Abstract
The objective of this work was to evaluate the effect of the ethyl acetate extract from A. ludoviciana (EALM) and artemisinin against adult parasites and eggs of F. hepatica. For the ovicidal assay, cell culture plates with 24 wells were used, and 90 to 110 F. hepatica eggs were placed in each well. The eggs were exposed to concentrations of 100, 200, 300, 400, and 500 mg/L EALM and incubated for 16 days. Additionally, triclabendazole (TCBZ) was used as a reference drug at concentrations of 10 and 50 mg, and the response of artemisinin at concentrations of 10 and 20 mg was simultaneously assessed. Adult flukes were exposed to concentrations of 125, 250, 375, and 500 mg/L EALM. The results of the ovicidal action of EALM on the eggs showed that concentrations greater than 300 mg/L were significant, with ovicidal percentages greater than 60% observed on day 16 of incubation (p < 0.05). The maximum efficiency of EALM on adult flukes was reached 72 h post-exposure at a concentration of 125 mg/L (p < 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fasciolosis is among the most serious liver diseases worldwide in the field of veterinary medicine and generates millions of dollars of economic losses (WHO 2020; FAO 2021). In addition, fasciolosis is a disease of serious public health concern, as it is an emerging zoonosis found in 70 countries; approximately 2.4 to 17 million people are infected worldwide and 1 billion more at risk (Sabourin et al. 2018; Fairweather et al. 2020). Over the years, fasciolosis has spread due to the growth of the global livestock industry and climatic factors favorable to bacterial adaptation and a greater geographical distribution of the intermediate host (Dargie 1987; Mas-Coma et al. 2005; Rojo et al. 2012; Rodríguez-Vivas et al. 2017; Sabourin et al. 2018; Alba et al. 2021; Chai and Jung 2022). Fasciolosis is caused by the fluke Fasciola hepatica. This parasite utilizes an indirect cycle and is located in the bile ducts of ruminants, swine, equines, rabbits, and humans (Javaregowda and Rani 2017). For decades, the main treatment for fasciolosis has involved chemotherapeutics (Olaechea et al. 2011). Unfortunately, due to the indiscriminate use of these drugs together with the poor prevention and diagnosis and genetic adaptation of the parasite, an increase in anthelmintic resistance to these drugs has occurred in different parts of the world (Moll et al. 2000; Olaechea et al. 2011; Kaplan and Vidyashankar 2012; Rojo et al. 2012; Ortiz et al. 2013; Hanna et al. 2015; Novobilsky et al. 2016; Ceballos et al. 2019; Kamaludeen et al. 2019; Romero et al. 2019; Fairweather et al. 2020; Kelley et al. 2020). An alternative substance to address this problem is natural products from plants with biological activity, which can be molecules or plant extracts. In Mexico, Artemisia ludoviciana Nutt. spp mexicana (estafiate), which belongs to the Asteraceae family, is recognized for its curative effect against diseases of the gastrointestinal tract (Andrade 2009; BDMTM/UNAM 2009). Extracts of different polarities obtained from the A. ludoviciana have demonstrated efficacy in vitro against young or recently excysted flukes, causing mortality greater than 90% at concentrations ranging from 125 to 500 mg/L (Álvarez et al. 2015; Ezeta et al. 2016). Recently, the effect of ethyl acetate extract from the A. ludoviciana on the tegument of recently excysted young flukes was demonstrated. Likewise, artemisinin was identified by HPLC/mass spectrometry as the major compound of the active extract (Ezeta et al. 2020). The objective of this work was to evaluate the effect of the ethyl acetate extract from A. ludoviciana and artemisinin against adult parasites and eggs of F. hepatica.
Methodology
Vegetal material
Healthy leaves of A. ludoviciana Nutt. spp. mexicana were collected in the vicinity of the Center for Teaching, Research and Extension in Tropical Livestock (CEIEGT), of the Faculty of Veterinary Medicine and Zootechnics (FMVZ), of the National Autonomous University of Mexico (UNAM), located in Martínez de la Torre—Tlapacoyan, municipality of Tlapacoyan, Veracruz, Mexico (19° 57′ 42″ N, 97° 12′ 39″ W). Taxonomic identification was performed in the herbarium of the Faculty of Higher Studies-Iztacala, UNAM (FESI-UNAM), and voucher number 2156 IZTA was assigned. The plant selection criteria were based on previous reports (Ibarra et al. 2012; Álvarez et al. 2015; Ezeta et al. 2020).
