82–93. https://doi.org/10.1179/2047773212Y.0000000016
Carlos BC, Rona LD, Christophides GK, Souza-Neto JA (2019) A comprehensive analysis of malaria transmission in Brazil. Pathog Glob Health 113:1–13. https://doi.org/10.1080/20477724.2019.1581463
Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF, Thomas MB (2013) ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc Biol Sci 280(1763):20130711. https://doi.org/10.1098/rspb.2013.0711
Cator LJ, Pietri JE, Murdock CC, Ohm JR, Lewis EE, Read AF, Luckhart S, Thomas MB (2015) Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Sci Rep 5:11947. https://doi.org/10.1038/srep11947
Charlwood JD, Jones MDR (1979) Mating behaviour in the mosquito, Anopheles gambiae. Physiol Entomol 4(2):111–120. https://doi.org/10.1111/j.1365-3032.1979.tb00185.x
Chiba Y, Yamamoto Y, Shimizu C, Zaitsu M, Uki M, Yoshii M, Tomioka K (1990) Insemination-dependent modification of circadian activity of the mosquito, Culex pipiens pallens. Zool Sci 7:895–906
Chiba Y, Shinkawa Y, Yoshii M, Matsumoto A, Tomioka K, Takahashi SYA (1992) Comparative study on insemination dependency of circadian activity pattern in mosquitoes. Physiol Entomol 17(3):213–218. https://doi.org/10.1111/j.1365-3032.1992.tb01013.x
Clements AN (1992) The biology of mosquitoes: Development, nutrition and reproduction. Chapman & Hall, London
Consoli RA, Oliveira RLD (1994) Principais mosquitos de importância sanitária no Brasil. FIOCRUZ, Brazil
Das S, Dimopoulos G (2008) Molecular analysis of photic inhibition of blood-feeding in Anopheles gambiae. BMC Physiol 8:23. https://doi.org/10.1186/1472-6793-8-23
Diabate A, Baldet T, Brengues C, Kengne P, Dabire KR, Simard F, Fontenille D (2003) Natural swarming behaviour of the molecular M form of Anopheles gambiae. Trans R Soc Trop Med Hyg 97(6):713–716. https://doi.org/10.1016/s0035-9203(03)80110-4
Donelson NC, Kim EZ, Slawson JB, Vecsey CG, Huber R, Griffith LC (2012) High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS One 7(5):e37250. https://doi.org/10.1371/journal.pone.0037250
Duffield GE, Acri DJ, George GF, Sheppard AD, Beebe NW, Ritchie SA, Burkot TR (2019) Diel flight activity of wild-caught Anopheles farauti (ss) and An. hinesorum malaria mosquitoes from northern Queensland Australia. Parasit Vectors 12:48. https://doi.org/10.1186/s13071-018-3271-0
Eaton JL (1980) An infrared LED-based electronic actograph for monitoring insect flight activity. Ann Entomol Soc Am 73(6):744–746. https://doi.org/10.1093/aesa/73.6.744
Feitoza TDS, Ferreira-de-Lima VH, Câmara DCP, Honório NA, Lounibos LP, Lima-Camara TN (2020) Interspecific mating effects on locomotor activity rhythms and refractoriness of Aedes albopictus (Diptera: Culicidae) Females. Insects 11(12):874. https://doi.org/10.3390/insects11120874
Ferguson HM, Read AF (2004) Mosquito appetite for blood is stimulated by Plasmodium chabaudi infections in themselves and their vertebrate hosts. Malar J 3:1–8. https://doi.org/10.1186/1475-2875-3-12
Forattini OP (1987) Comportamento exófilo de Anopheles darlingi Root, em região meridional do Brasil. Rev Saúde Pública 21(4):291–304. https://doi.org/10.1590/S0034-89101987000400002
Forattini OP (2002) Culicidologia Médica: Identificação, Biologia, Epidemiologia, 1st edn. USP, São Paulo
Fry SN, Bichsel M, Muller P, Robert P (2000) Tracking of flying insects using pan-tilt cameras. J Neurosci Methods 101(1):59–67. https://doi.org/10.1016/S0165-0270(00)00253-3
