Abstract
There are currently insufficient anthelmintic medications available for the treatment of toxocariasis. For instance, Albendazole (ABZ) is the preferred medication, but its effectiveness against tissue-dwelling parasites is limited. In addition, Metformin (MTF) is a widely used oral antidiabetic medication that is considered to be safe for treatment. This study aimed to investigate any potential effects of MTF, alone or in combination with ABZ, on mice infections caused by Toxocara canis (T. canis). The efficacy of the treatment was assessed in the acute and chronic phases of the infection by larval recovery and histopathological, immunohistochemical, and biochemical studies. The results showed that combined therapy significantly reduced larval counts in the liver, brain, and muscles and ameliorated hepatic and brain pathology. It reduced oxidative stress and TGF-β mRNA expression and increased FGF21 levels in the liver. It decreased TNF-α levels and MMP-9 expression in the brain. In addition, it increased serum levels of IL-12 and IFN-γ and decreased serum levels of IL-4 and IL-10. In the acute and chronic phases of the infection, the combined treatment was more effective than ABZ alone. In conclusion, this study highlights the potential role of MTF as an adjuvant in the treatment of experimental T. canis infection when administered with ABZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human toxocariasis is a chronic systemic parasitic infection with a worldwide distribution mainly caused by T. canis larvae (Ma et al. 2018). Humans may contract the infection either by unintentionally swallowing embryonated T. canis eggs (Błaszkowska et al. 2015) or by ingesting viable T. canis second-stage larvae present in raw or undercooked meat from other paratenic hosts (Nijsse et al. 2016). Larvae can persist in paratenic hosts, such as humans, for extended periods without undergoing further maturation and have the ability to infiltrate several parenchymal organs (Clinton et al. 2010).
The severity of the symptoms that arise during toxocariasis depends on how many larvae are in the tissues and where they are located (Maizels 2013). In addition to the direct damage caused by the parasite’s migration, a variety of factors contributes to the destruction of tissues that occurs in toxocariasis. Significant contributors to this damage include oxidative stress and the host's immune response. (Demirci et al. 2006; Othman et al. 2011; Resende et al. 2015; Waindok et al. 2019). Antioxidants and anti-inflammatory medications are therefore anticipated to aid in the host’s defense against these harmful factors (Rogerio et al. 2008; Sayiner et al. 2021).
There are currently inadequate anthelmintic drugs available for the treatment of human toxocariasis. For this purpose, ABZ is the drug of choice (Horton 2000; Magnaval et al. 2022). It is superior to other used drugs as it is widely available and has no significant side effects due to its administration in a short regimen. Moreover, it can cross the blood-brain barrier (Schneier and Durand 2011). However, its low bioavailability limits its effectiveness against tissue-dwelling parasites (Daniel-Mwambete et al. 2004; Rigter et al. 2004). Furthermore, Musa et al. (2011), Deshayes et al. (2016), and Kroten et al. (2018) demonstrated that its efficacy against T. canis infection is moderate. Consequently, it is crucial to investigate new or synergistic agents that may enhance the efficacy of ABZ against toxocariasis.
MTF is a bioavailable oral antidiabetic that is widely used and safe (Foretz et al. 2014). It is one of the derivatives of biguanides that was initially used in the treatment of malaria (Sweeney et al. 2003). The available evidence suggests that the utilization of this treatment has demonstrated positive outcomes in the management of various parasitic diseases, including schistosomiasis mansoni (Salama et al. 2021), cystic echinococcosis (Loos et al. 2017), experimental trichinellosis (Othman et al. 2016), leishmaniasis braziliensis (Lima et al. 2020), American trypanosomiasis, and African trypanosomiasis (Martínez-Flórez et al. 2020). It is also characterized by anti-oxidative stress (Othman et al. 2016), anti-fibrotic property (Salama et al. 2021), and anti-inflammatory property (Kim et al. 2014). The purpose of this study was to evaluate the efficacy of MTF in reducing the pathogenic effects of T. canis infection and MTF’s potential use as an adjuvant with ABZ for the treatment of toxocariasis.
Material and methods
Parasite
Adult female T. canis worms obtained from the small intestines of infected pups were dissected to isolate the eggs. According to Faz-López et al. (2013), the eggs were cultivated to produce the infective stage, the egg harboring the second-stage larva. Each mouse in the infected group was inoculated intragastrically with 1000 infectious T. canis eggs. Before use, the embryonated eggs’ viability was determined through a microscopic examination of larval movement (Barrera et al. 2010).
Drugs
Albendazole (ABZ): (Alzental) suspension (Eipico, Egypt) which contains 100 mg/5 ml was used. Each mouse received 100 mg/kg daily via oral gavage for five consecutive days (Nassef et al. 2014). Metformin (MTF): (Glucophage) 1000 mg/tablet (Minipharm, Egypt) was used. It was received via oral gavages to each mouse in a dosage of 150 mg/kg diluted in water once daily for 15 successive days (Salama et al. 2021).
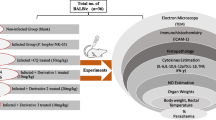
Animals and experimental design
In this study, 50 male Swiss albino laboratory mice (6–8 weeks old, 20–25 g in weight) were used. The mice were kept in typical cages at a temperature of 25 ± 2 °C, were fed a standard diet, and had free access to water. All mice were exposed to a 7-day acclimation period prior to the experiment.
Experimental design
The following groups of mice were used:
-
1.
Negative control group includes Group (I) (10 mice) uninfected control group.
-
2.
Acute toxocariasis groups (A) (5 mice each) include Group (A/II) acute infected untreated control group (acute positive control), Group (A/III) treated with ABZ alone starting on the 8th day PI. (acute ABZ), Group (A/IV) treated with MTF alone starting on the 8th day PI (acute MTF), and Group (A/V) treated with ABZ and MTF starting on the 8th day PI. MTF was given in parallel with ABZ and then continued for the following ten days (acute combined).
-
3.
Chronic toxocariasis groups (C) (5 mice each) include Group (C/II) chronic infected untreated control group (chronic positive control), Group (C/III) treated with ABZ alone starting on the 71st day PI (chronic ABZ), and Group (C/IV) treated with MTF alone starting on the 71st day PI (chronic MTF), and Group (C/V) treated with ABZ and MTF starting on the 71st day PI. MTF was given in parallel with ABZ and then continued for the following 10 days (chronic combined).
All mice of groups (A/II, A/III, A/IV, and A/V) and 5 mice from group (I) were sacrificed on the 22nd day PI, and all mice of groups (C/II, C/III, C/IV, and C/V) and 5 mice of group (I) were sacrificed on the 85th day PI. In order to do a parasitological investigation on all infected mice, larvae were counted in half of the liver, half of the brain, and muscles. For histopathological and immunohistochemical tests, identical brain and liver samples from each mouse were taken and stored in 10% formalin. Samples of each mouse’s brain, liver, and serum were collected and stored at −80 °C for biochemical analysis.