Preparation of crude extract of A. ludoviciana Nutt. spp mexicana (EALM)
The leaves were dried at a constant temperature of 60 °C for 3 days and then ground. The ground material was macerated at room temperature with ethyl acetate for one week. The extract was filtered and concentrated to dryness under reduced pressure in a Heidolph© Mod. Laborota 4000 rotary evaporator. Extracts were obtained once per week for 2 months and stored at 4 °C. The ethyl acetate extract obtained from A. ludoviciana (EALM) was used to perform the biological tests.
Collection of adult specimens of Fasciola hepatica and in vitro tests
F. hepatica adults were obtained directly from infected bovine livers. The collection was performed in the municipal slaughterhouse of Toluca, State of Mexico. The specimens were first obtained and washed with phosphate-buffered saline to remove excess blood and bile and then placed in Roswell Park Memorial Institute (RPMI)-1640 medium at 37 °C to be transported to the Helminth Experimental Chemotherapy Laboratory of the Parasitology Department FMVZ-UNAM. Once in the laboratory, the flukes were washed several times with RPMI-1640 medium and placed in 20-ml tissue culture dishes in a medium created with 50% RPMI 1640 medium and 50% bovine serum. A mixture of antibiotics (100 IU penicillin + 100 mg/ml streptomycin) was added to the medium to prevent bacterial growth. Finally, 4 flukes were placed in each box (ratio of 1 fluke for every 5 ml of medium).
A stock solution was prepared with EALM at a concentration of 500 mg/L, which was previously dissolved in 100 µL of solvent and calibrated with distilled water to form the stock solution, from which dilutions were performed to obtain the corresponding concentrations. Adult flukes were exposed in triplicate to concentrations of 125, 250, 375, and 500 mg/L EALM, incorporating the corresponding controls for each solvent to confirm that the solvent did not affect the parasite; in addition, negative controls without any treatment were used. Additionally, triclabendazole (TCBZ) was used as a reference drug at concentrations of 10 and 50 mg (TCBZ was donated by the Dept. of Biopharmacy of the Faculty of Chemistry, UNAM), and the response of artemisinin (SIGMA- ALDRICH©, 98% purity, Ref. 361593–100 mg) at concentrations of 10 and 20 mg was simultaneously assessed. Once the different groups were exposed, the samples were incubated at 37 °C in a 5% CO2 atmosphere. (Burden and Hammet 1980; Hegazi et al. 2007; Helmy et al. 2008; Aguayo et al. 2018; Rehman et al. 2020; Sánchez et al. 2020; Guo et al. 2021). The test readings were obtained at 24, 48, and 72 h postexposure. To evaluate the effectiveness of the extract and the drugs on adult parasites, the mobility and mortality of each were considered according to the methodology and motility criteria described by Jeyathilakan et al. (2010). The control groups were observed, washed, and placed daily in a fresh culture medium to maintain their viability. Each experiment was performed in triplicate (De Mello et al. 2023).
All the procedures described were performed under aseptic conditions using a BG© Mod. CFLV-130 laminar flow hood (Álvarez et al. 2015; WHO 2019; Ezeta et al. 2020, Rehman et al. 2020).
Fasciolicide efficacy
The anti-fasciola efficacy was determined by comparing the survival of the treated group in relation to the control group as follows (Wood et al. 1995):
Ovicidal activity of EALM
To obtain F. hepatica eggs, gallbladders were collected from the livers of sheep affected by fascioliasis in the municipal slaughterhouse of Toluca, State of Mexico. The livers were transported to the laboratory at a temperature of 4 to 8 °C. Once in the laboratory, the bile contents were obtained aseptically and mixed with 400 to 500 ml of distilled water to settle the mixture and eggs for approximately 20 to 30 min. After that period, 2/3 of the volume of the mixture was decanted, and the samples underwent further gauging with distilled water. Then, the samples were left to settle for the same amount of time, which was performed until the liquid was as clear as possible and the eggs could be recovered from the bottom. The eggs were left at 4 °C for 24 h before ovicidal evaluation (Moazeni and Khademolhoseini 2016; Ceballos et al. 2019; Reigate et al. 2021). For the ovicidal assay, NUNC© cell culture boxes with 24 wells were used, and 90 to 110 F. hepatica eggs were placed in each well. The eggs were exposed in triplicate to concentrations of 100, 200, 300, 400, and 500 mg/L EALM; control wells with solvent (ethyl acetate) and wells without treatment were used as control controls. Additionally, TCBZ was used at concentrations of 10 and 50 mg, and artemisinin at concentrations of 10 and 20 mg was assessed. Three replicates of each of the concentrations were carried out. Each of the boxes was covered with aluminum foil to protect them from light, and they were incubated for 14 and 16 days at a temperature of 28 °C and 80% humidity. After that period, they were exposed to 2 h of artificial light so that the miracidia would hatch. The ovicidal activity was evaluated according to formulas from the following (Najafi et al. 2017; Knepper et al. 2018; Machado et al. 2020):
Analysis of data
The data obtained were analyzed through the analysis of variance (ANOVA) test, Probit analysis, Dunnett’s test, and Kruskal‒Wallis test with a confidence interval of 95% to determine if there were statistically significant differences between the different treatments using SYSTAT v.12.0 32-bits (Systat Software, Inc. USA, 2008).