Gama RA, Santos RL, Santos FD, Silva IM, Resende MC, Eiras ÁE (2009) Periodicity of capture of the Anopheles darlingi Root (Diptera: Culicidae) in Porto Velho, Rondônia. Brazil Neotrop Entomol 38(5):677–682. https://doi.org/10.1590/S1519-566X2009000500019
Gentile C, Rivas GBDS, Lima JB, Bruno RV, Peixoto AA (2013) Circadian clock of Aedes aegypti: effects of blood-feeding, insemination and RNA interference. Mem Inst Oswaldo Cruz 108:80–87. https://doi.org/10.1590/0074-0276130471
Gil LHS, Alves FP, Zieler H, Salcedo JM, Durlacher RR, Cunha RPA, Tada MS, Camargo LMA, Camargo EP, Pereira-da-Silva LH (2003) Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol 40(5):636–641. https://doi.org/10.1603/0022-2585-40.5.636
Gil LHS, Tada MS, Katsuragawa TH, Ribolla PEM, Pereira-da-Silva LH (2007) Urban and suburban malaria in Rondônia (Brazilian Western Amazon) II: perennial transmissions with high anopheline densities are associated with human environmental changes. Mem Inst Oswaldo Cruz 102(3):271–276. https://doi.org/10.1590/S0074-02762007005000013
Gil LHS, Rodrigues MDS, Lima AAD, Katsuragawa TH (2015) Seasonal distribution of malaria vectors (Diptera: Culicidae) in rural localities of Porto Velho Rondônia, Brazilian Amazon. Rev Inst Med Trop São Paulo 57(3):263–267. https://doi.org/10.1590/S0036-46652015000300014
Gilestro GF, Cirelli C (2009) pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics 45(11):1466–1467. https://doi.org/10.1093/bioinformatics/btp237
Guo F, Chen X, Rosbash M (2017) Temporal calcium profiling of specific circadian neurons in freely moving flies. PNAS 114(41):8780–8787. https://doi.org/10.1073/pnas.1706608114
Hill SR, Ghaninia M, Ignell R (2019) Blood meal induced regulation of gene expression in the maxillary palps, a chemosensory organ of the mosquito Aedes aegypti. Front Ecol Evol 7:336. https://doi.org/10.3389/fevo.2019.00336
Jones MDR (1964) The automatic recording of mosquito activity. J Insect Physiol 10(2):349–351. https://doi.org/10.1016/0022-1910(64)90017-4
Jones MDR (1981) The programming of circadian flight-activity in relation to mating and the gonotrophic cycle in the mosquito, Aedes Aegypti. Physiol Entomol 6(3):307–313. https://doi.org/10.1111/j.1365-3032.1981.tb00275.x
Jones MDR, Gubbins SJ (1977) Modification of circadian flight activity in the mosquito Anopheles gambiae after insemination. Nature 268:731–732. https://doi.org/10.1038/268731a0
Jones MDR, Gubbins SJ (1978) Changes in the circadian flight activity of the mosquito Anopheles gambiae in relation to insemination, feeding and oviposition. Physiol Entomol 3(3):213–220. https://doi.org/10.1111/j.1365-3032.1978.tb00151.x
Jones MDR, Gubbins SJ (1979) Modification of female circadian flight-activity by a male accessory gland pheromone in the mosquito. Culex Pipiens Quinquefasciatus Physiol Entomol 4(4):345–351. https://doi.org/10.1111/j.1365-3032.1979.tb00626.x
Jones MDR, Hill M, Hope AM (1967) The circadian flight activity of the mosquito, Anopheles gambiae: Phase setting by the light regime. J Exp Biol 47(3):503–511. https://doi.org/10.1242/jeb.47.3.503
Jones MDR, Gubbins SJ, Cubbin CM (1974) Circadian flight activity in four sibling species of the Anopheles gambiae complex (Diptera, Culicidae). Bull Entomol Res 64:241–246. https://doi.org/10.1017/S0007485300031126
Klowden MJ (1999) The check is in the male: male mosquitoes affect female physiology and behavior. J Am Mosq Control Assoc 15(2):213–220