Larval burden recovery
According to Liao et al. (2008), larval counting was done with some modifications. Each infected mouse’s liver and brain were weighed and homogenized separately in 1 ml of saline using a mortar and pestle. The homogenized liver, homogenized brain, and whole-body muscles of each mouse were then separately digested for 3 hours at 37°C in artificial digestive juice containing 1% pepsin (activity 1:10000) (w/v) and 1% concentrated HCL (v/v) in warm tap water. The mixtures were centrifuged (at 250 g for 10 min), and sedimented larvae were counted using a Mc. Master counting chamber. The percentage of reduction in the larval counts in the liver, brain, and muscles of mice was calculated using the following formula:
Percentage of reduction = 100 × (NC − NT)/NC
NC is the mean larval count in the corresponding control group/mouse; NT is the mean larval count in the treated group/mouse.
Histopathological study
Tissue samples from the studied groups were fixed by 10% formalin, and then routine histologic processing was done by embedding in paraffin blocks, cutting by microtome, and staining with hematoxylin and eosin. Histopathological changes were observed in the study groups’ liver and brain. Granuloma formation was examined in hepatic tissues by counting the number and calculating their mean diameter. In each section, granulomas were counted in 10 high-power fields (×400) in 3 different fields per mouse to calculate the average. The largest diameter was measured using the ImageJ software (https://eliceirilab.org/software/imagej/). The mean diameter of granulomas per hepatic section was calculated (Klockiewicz et al. 2019).
Immunohistochemical study
Matrix metalloproteinase-9 (MMP-9) expression was assessed by immunohistochemistry in accordance with the manufacturer’s instructions using the ultra-vision detection Kit (TP-015-HD, Lab Vision, USA) and rabbit polyclonal anti-MMP-9 (Cat. No. ab38898; Abcam, USA).
Interpretation of MMP-9 positivity
A brown granular cytoplasmic stain was identified as MMP-9 positive immunostaining. The number of stained cells and the degree of staining were taken into account when calculating the score. Scores for staining intensity ranged from 0 to 3. 0 signified no staining, 1 slight staining, 2 moderate staining, and 3 high staining. On a scale of 1 to 4, the frequency of stained cells was rated as follows: 1 = (0–25%), 2 = (26–50%), 3 = (51–75%), and 4 = (75–100%). The intensity score and frequency score were multiplied to produce the final score. According to the final score, the staining was divided into five categories: score 0, absent expression; score +1 (1–2); score +2 (3–4); score +3 (6–8); and score +4 (9–12) (Niu et al. 2018).
Biochemical study
Spectrophotometric measurements of nitric oxide (NO) (CAT# NO 25 33), Malondialdehyde (MDA) (CAT# MD 25 29), superoxide dismutase (SOD) (CAT# SD 25 21), and catalase (CAT# CA 25 17) levels were determined in liver tissue homogenate using kits provided by Bio-diagnostic (Giza, Egypt). Using ELISA kits, the levels of liver homogenate fibroblast growth factor 21 (FGF21) (CAT# ab212160, Abcam, USA), brain homogenate tumor necrosis factor-alpha (TNF-α) (CAT# ab208348, Abcam, USA), serum interleukin-12 (IL-12) (CAT# BMS6004, Thermo Fisher Scientific, USA), serum interferon-gamma (IFN-γ) (CAT# E-EL-M0048, Elabscience, USA), serum interleukin-4 (IL-4) (CAT# BMS613, Thermo Fisher scientific, USA), and serum interleukin-10 (IL-10) (CAT# OKBB00194, Aviva Systems Biology, USA) were determined. All ELISA techniques were read using a microplate reader (Stat Fax®2100, Fisher Bio block Scientific, France) at 450 nm with a correction wavelength set at 570 nm per the manufacturer’s recommendations.
Real-time PCR analysis of liver homogenate relative transforming growth factor β (TGF β) mRNA expression
RNA was extracted from frozen liver homogenate using a Qiagen RNeasy Total RNA isolation kit (Qiagen, Hiden, Germany) and following the manufacturer’s instructions. The first strand of DNA was synthesized using the Super-Script III First-Strand Synthesis System for a real-time PCR kit (Life Technologies, Carlsbad, California, USA) and following the manufacturer’s instructions. PCR reactions were conducted using the Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, California, USA) according to the manufacturer’s instructions. The TGF-mRNA transcript amount was measured utilizing the housekeeping gene TGF-β as an internal control. These sequence-specific primers were developed as follows: mice TGF-β (Gene Bank accession NM_011577.2): up-stream: 5′-CTCTCTGCTCCTCCCTGTTCTA-3′, down-stream: 5′-ATAGATGGCGTTGTTGCGGT-3′ with amplicon size 202 bp, mice β-actin (Gene Bank accession NM_007393.5): up-stream: 5′-TTACAGGAAGTCCCTCACCC-3′, and down-stream: 5′-ACACAGAAGCAATGCTGTCAC-3′ with amplicon size 110 bp. Relative gene expression was calculated automatically by using the comparative threshold (Ct) method for the values of the target and the reference genes using the 2-ΔΔCT formula (Guo et al. 2021).
Statistical analysis
The data were displayed as means ± standard deviation. One-way ANOVA was used to compare more than two groups, and the post hoc test was used to find the likelihood of significant differences between the dual means of the groups. The chi-square test was used to compare qualitative data between the two groups. Values were considered significant when P < 0.05. Statistical Program of Social Sciences (SPSS), software for Windows, version 18.0, was used to perform the statistical analyses.
Results
T. canis larvae counts in the liver, brain, and muscles
At both time points PI, the mean number of larvae detected in the brain and muscles of the treated groups was significantly lower than that of the positive control group. The combined group had the best results, with reduction percentages in the brain (62.0% and 58.0%) and muscles (79.7% and 73.2%) during the acute and chronic phases of infection, respectively. The combined treatment was significantly more effective than each drug administered separately. In the acute phase of infection, either ABZ or MTF alone significantly decreased liver larval counts. Nevertheless, neither drug significantly reduced the number of hepatic larvae in the chronic phase of the infection. The combined treatment was successful in reducing the number of larvae in the liver, with reduction percentages of 82.6% and 84.6% in the acute and chronic stages, respectively (Table 1).
Histopathological and immunohistochemical results
Histopathological findings in the liver
In comparison to the positive control group, the mean number of granulomas per hepatic section decreased significantly in all ABZ-treated and MTF-treated groups. However, the difference between acute ABZ (G A/III) and acute MTF (G A/IV) groups was statistically insignificant. Furthermore, the combined groups significantly decreased when compared to the ABZ and MTF groups (Table 2).
There was a significant decrease in the mean diameter of hepatic granulomas in all treated groups compared to the matching positive control group. Compared to other treated groups, the combined group demonstrated significant decreases. The differences between each drug-treated group in the acute phase of the infection and its corresponding group in the chronic phase were not statistically significant (Table 2).
Multiple cellular granulomas composed of a collection of inflammatory cells, including macrophages, lymphocytes, and eosinophils, were observed in liver sections of the acute positive control group (Fig. 1a). The chronic positive control group showed multiple fibro cellular granulomas with excessive fibrous tissue (Fig. 1b). Granulomas were examined from acute ABZ (Fig. 1c) and acute MTF (Fig. 1e); they were cellular granulomas. Chronic ABZ (Fig 1d) and chronic MTF (Fig. 1f) showed fibrocellular granulomas formed of inflammatory cells with scanty fibrous tissue. Granuloma resolution was observed in the combined-treatment groups (Fig. 1g, h).