Results
EALM yields
Initially, 2.86 kg of green matter was collected, and when dried, 126 g of dry matter was obtained. At the end of the ethyl acetate extraction, a total of 17.85 g of EALM was obtained.
Ovicidal activity
The ovicidal activity was determined according to the number of hatched eggs in the different experimental groups. Regarding the percentages of ovicidal action, significant differences are observed between the reference compounds and EALM concentrations. Of the different concentrations of EALM, concentrations greater than 300 mg/L stand out, as ovicidal percentages greater than 60% were observed on both days of incubation (p < 0.05). The complete results of the percentage of ovicidal action of the eggs exposed to TCBZ, artemisinin, and EALM are shown in Table 1 and Fig. 1. The ovicidal effect increased according to the dose‒response relationship. The efficacy of EALM increased when the eggs were exposed for 2 more days, which represented a longer extract exposure time.
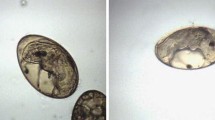
The observations obtained for the morphology of the eggs exposed to EALM identified changes in relation to the control group are shown in Fig. 2. When the eggs were exposed to 300 mg/L EALM (Fig. 2E), miracidium formation was observed. However, changes in the internal and external morphology of the egg were detected. Presumably, these changes affected the development and hatching of the miracidia. Eggs exposed to EALM at a concentration of 400 mg/L (Fig. 2F) showed a change in the external egg morphology, especially in one of the egg poles (indicated with the arrow), and a change in the germ cells within the egg started to become noticeable. In an area in the group treated with 500 mg/L EALM (Fig. 2G), the outline of the eggs is not adequately distinguished (indicated with the arrows), and their content has begun to emerge, suggesting that their morphology was damaged. Regarding the eggs treated with artemisinin, eggs without larval development and others with miracidium formation were observed. However, in both cases, small changes could be seen in the egg periphery, suggesting that the compound caused damage and the egg integrity was affected (Fig. 2H and I).
Observations obtained for the morphology of the eggs of F. hepatica exposed to EALM and artemisinin. A Eggs before being incubated; B Larval eggs after being incubated at 16 days; C miracidia emerging from the egg after being subjected to artificial light for 2 h; D empty egg after subjection to 2 h of artificial light; E egg of F. hepatica treated with 300 mg/L EALM; F egg of F. hepatica treated with 400 mg/L EALM; G egg of F. hepatica treated with 500 mg/L EALM; H egg of F. hepatica treated with 10 mg of artemisinin; and I egg of F. hepatica treated with 20 mg of artemisinin
Fasciolicidal activity in adult flukes
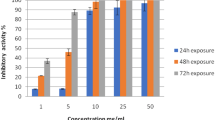
The controls without any treatment did not suffer mortality during the duration of the experiment. In the groups treated with TCBZ and artemisinin, 100% efficacy was observed from the first 24 h at the two respective concentrations (p < 0.05). The fasciolicidal efficiencies of EALM at different concentrations showed differences, and 500 mg/L EALM was the only group that began to exhibit 72% efficacy at 24 h postexposure (p < 0.05). At 48 h postexposure, 125 mg/L showed 94% efficacy, and 250, 375, and 500 mg/L resulted in 100% efficacy (p < 0.05). Finally, at a concentration of 125 mg/L, the maximum efficacy was reached 72 h postexposure (p < 0.05) (Fig. 3). In general, an effect was observed in relation to concentration and exposure time. The complete fasciolicidal activities of EALM, TCBZ, and artemisinin against adult parasites are shown in Table 2. Regarding the different exposure times, an increasing efficacy began to be seen in the first 24 h of exposure, with concentrations of 500 mg/L reaching almost 100% efficacy 48 h postexposure (p < 0.05).