Koella JC, Packer MJ (1996) Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitology 113(2):105–109. https://doi.org/10.1017/S0031182000066348
Koella JC, Sorensen FL, Anderson RA (1998) The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles Gambiae. Proc Biol Sci 265(1398):763–768. https://doi.org/10.1098/rspb.1998.0358
Lima-Camara TN, Bruno RV, Luz PM, Castro MG, Lourenço-De-Oliveira R, Sorgine MH, Peixoto AA (2011) Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS One 6:e17690. https://doi.org/10.1371/journal.pone.0017690
Lima-Camara TN, Codeço CT, Honório NA, Bruno RV, Peixoto AA, Lounibos LP (2013) Male accessory gland substances from Aedes albopictus affect the locomotor activity of Aedes aegypti females. Mem Inst Oswaldo Cruz 108:18–25. https://doi.org/10.1590/0074-0276130381
Lima-Camara TN, Lima JBP, Bruno RV, Peixoto AA (2014) Effects of insemination and blood-feeding on locomotor activity of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) females under laboratory conditions. Parasit Vectors 7:304. https://doi.org/10.1186/1756-3305-7-304
Maciel-De-Freitas R, Sylvestre G, Gandini M, Koella JC (2013) The influence of dengue virus serotype-2 infection on Aedes aegypti (Diptera: Culicidae) motivation and avidity to blood feed. PLoS One 8(6):e65252. https://doi.org/10.1371/journal.pone.0065252
Meireles-Filho ACA, Kyriacou CP (2013) Circadian rhythms in insect disease vectors. Mem Inst Oswaldo Cruz 108:48–58. https://doi.org/10.1590/0074-0276130438
Meireles-Filho ACA, Rivas GBS, Gesto JSM, Machado RC, Britto C, Souza NA, Peixoto AA (2006) The biological clock of an hematophagous insect: locomotor activity rhythms, circadian expression and downregulation after a blood meal. FEBS Lett 580(1):2–8. https://doi.org/10.1016/j.febslet.2005.11.031C
Moreno M, Tong C, Guzmán M, Chuquiyauri R, Llanos-Cuentos A, Rodriguez H, Gamboa D, Meister S, Winzeler EA, Maguina P, Conn JE, Vinetz JM (2014) Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am J Trop Med Hyg 90(4):612–616. https://doi.org/10.4269/ajtmh.13-0708
Newman CM, Anderson TK, Goldberg TL (2016) Decreased flight activity in Culex pipiens (Diptera: Culicidae) naturally infected with Culex flavivirus. J Med Entomol 53(1):233–236. https://doi.org/10.1093/jme/tjv161
Padilha KP, Resck MEB, Cunha OATD, Teles-De-Freitas R, Campos SS, Sorgine MHF, Lourenço-de-Oliveira R, Farnesi LC, Bruno RV (2018) Zika infection decreases Aedes aegypti locomotor activity but does not influence egg production or viability. Memo Inst Oswaldo Cruz 113(10):e180290. https://doi.org/10.1590/0074-02760180290
Peterson EL (1980) The temporal pattern of mosquito flight activity. Behaviour 72:1–25
Puchot N, Lecoq MT, Carinci R, Duchemin JB, Gendrin M, Bourgouin C (2022) Establishment of a colony of Anopheles darlingi from French Guiana for vector competence studies on malaria transmission. Front Trop Dis 05(24):493327. https://doi.org/10.3389/fitd.2022.949300
Rowland M (1989) Changes in the circadian flight activity of the mosquito Anopheles stephensi associated with insemination, blood-feeding, oviposition and nocturnal light intensity. Physiol Entomol 14(1):77–84. https://doi.org/10.1111/j.1365-3032.1989.tb00939.x
Rowland M, Boersma E (1988) Changes in the spontaneous flight activity of the mosquito Anopheles stephensi by parasitization with the rodent malaria Plasmodium yoelii. Parasitology 97(2):221–227. https://doi.org/10.1017/S003118200005842X