Hepatic sections (H&E x 200). a G A/II showed multiple cellular granulomas around T canis larvae. b G C/II group showed fibro cellular granulomas around T canis larvae. c G A/III showed a decrease in granuloma size and number, granulomas of cellular type. d G C/III showed a small fibro cellular granuloma. e G A/IV showed small cellular granuloma with a decrease in the number of granulomas. f G C/IV showed small fibro cellular granuloma with a decrease in the number of granulomas. g G A/V showed a tiny residual of cellular granuloma. h G C/V showed very small fibro cellular granuloma. Scale bar = 50 μm
Histopathological findings in the brain
G A/II and G C/II brain sections revealed scattered T. canis larvae in parenchymal tissue with few congested blood vessels. Nonetheless, these larvae did not induce any surrounding inflammatory cell infiltration (Fig. 2a, b). Sections of ABZ-treated groups (G A/III and G C/III) and MTF-treated groups (G A/IV and G C/ IV) showed few larvae with no congested blood vessels. (Figure 2c–f). Sections of the acute combined group (G A/V) revealed no larvae with no congested blood vessels (Fig. 2g). The chronic combined group (G C/V) showed few larvae and no congestion in blood vessels (Fig. 2h).
Brain sections (H&E × 400), a from G A/II showing scattered T. canis larvae (yellow arrows) with few congested blood vessels (red arrows), b from G C/II showing numerous longitudinal and transverse sections in T. canis larvae with no inflammatory cells around them, c from G A/III showing a decreased number of T. canis larvae and no obvious blood vessels congestion, d from G C/III showing similar findings as the G A/III, e from G A/IV showing a tangential section in a larva, f from G C/IV showing a longitudinal section in T. canis larva, g from G A/V showing no obvious larva, and h from G C/V showing a cross-section in T. canis larva. Scale bar = 50 μm
Immunohistochemical study of the MMP-9 reaction in brain tissues
The chronic positive control group (G C/II) had significantly higher MMP-9 reactivity in brain tissues than the acute positive control group (G A/II). In addition, its expression in the positive control groups (G A/II and G C/II) (Fig. 3a, b) was significantly higher than in the corresponding treated groups (Fig. 3c–h). ABZ-treated groups (Fig. 3c, d) and MTF-treated groups (Fig. 3e, f) exhibited decreased MMP-9 production at both time periods post-infection, with more pronounced effects seen during the acute phase rather than the chronic phase of the infection. The presence of MMP-9 reactivity was either not detected or found to be very limited in brain tissue obtained from the acute combined group (Fig. 3g). Similarly, the reactivity of MMP-9 was found to be minimal in the chronic combined group (Fig. 3h), as illustrated in Table 3.
Brain sections immunohistochemical expression of MMP 9 (immunoperoxidase × 400): a G A/II showed high cytoplasmic expression in astrocytes and glial cells in brain parenchyma around T. canis larva (+3). b G C/II showed higher cytoplasmic expression in the brain parenchyma (+4). c G A/III showed mild cytoplasmic expression (+1). d G C/III showed moderate cytoplasmic expression(+2). e G A/IV showed mild expression in brain cells (+1). f G C/IV showed moderate cytoplasmic expression in cells brain (+2). g G A/V showed negative expression in brain cells (0). h G C/V showed mild cytoplasmic expression in cells (+1). Scale bar = 50 μm
Biochemical results
NO and redox status markers in the liver
G C/II group showed a more significant increment in levels of NO and MDA than the G A/II group as compared to the negative control (G I). All treated groups exhibited a significant decline in NO levels compared to the corresponding positive control group. However, the G C/V group showed a more substantial elevation in NO levels than all infected groups except G C/IV. MDA levels in all treated groups except G A/III were significantly lower than the matching positive control group, as depicted in Table 4.
G A/II and G C/II groups exhibited a more pronounced increase in the levels of SOD & catalase than the negative control Group (G I). Both SOD and catalase levels were significantly higher in the G A/IV and G A/V groups compared to the G A/III group. Similarly, both markers were significantly elevated in the G C/IV and G C/V groups relative to the G C/III group. In treated groups relative to G A/II and G C/II, the levels of SOD and catalase did not differ significantly between G C/IV and G C/V (Table 4).
Levels of FGF21 in liver homogenate and TNF-α in brain homogenate
G A/II and G C/II had significantly lower levels of FGF21 compared to G I. All treated groups had significantly higher concentrations than the positive control groups. Additionally, levels of FGF21 in combined treated groups showed a significant increase in comparison with other treated groups. G A/II demonstrated a more significant increase in TNF-α levels than G C/II when compared to the negative control. However, G C/V demonstrated a higher decrease in TNF-α levels as compared to MTF- and ABZ-treated groups. TNF-α levels were decreased in G C/IV than in G A/IV. Moreover, G C/III had decreased levels of TNF-α than G A/III. There was a significant difference in TNF-α levels in all study groups (P < 0.001), as shown in Table 5.
Serum cytokine levels
IL-12 and IFN-γ levels showed significant decreases in G A/II and G C/II compared to G I. Treatment administration induced significant elevation in levels of both markers in comparison with G A/II and G C/II. The differences in levels of both markers were non-significant between G I and G A/V. Likewise, no significant change was detected in levels of IL-12 between G A/III and G A/V. Also, IFN-γ levels in G C/V did not differ significantly from those in G I (Table 6).
In contrast, levels of IL-4 and IL-10 demonstrated a significant rise in G A/II and G C/II compared to G I. All treated groups had significantly lower levels than the corresponding positive control group. IL-10 levels did not differ significantly between G A/V and G I. During the acute and chronic phases of the infection, the levels of both IL-4 and IL-10 in the combined groups were significantly lower than those in the groups receiving either ABZ or MTF alone (Table 6).
TGF β mRNA expression in liver homogenate
There were significant differences in relative liver TGF-β gene mRNA expression between all treatment groups and the corresponding positive control group (P < 0.001). There was a statistically significant increase in its expression in the chronic positive control group compared to other groups (P < 0.001). However, the acute combined group showed a significant decrease compared to other studied groups. There were non-significant differences between acute ABZ and acute MTF (P < 0.420) as well as between chronic ABZ and chronic MTF (P < 1.0) (Fig. 4).
Relative TGF- β mRNA expression in liver tissue homogenates of different groups. vertical bar illustrates the means ± standard deviation. a indicates significant vs. G I, b indicates significant vs. G A/II, c indicates significant vs. G A/III, d indicates significant vs. G A/IV, e indicates significant versus G A/V, f indicates significant vs. G C/II, g indicates significant vs. G C/III, h indicates significant vs. G C/IV, and i indicates significant vs. G C/V
Discussion
Currently, the efficacy of the available anti-toxocariasis drugs remains suboptimal. ABZ has emerged as one of the recommended drugs (Magnaval et al. 2022). Nevertheless, its poor solubility and limited tissue absorption are factors that limit its efficacy (Márquez-Navarro et al. 2009). Accordingly, finding novel therapeutic agents or adjuvants that could enhance the efficacy of ABZ is appropriate for the treatment of visceral toxocariasis.