Lethal concentration (LCs) 50, 90, and 99 estimates from EALM
Probit analysis showed that the LC50, LC90, and LC99 values for the EALM with fasciolicidal efficacy in F. hepatica adults in vitro determined in this study are 80.1 mg/L, 310.9 mg/L, and 362.9 mg/L, respectively.
Discussion
Previously, ovicidal assays of different parasites have been used to evaluate the efficacy of various compounds and plant extracts; recently, they have begun to be evaluated against F. hepatica eggs (Tunc et al. 2000; Kamaraj and Rahuman 2011; Moazeni and Khademolhoseini 2016; Moazeni et al. 2017; Najafi et al. 2017; Knepper et al. 2018; Machado et al. 2020). EALM had not been evaluated in this way, and this experiment showed an ovicidal effect on F. hepatica eggs. Presumably, there was damage to the eggshell, which may have affected its permeability. However, it is still necessary to determine in greater depth the type of damage that EALM has on the eggs to try to elucidate its mechanism of ovicidal action. In addition, certain drugs have been evaluated against F. hepatica eggs, such as ivermectin, artemisinin, and albendazole, all of which show ovicidal action, but the mechanism of action is still not clear (Moazeni et al. 2017). Regarding the evaluation of artemisinin and its ovicidal action, the observed damage was sufficient to alter egg integrity and prevent the development of miracidia and their hatching, although further studies are still necessary to visualize the nature of the damage to F. hepatica. In this experiment, although TCBZ was selected as the reference drug, it did not exhibit a considerable ovicidal percentage, in alignment with the results obtained by Álvarez et al. (2009). Additionally, the fasciolicidal effect of EALM shows its efficacy on adult parasites. Previously, its efficacy has been shown in newly excysted flukes, reaching fasciolicidal efficacy greater than 90% from the first 24 h postexposure (Ezeta et al. 2016, 2020). It is important to emphasize the differences that exist between young and adult flukes. From the time the definitive host is infected until the adult forms reach the liver, approximately 6 to 7 weeks pass. During this time, the changes in its tegument are influenced by the progression through the intestinal mucosa, peritoneum, and liver (Robinson et al. 2022; González et al. 2021). During their migration, young flukes, with the help of their glycocalyx, proteolytic systems, genetic expression, and changes in their energy metabolism, are able to better evade the host’s immune response, achieving greater adaptation and survival. All of this results in better development of the tegument, giving the flukes, among other things, better environmental protection (González et al. 2021). This may explain the differences between the fasciolicidal efficiencies of EALM in immature and adult forms of F. hepatica, especially at different exposure times where 100% efficacy is reached.
Additionally, the fasciolicidal effect of artemisinin has been demonstrated. Taking into account the previous work of Ezeta et al. (2020), the ethyl acetate extract of A. ludoviciana and its fractions point to the presence of artemisinin within them as the probable compound that generates the effect on parasites. Artemisinin is a constant metabolite within the genus Artemisia spp. and has been useful in treating Plasmodium falciparum infections in humans (Klayman 1985; Keizer and Utzinger 2007; Ferreira et al. 2011). Studies have been carried out with artesunate and artemether, which are semisynthetic derivatives of artemisinin, against F. hepatica, and an interruption in spermatogenesis in the adult flukes, affecting their reproductive system, was observed (O’Neill et al. 2009, 2015a, b, 2017). In another experiment, artemether caused extensive damage to the tegument of F. hepatica (Keiser and Morson 2008). However, none of these studies have directly evaluated artemisinin. Taking this into account, it is necessary to continue with studies that allow us to see the real damage to adult flukes caused by EALM and artemisinin, the compounds identified within the A. ludoviciana extract (Ezeta et al. 2020), to determine the affected area and compare the effect on the tegument of young and adult flukes. This would help in the ongoing effort to propose in vitro EALM as an integral option for the control of fasciolosis, covering different stages of parasite development and providing new insights into strategies against the fluke eggs.