Rowley WA, Jones MDR, Jacobson DW, Clarke JL (1987) A microcomputer-monitored mosquito flight activity system. Ann Entomol Soc Am 80(4):534–538. https://doi.org/10.1093/aesa/80.4.534
Rund SS, Hou TY, Ward SM, Collins FH, Duffield GE (2011) Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA 108(32):421–430. https://doi.org/10.1073/pnas.110058410
Rund SS, Lee SJ, Bush BR, Duffield GE (2012) Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J Insect Physiol 58(12):1609–1619. https://doi.org/10.1016/j.jinsphys.2012.09.016
Rund SS, O’Donnell AJ, Gentile JE, Reece SE (2016) Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7(2):14. https://doi.org/10.3390/insects7020014
Sampaio VS, Rivas GBS, Kobylinski K, Pinilla Y, Pimenta PFP, Lima JBP, Bruno RV, Lacerda MVG, Monteiro WM (2017) What does not kill it makes it weaker: effects of sub-lethal concentrations of ivermectin on the locomotor activity of Anopheles aquasalis. Parasit Vectors 10:623. https://doi.org/10.1186/s13071-017-2563-0
Sangare I, Michalakis Y, Yameogo B, Dabire R, Morlais I, Cohuet A (2013) Studying fitness cost of Plasmodium falciparum infection in malaria vectors: validation of an appropriate negative control. Malar J 12:2. https://doi.org/10.1186/1475-2875-12-2
Smallegange RC, Van Gemert GJ, Van De Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW, Logan JG (2013) Malaria infected mosquitoes express enhanced attraction to human odor. PLoS One 8:e563602. https://doi.org/10.1371/journal.pone.0063602
Spitzen J, Takken W (2018) Keeping track of mosquitoes: a review of tools to track, record and analyse mosquito flight. Parasit Vectors 11:123. https://doi.org/10.1186/s13071-018-2735-6
Sylvestre G, Gandini M, Maciel-De-Freitas R (2013) Age-Dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) feeding behavior, survival, oviposition success and fecundity. PLoS One 8:e59933. https://doi.org/10.1371/journal.pone.0059933
Tadei WP, Thatcher DB (2000) Malaria vectors in the Brazilian amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop São Paulo 42(2):87–94. https://doi.org/10.1590/S0036-46652000000200005
Tadei WP, Thatcher BD, Santos JMM, Scarpassa VM, Rodrigues IB, Rafael MS (1998) Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg 59:325–335. https://doi.org/10.4269/ajtmh.1998.59.325
Takken W, Van Loon JJA, Adam W (2001) Inhibition of host-seeking response and olfactory responsiveness in Anopheles gambiae following blood feeding. J Insect Physiol 47(3):303–310. https://doi.org/10.1016/S0022-1910(00)00107-4
Tallon AK, Lorenzo MG, Moreira LA, Martinez Villegas LE, Hill SR, Ignell R (2020) Dengue infection modulates locomotion and host seeking in Aedes aegypti. Plos Negl Trop Dis 14:e0008531. https://doi.org/10.1371/journal.pntd.0008531
Taparia T, Ignell R, Hill SR (2017) Blood meal induced regulation of the chemosensory gene repertoire in the southern house mosquito. BMC Genomics 18:393. https://doi.org/10.1186/s12864-017-3779-2
Thiévent K, Zilio G, Hauser G, Koella JC (2019) Malaria load affects the activity of mosquito salivary apyrase. J Insect Physiol 116:10–16. https://doi.org/10.1016/j.jinsphys.2019.04.003
Traoré AS, Porciani A, Moiroux N, Simard F, Contantini C, Mouline K (2021) Effects of insemination and blood-feeding on locomotor activity of wild-derived females of the malaria mosquito Anopheles coluzzii. Parasit Vectors 14:457. https://doi.org/10.1186/s13071-021-04967-0