In the current study, combined treatment was more effective on T. canis larvae in the brains, livers, and muscles than administration of ABZ alone during the acute and chronic stages of the infection. Additionally, MTF-treated groups showed significant reductions compared to the positive control groups. To our knowledge, it is the first time to investigate the efficacy of MTF on toxocariasis. However, these results were not surprising as previous studies on the efficacy of MTF in the treatment of other parasitic infections reported its effectiveness in reducing parasite burdens. For instance, MTF could reduce the total number of larvae in the muscles of Trichinella spiralis-infected mice (Othman et al. 2016). In addition, levels of parasitemia in Plasmodium yoelii-infected mice were significantly reduced by MTF administration (Miyakoda et al. 2018). Furthermore, the synergistic effects of combined therapy of both ABZ and MTF were previously reported in the treatment of cystic echinococcosis, where the combined therapy induced a significant effect on both metacestodes and protoscoleces than ABZ alone (Loos et al. 2017).
The liver is one of the visceral organs most frequently impacted in visceral T. canis larval migration. Larvae actively moving through the liver generate tissue disorganization and granulomatous inflammation, which can result in granulomatous hepatitis (Kayes 1997). In terms of histopathological findings in the liver, the current study revealed that treatment of T. canis-infected mice with ABZ and MTF significantly decreased granuloma numbers and granuloma diameter and fibrosis compared to ABZ-treated groups. This observation was true for the infection’s acute and chronic phases. The decrease in granuloma counts paralleled the decrease in hepatic larval counts. Comparatively, the MTF-treated groups had significantly smaller granulomas than the positive control groups. Salama et al. (2021) demonstrated the efficacy of MTF in reducing the size of hepatic granuloma and ameliorating hepatic fibrosis in Schistosoma mansoni-infected mice.
In an effort to determine the underlying mechanisms by which MTF alleviates hepatic pathology, the redox status, TGF-β, and FGF21 levels in the hepatic tissues were measured. In comparison to the positive control groups, the MTF, and combined groups exhibited a significant reduction in oxidative stress, as evidenced by lower levels of MDA and NO and higher levels of SOD and catalase (Hou et al. 2010; Esteghamati et al. 2013; Ashabi et al. 2015). Several previous studies, such as Marchetti et al. (2004) and Kelly et al. (2015), have reported that MTF lowered levels of MDA by inhibiting the mitochondrial complex-I of the respiratory chain and reducing the formation of reactive oxygen species. Additionally, it could halt the production of reactive nitrogen species like NO at the intracellular level (Esteghamati et al. 2013).
NO is a highly reactive chemical that plays a significant role in hepatic cell damage. Different cells, including hepatocytes, kupffer cells, macrophages, and endothelial cells, secrete it in the liver (Aksu et al. 2010). Fan et al. (2004) earlier established the role of NO in the pathophysiology of T. canis-induced hepatic disease. Therefore, inhibiting NO production could aid in mitigating the damage caused by T. canis infection. This finding was demonstrated by Espinoza et al. (2002), who reported that inhibition of NO production significantly reduced lung pathology in T. canis-infected rats.
Fibrosis is considered to be primarily regulated by TGF-β signaling. According to Zhang (2017) and Mi et al. (2019), it controls multiple signaling pathways contributing to liver fibrosis. Additionally, FoxO3a (Forkhead box O3) and the DNA demethylase TET3 (Tet methylcytosine dioxygenase 3) are upregulated by TGF-β in hepatic stellate cells (HSC), which promotes hepatic fibrogenesis (Xu et al. 2020; Kim et al. 2021). Therefore, the inactivation of TGF-β prevents HSC activation and liver fibrosis (Fan et al. 2019).
In contrast, FGF21 is predominantly expressed in hepatocytes, and its administration as a treatment has multiple direct and indirect effects on the liver (Coskun et al. 2008). It has anti-steatotic and anti-inflammatory properties (Keinicke et al. 2020; Seitz and Hellerbrand 2021). It also inhibits HSC activation via TGF-β and NF-κB pathways, and it can induce HSC apoptosis through caspase-3, thereby reducing hepatic fibrogenesis (Xu et al. 2016). FGF21 ameliorates hepatic fibrosis by multiple mechanisms, such as inhibition of TGF-β (Meng et al. 2021).
In the present study, we examined changes in the expression of TGF β-mRNA and FGF21 levels in the liver of T. canis-infected mice. TGF-β mRNA expression was enhanced in the infected untreated mice compared to the negative control. In acute and chronic phases of infection, Its expression was significantly decreased under the effect of combination therapy compared to the groups treated with either ABZ or MTF alone. These results are consistent with Li et al. (2015) and Lu et al. (2015), who illustrated that MTF had inhibitory effects on the TGF β signaling pathway.
Regarding levels of FGF21 in the current study, all treatment groups showed significant increases compared to the positive control groups. MTF and combined treated groups showed the highest levels. As previously reported by Nygaard et al. (2012), metformin is a potent inducer of FGF21 expression in primary rat and human hepatocytes through activating 5′ adenosine monophosphate-activated protein kinase (AMPK). Based on the previously reported results, we could attribute the significant improvement in hepatic pathology in the groups that received MTF or combined treatment in the present study to the potent anti-oxidant effects of MTF together with its effects in inhibition of TGF-β expression and enhancement of FGF21 expression.
During the course of T. canis infection, the larvae travel across all body organs. As the infection progresses, they tend to leave the different organs, such as the liver and lungs, and frequently build up in the muscles and the brain (Chou et al. 2017). According to Holland and Hamilton (2013), the brain is seen as a sanctuary for these larvae, protecting them from the host’s immunological response, which aligns with the findings of this work. As larval counts in the positive control group’s brain and muscles increased in the infection's chronic phase compared to the acute phase. However, their counts in the liver in the chronic phase were significantly lower than in the acute phase.
Previous research on T. canis infection has shown that the absence of inflammation around the larvae in the brain is a common observation in the mouse model (Liao et al. 2008; Eid et al. 2015; Chou et al. 2017). In the same context, Resende et al. (2015) reported the same observation, but they stated the presence of hemorrhagic areas as a sign of active larval movement in the brain. Furthermore, Othman et al. (2010) reported no discernible inflammatory response around the larvae. However, they identified the existence of vascular congestion close to some larvae. In the current study, we have noticed similar results indicating clogged blood arteries close to the larvae inside the positive control group.
The present investigation revealed that infection with T. canis resulted in a significant increase in the levels of TNF-α inside the brain tissues of the infected mic. Similarly, Othman et al. (2010) showed upregulation of the expression of TNF-α mRNA in the brain of T. canis-infected mice with the progression of the infection. TNF-α is a pro-inflammatory cytokine that is released by a wide variety of brain cells such as neurons, astrocytes, microglia, and oligodendrocytes. Its receptors are distributed throughout the CNS (Probert 2015). Its expression is typically increased in numerous CNS diseases. It has been proved that TNF-α has a significant role in the pathogenesis of parasitic infections affecting the brain such as African trypanosomiasis and cerebral malaria (Kinra and Dutta 2013; Kitwan et al. 2023).