Conclusion
The extract of A. ludoviciana obtained from ethyl acetate has an ovicidal effect on F. hepatica eggs and fasciolicidal efficacy in adult forms of the parasite.
Data availability
All data supporting the findings of this study are available in the document.
References
Aguayo V, Valdes B, Espino AM (2018) Assessment of Fasciola hepatica glutathione S-transferase as an antigen for serodiagnosis of human chronic fascioliasis. Acta Trop 186:41–49. https://doi.org/10.1016/j.actatropica.2018.07.002
Álvarez L, Moreno G, Moreno L, Ceballos L, Shaw L, Fairweather I, Lanusse C (2009) Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet Parasitol 164:211–216. https://doi.org/10.1016/j.vetpar.2009.05.014
Álvarez JM, Ibarra F, Alonso MA, Vera Y, Ávila JG, García AM (2015) In vitro anthelmintic effect of fifteen tropical plant extracts on excysted flukes of Fasciola hepatica. BMC Vet Res 11:45. https://doi.org/10.1186/s12917-015-0362-4
Alba A, Vazquez AA, Hurtrez-Boussès S (2021) Towards the comprehension of fasciolosis (re)emergence: an integrative overview. Parasitol 148:385–407. https://doi.org/10.1017/S0031182020002255
Andrade CA (2009) Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J Ethnopharmacol 122:163–171. https://doi.org/10.1016/j.jep.2008.12.008
Biblioteca Digital de la Medicina Tradicional (BDMTM). Universidad Nacional Autónoma de México (UNAM) (2009) Atlas de las Plantas de la Medicina Tradicional Mexicana (APMTM). Monografías, Estafiate. http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=artemisia-ludoviciana. Accessed 23 February 2023
Burden DJ, Hammet NC (1980) Fasciola hepatica: attempts to immunise rats using fluke eggs and in vitro culture products. Vet Parasitol 7:51–57. https://doi.org/10.1016/0304-4017(80)90009-6
Ceballos L, Canton C, Pruzzo C, Sanabria R, Moreno L, Sanchis J, Suarez G, Ortiz P, Fairweather I, Lanusse C, Alvarez L, Martinez VM (2019) The egg hatch test: a useful tool for albendazole resistance diagnosis in Fasciola hepatica. Vet Parasitol 271:7–13. https://doi.org/10.1016/j.vetpar.2019.06.001
Chai JY, Jung BK (2022) General overview of the current status of human foodborne trematodiasis. Parasitol 149:1262–1285. https://doi.org/10.1017/S0031182022000725
Dargie JD (1987) The impact on production and mechanisms of pathogenesis of trematode infections in cattle and sheep. Int J Parasitol 17:453–463. https://doi.org/10.1016/0020-7519(87)90121-4
De Mello AB, Baccega BF, Martins FO, Da Rosa FNA, De Giacometi M, Da Fonseca RN, De Oliveira HS, Soares MP, Oliveira CB (2023) Microscopic alterations in Fasciola hepatica treated with the essential oils of Pelargonium graveolens and Citrus aurantium. Vet Parasitol 314:109863. https://doi.org/10.1016/j.vetpar.2022.109863
Ezeta MA, Vera MY, Ávila AJG, Álvarez MJM, Francisco MG (2016) In vitro fasciolicide activity of the raw extract of estafiate (Artemisia ludoviciana Nutt. spp mexicana). Mex J Biotechnol 1(1):177–183
Ezeta MA, Vera MY, Ávila AJG, García BAM, Estrella PEA, Francisco MG, Ibarra VF (2020) Efficacy of purified fractions of Artemisia ludoviciana Nutt. mexicana and ultrastructural damage to newly excysted juveniles of Fasciola hepatica in vitro. Vet Parasitol 285:109184. https://doi.org/10.1016/j.vetpar.2020.109184
Fairweather I, Brennan GP, Hanna REB, Robinson MW, Skuce PJ (2020) Drug resistance in liver flukes. Int J Parasitol Drugs Drug Resist 12:39–49. https://doi.org/10.1016/j.ijpddr.2019.11.003
Ferreira JFS, Peaden P, Keiser J (2011) In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis. Trematocidal Plant Alcoholic Extracts Parasitol Res 109:1585–1592. https://doi.org/10.1007/s00436-011-2418-0