Vantaux A, De Sales Hien DF, Yameogo B, Dabiré KR, Thomas F, Cohuet A, Lefevre T (2015) Host-seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: no evidence for host manipulation. Front Ecol Evol 3:86. https://doi.org/10.3389/fevo.2015.00086
Villarreal-Treviño C, Vásquez GM, López-Sifuentes VM, Escobedo-Vargas K, Huayanay-Repetto A, Linton YM, Flores-Mendoza C, Lescano AG, Stell FM (2015) Establishment of a free-mating, long-standing and highly productive laboratory colony of Anopheles darlingi from the Peruvian Amazon. Malar J 14:1–12. https://doi.org/10.1186/s12936-015-0733-0
Wekesa JW, Copeland RS, Mwangi RW (1992) Effect of Plasmodium falciparum on blood feeding behavior of naturally infected Anopheles mosquitoes in western Kenya. Am J Trop Med Hyg 47(4):484–488. https://doi.org/10.4269/ajtmh.1992.47.484
Wolkoff M, Fyie L, Meuti M (2023) Light pollution disrupts seasonal differences in the daily activity and metabolic profiles of the northern house mosquito Culex pipiens. Insects 14(1):64. https://doi.org/10.3390/insects14010064
Ziegler R, Blanckenhorn WU, Mathis A, Verhulst NO (2022) Video analysis of the locomotory behaviour of Aedes aegypti and Ae. japonicus mosquitoes under different temperature regimes in a laboratory setting. J Therm Biol 105:103205. https://doi.org/10.1016/j.jtherbio.2022.103205
Acknowledgements
The authors gratefully acknowledge Michael Rosbash for the donation of FlyBox.
Funding
This work was supported, in part, by the Brazilian Ministry of Health/DECIT/CNPq No. 23/2019 (grant number 442653/2019–0) and the Bill & Melinda Gates Foundation (INV-003970). Under the grant conditions of the foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the author-accepted manuscript version that might arise from this submission. ASB and NACS expresses their appreciation for the fellowship granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (88887.507471/2020–00 and 88887.633027/2021–00). JFM is a CNPq research productivity fellow (process number: 304830/2022–4).
Author information
Authors and Affiliations
Contributions
MSA and ASB conceived and designed the experiments. ASB performed the experiments. ASB, AOA, JDP, and JEA maintained the production of mosquitoes from PIVEM to experiments. ASB analyzed the data and drafted the first version of the manuscript. MSA revised the manuscript. MSA and JFM managed the project and obtained the funding for the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were performed with the approval of the Ethics Committee at the Centro de Pesquisa em Medicina Tropical (CEPEM) (no 2.641.046). At CEPEM, patients diagnosed with P. vivax by microscopy, with parasitemia level of 500 or more parasites per µL, and who were ≥ 18 years of age, were invited to participate in the study. After the informed consent was read and signed by each volunteer, a sample of blood was collected by venipuncture using a heparinized vacutainer tube (10 mL). The following exclusion criteria were adopted: patients with severe or complicated malaria, patients with serious comorbid conditions (e.g., HIV/AIDS or malnutrition), pregnant women, children, and Native American people.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Silva Bastos, A., dos Santos, N.A.C., Andrade, A.O. et al. Evaluation of insemination, blood feeding, and Plasmodium vivax infection effects on locomotor activity patterns of the malaria vector Anopheles darlingi (Diptera: Culicidae). Parasitol Res 123, 15 (2024). https://doi.org/10.1007/s00436-023-08053-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08053-5