Although increased production of pro-inflammatory cytokines, such as TNF-α, is helpful in boosting clearance of invasive infections and phagocytosis of cell debris, prolonged inflammatory processes can cause harmful brain damage and neurodegeneration (Lucas et al. 2006; Becher et al. 2017). Our results showed a significant reduction in TNF-α levels in the brains of mice that received MTF or combined treatment. MTF was previously reported to induce neuroprotective effects on traumatic brain injury in rats via inhibiting microglial activation and decreasing TNF-α production in the brain (Tao et al. 2018). The existing body of evidence indicates that MTF exhibits robust neuroprotective properties when used to treat various neurological disorders, such as brain ischemia (Zhang et al. 2022), traumatic brain injury (Tao et al. 2018), an Alzheimer’s disease model induced by streptozotocin (Saffari et al. 2020), and brain injury induced by sepsis (Tang et al. 2017).
A comprehensive investigation was conducted to examine the function of MMP-9 in the etiology of various neurological diseases. For instance, previous studies have shown its involvement in several types of brain traumas, including traumatic, hemorrhagic, and ischemic injuries (Chaturvedi and Kaczmarek 2014; Shao et al. 2014; Pijet et al. 2018). Moreover, it is well acknowledged that it serves as a reliable marker for neuroinflammation in different central nervous system disorders, including viral and bacterial meningitis as well as multiple sclerosis (Sulik and Chyczewski 2008; Aung et al. 2015).
In the present research, an investigation was conducted to compare the expression of MMP-9 in the brain of mice across various study groups. The results revealed a substantial increase in MMP-9 expression in the positive control group compared to the negative control group. A decrease in expression was seen in mice subjected to treatment with either MTF or a combination of treatments, regardless of whether it was during the acute or chronic stages of the infection. The findings of this study demonstrate the significant impact of MTF on the expression of MMP-9. Prior research conducted by Zhang et al. (2017) demonstrated a decrease in the expression of MMP-9 after the injection of MTF in the treatment of traumatic brain injury.
Migration of Toxocara canis larvae induces dynamic changes in the systemic immune response of the host. In the current study, T. canis infection induced a predominant decrease in IL-12 and IFN-γ levels and an increase in IL-4 along the course of the infection. Additionally, IL-10 levels increased significantly more during the chronic phase than during the acute phase. These findings are supported by prior research (Kuroda et al. 2001; Resende et al. 2015; Ruiz-Manzano et al. 2019). The findings of this research suggest that the immune mediators’ expression pattern was altered by the administration of MTF or combined treatment. There was an increase in levels of IL-12 and IFN-γ and a reduction in IL-4 and IL-10 levels. The results presented in this study align with the previous research conducted by Wang et al. (2020) and Nicolao et al. (2023), which also showed similar effects of MTF.
T. canis larvae use crucial immunological mechanisms, including the suppression of IL-12 and IFN-γ, as survival mechanisms. This suppression hinders the recruitment of macrophages and prolongs the lifespan of the parasite (Kuroda et al. 2001). Moreover, increased IL-10 expression is another contributor to the development and extension of toxocariasis pathology (Othman et al. 2011). Findings suggest that immunomodulation is involved in MTF’s protection against toxocariasis. The increase in IL-12 levels under the influence of MTF suggests that this drug prevents T. canis from inhibiting IL-12 and contributes to the body’s anti-infection response. This result is consistent with previous research indicating that an increase in IL-12 and IFN-γ may enhance the protective host response against T. canis (Pilarczyk et al. 2008; de Avila et al. 2016). The elevated IL-12 levels and decreased infection development in the MTF or combined treated groups also increased. This finding may have been due to the MTF-induced suppression of IL-10 expression, as both IL-10 and IL-12 have an antagonistic interaction (Torina et al. 2005; de Avila et al. 2016).
Conclusion
Our findings indicate that MTF has a positive effect on reducing the pathological changes caused by T. canis infection in mice. It decreased recovery in the liver, brain, and muscles of developing larvae. Combined therapy was superior to ABZ alone in treating hepatic and cerebral pathologies. In addition, MTF was able to regulate the host’s immune response and oppose the immunomodulatory processes induced by T. canis infection. Identical effects were observed during both the acute and chronic phases of infection. We hypothesize that metformin exerts its anti-toxocara effects by modulating the immune response and ameliorating infection-related pathology. Therefore, MTF should be considered an adjuvant therapy for the treatment of experimental toxocariasis.
Data availability
Not applicable.
Abbreviations
- T. canis :
-
Toxocara canis
- ABZ:
-
Albendazole
- MTF:
-
Metformin
- PI:
-
Post-infection
- HCL:
-
Hydrochloric acid
- H&E:
-
Hematoxylin and eosin
- MMP-9:
-
Matrix metalloproteinase
- NO:
-
Nitric oxide
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- ELISA:
-
Enzyme-linked immunosorbent assay
- FGF21:
-
Fibroblast growth factor 21
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-12:
-
Interleukin-12
- IFN-γ:
-
Interferon-gamma
- IL-4:
-
Interleukin-4
- IL-10:
-
Interleukin-10
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- TGF-β:
-
Transforming growth factor β
References
Aksu B, Umit H, Kanter M, Guzel A, Aktas C, Civelek S, Uzun H (2010) Effects of methylene blue in reducing cholestatic oxidative stress and hepatic damage after bile-duct ligation in rats. Acta Histochem 112(3):259–269. https://doi.org/10.1016/j.acthis.2008.12.002
Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A (2015) Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis 30:747–754. https://doi.org/10.1007/s11011-014-9632-2
Aung LL, Mouradian MM, Dhib-Jalbut S, Balashov KE (2015) MMP-9 expression is increased in B lymphocytes during multiple sclerosis exacerbation and is regulated by microRNA-320a. J Neuroimmunol 278:185–189. https://doi.org/10.1016/j.jneuroim.2014.11.004