Food and Agriculture Organization of the United Nations (FAO) (2021) Fasciolasis (liver fluke). Foodborne parasitic infections. https://www.fao.org/documents/card/en/c/cb1127en. Accessed 27 February 2023
González MJ, Becerro RD, Siles LM (2021) Insights into Fasciola hepatica juveniles: crossing the fasciolosis rubicon. Trends Parasitol 37:1. https://doi.org/10.1016/j.pt.2020.09.007
Guo A, Wang L, Meng X, Zhang S, Sheng S, Luo X, Huang W, Wang S, Cai X (2021) Extracellular vesicles from Fasciola gigantica induce cellular response to stress of host cells. Exp Parasitol 231:108173. https://doi.org/10.1016/j.exppara.2021.108173
Hanna REB, Mc Mahon C, Ellison S, Edgar HW, Kajugu PE, Gordon A, Irwin D, Barley JP, Malone FE, Brennan GP, Fairweather I (2015) Fasciola hepatica: a comparative survey of adult fluke resistance to triclabendazole, nitroxynil and closantel on selected upland and lowland sheep farms in Northern Ireland using faecal egg counting, coproantigen ELISA testing and fluke histology. Vet Parasitol 207:34–43. https://doi.org/10.1016/j.vetpar.2014.11.016
Hegazi AG, Abd El Hady FK, Shalaby HA (2007) An in vitro effect of propolis on adult worms of Fasciola gigantica. Vet Parasitol 144:279–286. https://doi.org/10.1016/j.vetpar.2006.10.006
Helmy MMF, Fahmy ZH, Sabry HY (2008) Fasciola gigantica: evaluation of the effect of phenyl vinyl sulfone in vitro. Exp Parasitol 119:125–134. https://doi.org/10.1016/j.exppara.2008.01.002
Ibarra MS, Ibarra VF, Ávila AJG (2012) In vitro evaluation of fasciolicide activity with hexane, methanol and ethyl acetate with extracts processed and obtained from some mexican plants used in traditional medicine based on ethno botanical studies. Am J Plant Sci 3:506–511
Javaregowda AK, Rani KB (2017) Chronic bovine fasciolosis associated cholangiolithiasis: a case report. J Parasit Dis 41(1):90–92. https://doi.org/10.1007/s12639-016-0755-8
Jeyathilakan N, Murali K, Anandaraj A, Latha AR, AbdulBasith S (2010) Anthelmintic activity of essential oils of Cymbopogan nardus and Azadirachta indica on Fasciola gigantica. Vet Anim Sci 6(5):204–209
Kamaludeen J, Graham BJ, Stephens N, Miller J, Howell A, Beesley N, Hodgkinson J, Learmount J, Williams D (2019) Lack of efficacy of triclabendazole against Fasciola hepatica is present on sheep farms in three regions of England, and Wales. Vet Res 184:502–507. https://doi.org/10.1136/vr.105209
Kamaraj C, Rahuman AA (2011) Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res Vet Sci 91:400–404. https://doi.org/10.1016/j.rvsc.2010.09.018
Kaplan RM, Vidyashankar AN (2012) An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol 186:70–78. https://doi.org/10.1016/j.vetpar.2011.11.048
Keiser J, Utzinger J (2007) Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol 23(11):555–562. https://doi.org/10.1016/j.pt.2007.07.012
Keiser J, Morson G (2008) Fasciola hepatica: tegumental alterations in adult flukes following in vitro and in vivo administration of artesunate and artemether. Exp Parasitol 118:228–237. https://doi.org/10.1016/j.exppara.2007.08.007
Kelley JM, Rathinasamy V, Elliot TP, Rawlin G, Beddoe T, Stevenson MA, Spithill TW (2020) Determination of the prevalence and intensity of Fasciola hepatica infection in dairy cattle from six irrigation regions of Victoria, South-eastern Australia, further identifying significant triclabendazole resistance on three properties. Vet Parasitol 277:109019. https://doi.org/10.1016/j.vetpar.2019.109019
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055. https://doi.org/10.1126/science.3887571
Knepper ZF, Machado PMA, Massia PK, Aires BME, Cunico W, Campos JJC, Pires GD, Silva NP, Oliveira HS, Machado SG (2018) Ovicidal in vitro activity of 2-aryl-3-(2-morpholinoethyl)thiazolidin-4-ones and 2-aryl-3-(3-morpholinopropyl)thiazolidin-4-ones against Fasciola hepatica (Linnaeus, 1758). Exp Parasitol 192:60–64. https://doi.org/10.1016/j.exppara.2018.07.012
Machado PMA, Knepper ZF, Massia PK, Silveira PB, Antonio FR, Berne PN, Helena MR, Villareal VJP, Oliveira HS, Aires BME, Silva NP (2020) Ovicidal in vitro activity of the fixed oil of Helianthus annus L. and the essential oil of Cuminum cyminum L. against Fasciola hepatica (Linnaeus, 1758). Exp Parasitol 218:107984. https://doi.org/10.1016/j.exppara.2020.107984
Mas-Coma S, Bargues MD, Valero MA (2005) Fasciolasis and other plant-borne trematode zoonoses. Int J Parasitol 35:1255–1278. https://doi.org/10.1016/j.ijpara.2005.07.010
Moazeni M, Khademolhoseini AA (2016) Ovicidal effect of the methanolic extract of ginger (Zingiber officinale) on Fasciola hepatica eggs: an in vitro study. J Parasit Dis 40(3):662–666. https://doi.org/10.1007/s12639-014-0554-z
Moazeni M, Saadaty AZS, Saharkhiz MJ, Jalaei J, Khademolhoseini AA, Esfand ASS, Alavi AM (2017) In vitro ovicidal activity of Peganun harmala seeds extracton the eggs of Fasciola hepatica. J Parasit Dis 41(2):467–472. https://doi.org/10.1007/s12639-016-0830-1
Moll L, Gaasenbeek CPH, Vellema P, Borgsteede FHM (2000) Resistance of Fasciola hepatica against triclabendazole in cattle and sheep in The Netherlands. Vet Parasitol 91:153–158. https://doi.org/10.1016/S0304-4017(00)00267-3
Najafi F, Rezaie S, Kia EB, Mahmoudi M, Khodavaisy S, Mohebali M, Gharagozlou MJ, Rokni MB, Mowlavi G (2017) In vitro assay of Paecilomyces lilacinus biocontrol effects on Fasciola hepatica eggs illustrated in scanning electron micrographs. Iran J Parasitol 12(1):22–28
Novobilsky A, Solis NA, Skarin M, Höglund J (2016) Assessment of flukicide efficacy against Fasciola hepatica in sheep in Sweden in the absence of a standardised test. Int J Parasitol Drugs Drug Resist 6:141–147. https://doi.org/10.1016/j.ijpddr.2016.06.004
Olaechea F, Lovera V, Larroza M, Raffo F, Cabrera R (2011) Resistance of Fasciola hepatica against triclabendazole in cattle in Patagonia (Argentina). Vet Parasitol 178:364–366. https://doi.org/10.1016/j.vetpar.2010.12.047
O’Neill JF, Johnston RC, Halferty L, Brennan GP, Keiser J, Fairweather I (2009) Adult triclabendazole-resistant Fasciola hepatica: morphological changes in the tegument and gut following in vivo treatment with artemether in the rat model. J Helminthol 83:151–163. https://doi.org/10.1017/S0022149X09344934
O’Neill JF, Johnston RC, Halferty L, Brennan GP, Fairweather I (2015a) Ultrastructural changes in the tegument and gut of adult Fasciola hepatica following in vivo treatment with artesunate. Exp Parasitol 154:143–154. https://doi.org/10.1016/j.exppara.2015.04.012
O’Neill JF, Johnston RC, Halferty L, Hanna REB, Brennan GP, Fairweather I (2015b) A comparative study on the impact of two artemisinin derivatives, artemether and artesunate, on the female reproductive system of Fasciola hepatica. Vet Parasitol 211:182–194. https://doi.org/10.1016/j.vetpar.2015.05.027
O’Neill JF, Johnston RC, Halferty L, Hanna REB, Brennan GP, Fairweather I (2017) Disruption of spermatogenesis in the liver fluke, Fasciola hepatica by two artemisinin derivates, artemether and artesunate. J Helminthol 91:55–71. https://doi.org/10.1017/S0022149X16000079
Ortiz P, Scarcella S, Cerna C, Rosales C, Cabrera M, Guzmán M, Lamenza P, Solana H (2013) Resistance of Fasciola hepatica against triclabendazole in cattle in Cajamarca (Perú): a clinical trial and an in vivo efficacy test in sheep. Vet Parasitol 195:118–121. https://doi.org/10.1016/j.vetpar.2013.01.001
Rehman A, Ullah R, Gupta D, Khan MAH, Rehman L, Beg MA, Khan AU, Abidi SMA (2020) Generation of oxidative stress and induction of apoptotic like events in curcumin and thymoquine treated adult Fasciola gigantica worms. Exp Parasitol 209:107810. https://doi.org/10.1016/j.exppara.2019.107810