Barrera MG, Leonardi D, Bolmaro RE, Echenique CG, Olivieri AC, Salomon CJ, Lamas MC (2010) In vivo evaluation of albendazole microspheres for the treatment of Toxocara canis larva migrans. Eur J Pharm Biopharm 75(3):451–454. https://doi.org/10.1016/j.ejpb.2010.03.017
Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17(1):49–59. https://doi.org/10.1038/nri.2016.123
Błaszkowska J, Góralska K, Wójcik A, Kurnatowski P, Szwabe K (2015) Presence of Toxocara spp. eggs in children's recreation areas with varying degrees of access for animals. Ann Agric Environ Med 22(1). https://doi.org/10.5604/12321966.1141363
Chaturvedi M, Kaczmarek L (2014) Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49:563–573. https://doi.org/10.1007/s12035-013-8538-z
Chou CM, Lee YL, Liao CW, Huang YC, Fan CK (2017) Enhanced expressions of neurodegeneration-associated factors, UPS impairment, and excess Aβ accumulation in the hippocampus of mice with persistent cerebral toxocariasis. Parasit Vectors 10:1–14. https://doi.org/10.1186/s13071-017-2578-6
Clinton RM, Carabin H, Little SE (2010) Emerging zoonoses in the southern United States: toxocariasis, bovine tuberculosis and southern tick-associated rash illness. Am J Med Sci 340(3):187–193. https://doi.org/10.1097/maj.0b013e3181e937e5
Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Kharitonenkov A (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149(12):6018–6027. https://doi.org/10.1210/en.2008-0816
Daniel-Mwambete K, Torrado S, Cuesta-Bandera C, Ponce-Gordo F, Torrado JJ (2004) The effect of solubilization on the oral bioavailability of three benzimidazole carbamate drugs. Int J Pharm 272(1-2):29–36. https://doi.org/10.1016/j.ijpharm.2003.11.030
de Avila LDC, De Leon PMM, De Moura MQ, Berne MEA, Scaini CJ, Leivas Leite FP (2016) Modulation of IL-12 and IFN γ by probiotic supplementation promotes protection against Toxocara canis infection in mice. Parasite Immunol 38(5):326–330. https://doi.org/10.1111/pim.12314
Demirci C, Gargili A, Kandil A, Cetinkaya H, Uyaner I, Boynuegri B, Gumustas MK (2006) Inhibition of inducible nitric oxide synthase in murine visceral larva migrans: effects on lung and liver damage. Chin J Physiol 49(6):326
Deshayes S, Bonhomme J, de La Blanchardière A (2016) Neurotoxocariasis: a systematic literature review. Infection 44:565–574. https://doi.org/10.1007/s15010-016-0889-8
Eid MM, El-Kowrany SI, Othman AA, El Gendy DI, Saied EM (2015) Immunopathological changes in the brain of immunosuppressed mice experimentally infected with Toxocara canis. Korean J Parasitol 53(1):51. https://doi.org/10.3347/kjp.2015.53.1.51
Espinoza EY, PérezArellano JL, Carranza C, Collía F, Muro A (2002) In vivo inhibition of inducible nitric oxide synthase decreases lung injury induced by Toxocara canis in experimentally infected rats. Parasite Immunol 24(11-12):511–520. https://doi.org/10.1046/j.1365-3024.2002.00598.x
Esteghamati A, Eskandari D, Mirmiranpour H, Noshad S, Mousavizadeh M, Hedayati M, Nakhjavani M (2013) Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin Nutr 32(2):179–185. https://doi.org/10.1016/j.clnu.2012.08.006
Fan CK, Lin YH, Hung CC, Chang SF, Su KE (2004) Enhanced inducible nitric oxide synthase expression and nitrotyrosine accumulation in experimental granulomatous hepatitis caused by Toxocara canis in mice. Parasite Immunol 26(6-7):273–281. https://doi.org/10.1111/j.0141-9838.2004.00708.x
Fan W, Liu T, Chen W, Hammad S, Longerich T, Hausser I et al (2019) ECM1 prevents activation of transforming growth factor β, hepatic stellate cells, and fibrogenesis in mice. Gastroenterol 157(5):1352–1367. https://doi.org/10.1053/j.gastro.2019.07.036
Faz-López B, Ledesma-Soto Y, Romero-Sánchez Y, Calleja E, Martínez-Labat P, Terrazas LI (2013) Signal transducer and activator of transcription factor 6 signaling contributes to control host lung pathology but favors susceptibility against Toxocara canis infection. Biomed Res Int 2013:696343. https://doi.org/10.1155/2013/696343
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metab 20(6):953–966. https://doi.org/10.1016/j.cmet.2014.09.018
Guo H, Jian Z, Liu H, CuiH DH, Fang J, Tang H (2021) TGF-β1-induced EMT activation via both Smad-dependent and MAPK signaling pathways in Cu-induced pulmonary fibrosis. Toxicol Appl Pharmacol 418:115500. https://doi.org/10.1016/j.taap.2021.115500
Holland CV, Hamilton CM (2013) The significance of cerebral toxocariasis: a model system for exploring the link between brain involvement, behaviour and the immune response. J Exp Biol 216(1):78–83. https://doi.org/10.1242/jeb.074120
Horton J (2000) Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitol 121(S1):S113–S132. https://doi.org/10.1017/s0031182000007290
Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH (2010) Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 396(2):199–205. https://doi.org/10.1016/j.bbrc.2010.04.017
Kayes SG (1997) Human toxocariasis and the visceral larva migrans syndrome: correlative immunopathology. Chem Immunol 66:99–124. https://doi.org/10.1159/000058667
Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B, Kjaergaard M (2020) FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect 9(8):755. https://doi.org/10.1530/ec-20-0152
Kelly B, Tannahill GM, Murphy MP, O'NeillL A (2015) Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem 290(33):20348–20359. https://doi.org/10.1074/jbc.m115.662114
Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG (2014) Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem 289(33):23246–23255. https://doi.org/10.1074/jbc.M114.577908
Kim SJ, Kim KM, Yang JH, Cho SS, Jeong EH, Kim JH, Ki SH (2021) Transforming growth factor beta-induced Foxo3a acts as a profibrotic mediator in hepatic stellate cells. Toxicol Sci 179(2):241–250. https://doi.org/10.1093/toxsci/kfaa185
Kinra P, Dutta V (2013) Serum TNF alpha levels: a prognostic marker for assessment of severity of malaria. Trop Biomed 30(4):645–653
Kitwan L, Makobe C, Mdachi R, Maranga DN, Isaac AO, Nyariki JN (2023) Coenzyme Q10 prevented Trypanosoma brucei rhodesiense-mediated breach of the blood brain barrier, inflammation and organ damage in late stage of Human African Trypanosomiasis. J Parasit Dis 47(1):167–184. https://doi.org/10.1007/s12639-022-01553-8
Klockiewicz M, Jakubowski T, Sobczak-Filipiak M, Bartosik J, Długosz E (2019) Experimental infection with T. canis and T. leonina in farm mink (Neovison vison). J Vet Res 63(2):197–203 https://doi.org/10.2478/jvetres-2019-0033
Kroten A, Toczylowski K, Oldak E, Sulik A (2018) Toxocarosis in children: poor hygiene habits and contact with dogs is related to longer treatment. Parasitol Res 117:1513–1519. https://doi.org/10.1007/s00436-018-5833-7
Kuroda E, Yoshida Y, Shan BE, Yamashita U (2001) Suppression of macrophage interleukin-12 and tumour necrosis factor-α production in mice infected with Toxocara canis. Parasite Immunol 23(6):305–311. https://doi.org/10.1046/j.1365-3024.2001.00387.x
Li L, Huang W, Li K, Zhang K, Lin C, Han R, He Y (2015) Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway. Oncotarget 6(41):43605. https://doi.org/10.18632/oncotarget.6186
Liao CW, Fan CK, Kao TC, Ji DD, Su KE, Lin YH, Cho WL (2008) Brain injury-associated biomarkers of TGF-beta1, S100B, GFAP, NF-L, tTG, AbetaPP, and tau were concomitantly enhanced and the UPS was impaired during acute brain injury caused by Toxocara canis in mice. BMC Infect Dis 8(1):1–15. https://doi.org/10.1186/1471-2334-8-84
Lima FR, Ferreira LDM, Malta TA, Bonyek-Silva I, Santos RL, Tavares NM, Arruda S (2020) Metformin promotes susceptibility to experimental Leishmania braziliensis infection. Memórias do Instituto Oswaldo Cruz 115. https://doi.org/10.1590/0074-02760200272
Loos JA, Dávila VA, Rodrígues CR, Petrigh R, Zoppi JA, Crocenzi FA, Cumino AC (2017) Metformin exhibits preventive and therapeutic efficacy against experimental cystic echinococcosis. PLOS Negl Trop Dis 11(2):e0005370. https://doi.org/10.1371/journal.pntd.0005370
Lu J, Shi J, Li M, Gui B, Fu R, Yao G, Tian L (2015) Activation of AMPK by metformin inhibits TGF-β-induced collagen production in mouse renal fibroblasts. Life Sci 127:59–65. https://doi.org/10.1016/j.lfs.2015.01.042
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(S1):S232–S240. https://doi.org/10.1038/sj.bjp.0706400
Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, Gasser RB (2018) Human toxocariasis. Lancet Infect Dis 18(1):e14–e24. https://doi.org/10.1016/s1473-3099(17)30331-6
Magnaval JF, Bouhsira E, Fillaux J (2022) Therapy and prevention for human toxocariasis. Microorganisms 10(2):241. https://doi.org/10.3390/microorganisms10020241
Maizels RM (2013) Toxocara canis: molecular basis of immune recognition and evasion. Vet Parasitol 193(4):365–374 https://doi.org/10.1016/j.vetpar.2012.12.032
Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Del Prato S (2004) Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab 89(11):5535–5541. https://doi.org/10.1210/jc.2004-0150
Márquez-Navarro A, Nogueda-Torres B, Hernández-Campos A, Soria-Arteche O, Castillo R, Rodríguez-Morales S, Hernández-Luis F (2009) Anthelmintic activity of benzimidazole derivatives against Toxocara canis second-stage larvae and Hymenolepis nana adults. Acta Trop 109(3):232–235. https://doi.org/10.1016/j.actatropica.2008.11.014
Martínez-Flórez A, Galizzi M, Izquierdo L, Bustamante JM, Rodriguez A, Rodriguez F, Alberola J (2020) Repurposing bioenergetic modulators against protozoan parasites responsible for tropical diseases. Int J Parasitol Drugs Drug Resist 14:17–27. https://doi.org/10.1016/jijpddr.2020.07.002
Meng F, Khoso MH, Kang K, He Q, Cao Y, Jiang X, Li D (2021) FGF21 ameliorates hepatic fibrosis by multiple mechanisms. Mol Biol Rep 48:7153–7163. https://doi.org/10.1007/s11033-021-06707-0
Mi XJ, Hou JG, Jiang S, Liu Z, Tang S, Liu XX, Li W (2019) Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem 67(5):1392–1401. https://doi.org/10.1021/acs.jafc.8b05943
Miyakoda M, Bayarsaikhan G, Kimura D, Akbari M, Udono H, Yui K (2018) Metformin promotes the protection of mice infected with Plasmodium yoelii independently of γδ T cell expansion. Front Immunol 9:2942. https://doi.org/10.3389/fimmu.2018.02942
Musa D, Senocak G, BorazanG AM, Ozgonul A, Sogut O, Emin Güldür M (2011) Effects of Nigella sativa and albendazole alone and in combination in Toxocara canis infected mice. J Pak Med Assoc 61(9):866–870
Nassef NA, El-Kersh WM, El-Nahas NS, El-Din SAS, Oshiba SF, Nosseir MM (2014) Parasitological, histopathological, and immunohistochemical assessment of nitric oxide synthase inhibitor: aminoguanidine versus albendazole in the treatment of experimental murine toxocariasis. Menoufia Med J 27(1):103–114. https://doi.org/10.4103/1110-2098.132778
Nicolao MC, Rodrigues CR, Coccimiglio MB, Ledo C, Docena GH, Cumino AC (2023) Echinococcus granulosus extracellular vesicles and their cargo in drug response: influence on initial immune response. Res Square. https://doi.org/10.21203/rs.3.rs-2693470/v1
Nijsse R, Ploeger HW, Wagenaar JA, Mughini-Gras L (2016) Prevalence and risk factors for patent Toxocara infections in cats and cat owners’ attitude towards deworming. Parasitol Res 115(12):4519–4525. https://doi.org/10.1007/s00436-016-5242-8
Niu H, Li F, Wang Q, Ye Z, Chen Q, Lin Y (2018) High expression level of MMP9 is associated with poor prognosis in patients with clear cell renal carcinoma. PeerJ. https://doi.org/10.7717/peerj.5050
Nygaard EB, Vienberg SG, Ørskov C, Hansen HS, Andersen B (2012) Metformin stimulates FGF21 expression in primary hepatocytes. Exp Diabetes Res 2012:465282. https://doi.org/10.1155/2012/465282
Othman AA, Abdel-Aleem GA, Saied EM, Mayah WW, Elatrash AM (2010) Biochemical and immunopathological changes in experimental neurotoxocariasis. Mol Biochem Parasitol 172(1):1–8. https://doi.org/10.1016/j.molbiopara.2010.03.006
Othman AA, Abou Rayia DM, Ashour DS, Saied EM, Zineldeen DH, El-Ebiary AA (2016) Atorvastatin and metformin administration modulates experimental Trichinella spiralis infection. Parasitol Int 65(2):105–112. https://doi.org/10.1016/j.parint.2015.11.001
Othman AA, El-Shourbagy SH, Soliman RH (2011) Kinetics of Foxp3-expressing regulatory cells in experimental Toxocara canis infection. Exp Parasitol 127(2):454-459. 11. https://doi.org/10.1016/j.exppara.2010.10.005
Pijet B, Stefaniuk M, Kostrzewska-Ksiezyk A, Tsilibary PE, Tzinia A, Kaczmarek L (2018) Elevation of MMP-9 levels promotes epileptogenesis after traumatic brain injury. Mol Neurobiol 55:9294–9306. https://doi.org/10.1007/s12035-018-1061-5
Pilarczyk B, Doligalska MJ, Donskow-Schmelter K, Balicka-Ramisz A, Ramisz A (2008) Selenium supplementation enhances the protective response to Toxocara canis larvae in mice. Parasite Immunol 30(8):394–402. https://doi.org/10.1111/j.1365-3024.2008.01039.x
Probert L (2015) TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302:2–22. https://doi.org/10.1016/j.neuroscience.2015.06.038
Resende NM, Gazzinelli-Guimarães PH, Barbosa FS, Oliveira LM, Nogueira DS, Gazzinelli-Guimarães AC, Fujiwara RT (2015) New insights into the immunopathology of early Toxocara canis infection in mice. Parasit vectors 8:1–11. https://doi.org/10.1186/s13071-015-0962-7