Reigate C, Williams HW, Denwood MJ, Morphew RM, Thomas ER, Brophy PM (2021) Evaluation of two Fasciola hepatica faecal egg counting protocols in sheep and cattle. Vet Parasitol 294:109435. https://doi.org/10.1016/j.vetpar.2021.109435
Robinson MW, Hanna REB, Fairweather I (2022) Development of Fasciola hepatica in the mammalian host. In: Dalton JP (ed) Fasciolosis, 2nd edn. CABI Publishing, UK, pp 65–111
Rodríguez-Vivas RI, Grisi L, Pérez de León AA, Silva VH, Torres-Acosta JF, Fragoso SH, Romero SD, Rosario CR, Saldierna F, García CD (2017) Potential economic impact assessment for cattle parasites in Mexico. Review. Rev Mex Cienc Pecu 8(1):61–74. https://doi.org/10.22319/rmcp.v8i1.4305
Rojo VFA, Meana A, Valcárcel F, Martínez VM (2012) Update on trematode infections in sheep. Vet Parasitol 189:15–38. https://doi.org/10.1016/j.vetpar.2012.03.029
Romero J, Villaguala C, Quiroz F, Laudaeta-Aqueveque C, Alfaro G, Pérez R (2019) Flukicide efficacy against Fasciola hepatica of triclabendazole and nitroxynil in cattle of the central valley of Chile. Brazil J Vet Parasitol 28:164–167. https://doi.org/10.1590/S1984-296120180089
Sánchez LCM, Trelis M, Jara L, Cantalapiedra F, Marcilla A, Bernal D (2020) Diversity of extracellular vesicles from different development stages of Fasciola hepatica. Int J Parasitol 50:663–669. https://doi.org/10.1016/j.ijpara.2020.03.011
Sabourin E, Alda P, Vázquez A, Hurtrez-Bousses S, Vittecoq M (2018) Impact of human activities on fasciolosis transmission. Trends Parasitol 34:10. https://doi.org/10.1016/j.pt.2018.08.004
Tunc IB, Berger BM, Erler F, Dagli F (2000) Ovicidal activity of essential oils from five plants against two stored-product insects. J Stored Prod Res 36:161–168. https://doi.org/10.1016/S0022-474X(99)00036-3
Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB Jr, Pankavic JA, Reinecke RK, Slocombe O, Taylor SM, Vercruysse J (1995) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol 58:181–213. https://doi.org/10.1016/0304-4017(95)00806-2
World Health Organization (WHO) (2019) Technical document of the use of non-pharmaceutical forms of Artemisia. WHO Headquarters, Geneva, Switzerland, October, pp 1–21. Access https://www.who.int/publications/i/item/WHO-CDS-GMP-2019.14. Accesed 3 Mar 2023
World Health Organization (WHO) (2020) Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization, pp 106–109. Access https://www.who.int/publications/i/item/9789240010352. Accesed 5 Mar 2023
Funding
This work was supported by the UNAM Postdoctoral Program (POSDOC).
Author information
Authors and Affiliations
Contributions
Alonso Ezeta Miranda: term, conceptualization, methodology, validation, formal analysis, investigation, data curation, and writing original draft; José Guillermo Ávila Acevedo: term, conceptualization, methodology, validation, resources, writing review and editing, and supervision; Yolanda Vera Montenegro: term, conceptualization, and resources; Gerardo Francisco Márquez: conceptualization and investigation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
The authors give their consent to participate in the editorial process by the journal.
Consent for publication
The authors give their consent to publish the research work in the journal.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ezeta-Miranda, A., Avila-Acevedo, J.G., Vera-Montenegro, Y. et al. Evaluation of the ovicidal activity and fasciolicidal activity of the extract of ethyl acetate from Artemisia ludoviciana Nutt. spp. mexicana and of artemisinin against adult parasites of Fasciola hepatica. Parasitol Res 123, 71 (2024). https://doi.org/10.1007/s00436-023-08052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08052-6