Rigter IM, Schipper HG, Koopmans RP, Van Kan HJM, Frijlink HW, Kager PA, Guchelaar HJ (2004) Relative bioavailability of three newly developed albendazole formulations: a randomized crossover study with healthy volunteers. Antimicrob Agents Chemother 48(3):1051–1054. https://doi.org/10.1128/aac.48.3.1051-1054.2004
Rogerio AP, Sá-Nunes A, Albuquerque DA, Soares EG, Faccioli LH (2008) Anti-eosinophilic effect of Lafoensia pacari in toxocariasis. Phytomedicine 15(5):348–357. https://doi.org/10.1016/j.phymed.2007.09.012
Ruiz-Manzano RA, Hernández-Cervantes R, Del Río-Araiza VH, Palacios-Arreola MI, Nava-Castro KE, Morales-Montor J (2019) Immune response to chronic Toxocara canis infection in a mice model. Parasite Immunol 41(12):e12672. https://doi.org/10.1111/pim.12672
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, Partoazar A (2020) Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer's disease model. Life Sci 255:117861. https://doi.org/10.1016/j.lfs.2020.117861
Salama WM, El-Naggar SA, Harras SF, El-Said KS (2021) An adjuvant effect of metformin as an anti-fibrotic agent when administered with the anti-schistosomal Praziquantel in Schistosoma mansoni infected mice. Trop Biomed 38(2):205–213. https://doi.org/10.47665/tb.38.2.059
Sayiner S, Altaş M, Camkerten G, ŞehirliA Ö, Paksoy N, Balkan BM, Soran GŞ (2021) Kidney tissue selenium levels of Toxocara canis infected mice given Nigella sativa. Vet Bio Sci Tech 6(3):278–283. https://doi.org/10.31797/vetbio.977962
Schneier AJ, Durand ML (2011) Ocular toxocariasis: advances in diagnosis and treatment. Int Ophthalmol Clin 51(4):135–144. https://doi.org/10.1097/iio.0b013e31822d6a5a
Seitz T, Hellerbrand C (2021) Role of fibroblast growth factor signalling in hepatic fibrosis. Liver Int 41(6):1201–1215. https://doi.org/10.1111/liv.14863
Shao AW, Wu HJ, Chen S, Ammar AB, Zhang JM, Hong Y (2014) Resveratrol attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-κB-dependent inflammatory/MMP-9 pathway. CNS Neurosci Ther 20(2):182–185. https://doi.org/10.1111/cns.12194
Sulik A, Chyczewski L (2008) Immunohistochemical analysis of MMP-9, MMP-2 and TIMP-1, TIMP-2 expression in the central nervous system following infection with viral and bacterial meningitis. Folia Histochem Cytobiol 46(4):437–442. https://doi.org/10.2478/v10042-008-0058-8
Sweeney D, Raymer ML, Lockwood TD (2003) Antidiabetic and antimalarial biguanide drugs are metal-interactive antiproteolytic agents. Biochem Pharmacol 66(4):663–677. https://doi.org/10.1016/s0006-2952(03)00338-1
Tang G, Yang H, Chen J, Shi M, Ge L, Ge X, Zhu G (2017) Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget 8(58):97977. https://doi.org/10.18632/oncotarget.20105
Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y, Gao R, Chen G (2018) Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull 140:154–161. https://doi.org/10.1016/j.brainresbull.2018.04.008
Torina A, Caracappa S, Barera A, Dieli F, Sireci G, Genchi C, Deplazes P, Salerno A (2005) Toxocara canis infection induces antigen-specific IL-10 and IFNγ production in pregnant dogs and their puppies. Vet Immunol Immunopathol 108(1-2):247–251. https://doi.org/10.1016/j.vetimm.2005.08.006
Waindok P, Janecek-Erfurth E, Lindenwald D, Wilk E, Schughart K, Geffers R, Balas L, Durand T, Rund KM, Schebb NH, Strube C (2019) Multiplex profiling of inflammation-related bioactive lipid mediators in Toxocara canis and Toxocara cati-induced neurotoxocarosis. PLoS Negl Trop Dis 13(9):e0007706. https://doi.org/10.1371/journal.pntd.0007706
Wang Y, Meng J, Men L, An B, Jin X, He W, Lu S, Li N (2020) Rosmarinic acid protects mice from concanavalin A-induced hepatic injury through AMPK signaling. Biol Pharm Bull 43(11):1749–1759. https://doi.org/10.1248/bpb.b20-00477
Xu P, Zhang Y, Liu Y, Yuan Q, Song L, Liu M, Liu Z, Yang Y, Li J, Li D, Ren G (2016) Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways. Toxicol Appl Pharmacol 290:43–53. https://doi.org/10.1016/j.taap.2015.11.012
Xu Y, Sun X, Zhang R, Cao T, Cai SY, Boyer JL, Zhang X, Li D, Huang Y (2020) A positive feedback loop of TET3 and TGF-β1 promotes liver fibrosis. Cell Rep 30(5):1310–1318. https://doi.org/10.1016/j.celrep.2019.12.092
Zhang D, Tang Q, Zheng G, Wang C, Zhou Y, Wu Y, Xuan J, Tian N, Wang X, Wu Y, Xu H, Zhang X (2017) Metformin ameliorates BSCB disruption by inhibiting neutrophil infiltration and MMP-9 expression but not direct TJ proteins expression regulation. J Cell Mol Med 21(12):3322–3336. https://doi.org/10.1111/jcmm.13235
Zhang G, Chen S, Jia J, Liu C, Wang W, Zhang H, Zhen X (2022) Development and evaluation of novel metformin derivative metformin threonate for brain ischemia treatment. Front Pharmacol 13:879690. https://doi.org/10.3389/fphar.2022.879690
Zhang YE (2017) Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 9(2):a022129. https://doi.org/10.1101/cshperspect.a022129
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Amina M. Salama and Dina I. Elgendy conceived and designed the experiments. Rasha A. Elmahy, Amina M. Salama, and Dina I. Elgendy Performed the experiments. Hoda A. Ibrahim performed Biochemical tests. Histopathological examinations were done by Alaa Ibrahim Mohamed Amer. Asmaa Fawzy Eltantawy performed statistical analyses of the data. Dina I. Elgendy, Alaa Ibrahim Mohamed Amer, Hoda A. Ibrahim, Amina M. Salama, and Rasha A. Elmahy wrote the manuscript. All authors reviewed the manuscript and approved the version for publication.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was designed following the World Health Organization’s good laboratory practice rules. Experiments on mice were conducted in compliance with the ethical rules and regulation guidelines of the animal care committee of Tanta Faculty of Medicine, along with the internationally acknowledged principles for laboratory animal use and care. The Laboratory Animal Center for Research Ethics Committee, Faculty of Medicine, Tanta University, approved the study protocol (Approval code: 36088/11/22). All of the remains of the sacrificed animals were disposed of at the Tanta Faculty of Medicine general incinerator in accordance with the faculty research and safe disposal regulations.
Consent to participate
Consent to participate was done and confirmed.
Consent for publication
Consent for publication was done and confirmed.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salama, A.M., Elmahy, R.A., Ibrahim, H.A. et al. Effects of metformin on parasitological, pathological changes in the brain and liver and immunological aspects during visceral toxocariasis in mice. Parasitol Res 122, 3213–3231 (2023). https://doi.org/10.1007/s00436-023-08011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-08011-1