Abstract
Cryptosporidium is a major cause of diarrhoeal disease and mortality in young children in resource-poor countries, for which no vaccines or adequate therapeutic options are available. Infection in humans is primarily caused by two species: C. hominis and C. parvum. Despite C. hominis being the dominant species infecting humans in most countries, very little is known about its growth characteristics and life cycle in vitro, given that the majority of our knowledge of the in vitro development of Cryptosporidium has been based on C. parvum. In the present study, the growth and development of two C. parvum isolates (subtypes Iowa-IIaA17G2R1 and IIaA18G3R1) and one C. hominis isolate (subtype IdA15G1) in HCT-8 cells were examined and compared at 24 h and 48 h using morphological data acquired with scanning electron microscopy. Our data indicated no significant differences in the proportion of meronts or merozoites between species or subtypes at either time-point. Sexual development was observed at the 48-h time-point across both species through observations of both microgamonts and macrogamonts, with a higher frequency of macrogamont observations in C. hominis (IdA15G1) cultures at 48-h post-infection compared to both C. parvum subtypes. This corresponded to differences in the proportion of trophozoites observed at the same time point. No differences in proportion of microgamonts were observed between the three subtypes, which were rarely observed across all cultures. In summary, our data indicate that asexual development of C. hominis is similar to that of C. parvum, while sexual development is accelerated in C. hominis. This study provides new insights into differences in the in vitro growth characteristics of C. hominis when compared to C. parvum, which will facilitate our understanding of the sexual development of both species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteric protozoan parasites of the Cryptosporidium genus are a major cause of diarrhoea-related morbidity and mortality, especially in young children, with higher endemicity occurring in resource-poor countries (Yang et al. 2021). In industrialised countries, the burden of cryptosporidiosis is primarily associated with waterborne outbreaks (Gharpure et al. 2019; Ryan et al. 2022). Currently, at least 45 Cryptosporidium species are recognised (Prediger et al. 2021; Ryan et al. 2021), with two main species, C. hominis and C. parvum, responsible for over 90% of infections reported in humans globally (Chalmers et al. 2019; Feng et al. 2018; Ryan et al. 2021). While most species of Cryptosporidium exhibit a high level of host specificity, C. parvum is one of the few species that has a broad host range (Feng et al. 2018). Zoonotic transmission of C. parvum is common and represents an important aspect of the epidemiology of Cryptosporidium infections. This contrasts with C. hominis which, despite being reported in numerous hosts, is primarily a human pathogen (Widmer et al. 2020; Yang et al. 2021). There is no vaccine to prevent Cryptosporidium infection, and effective treatments for cryptosporidiosis are lacking, with only one anti-cryptosporidial drug (nitazoxanide) that is used clinically (Rahman et al. 2022). The efficacy of nitazoxanide for the treatment of cryptosporidiosis in immunocompromised and young patients, who are most vulnerable to severe disease and death from infection, is significantly lower than in immunocompetent patients. This poses a major challenge for mitigating the health burden of human cryptosporidiosis (Diptyanusa and Sari 2021; Khan and Witola 2023).
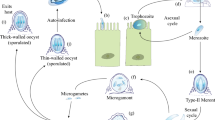
Cryptosporidium has a complex monoxenous life cycle where the infectious stage of the parasite, the oocyst, is ingested by the susceptible host. Each sporulated oocyst contains four sporozoites that are released after the oocyst exits the stomach and reaches the intestinal lumen, where they can subsequently invade the intestinal epithelium from the apical surface (Wetzel et al. 2005). The sporozoites then inhabit a niche within an intracellular but extracytoplasmic location of the host cell and develop a feeder organelle which anchors the parasite to the host epithelial cell (Forney et al. 1999). Upon invasion of the host cell, the sporozoite will rapidly mature into a trophozoite and later undergo merogony to form a meront (Edwinson et al. 2016). The meronts then release uninucleated merozoites into the intestinal lumen where they subsequently invade surrounding intestinal epithelial cells (Guérin et al. 2021). Merozoites will continue to cycle through the asexual phases of development (trophozoites, meronts, merozoites) three times, before initiating sexual development through the formation of microgamonts and macrogamonts (English et al. 2022). Fertilisation will then occur, resulting in the formation of new oocysts that are shed in the faeces. Consequently, transmission of Cryptosporidium occurs most commonly through the faecal–oral route (Feng et al. 2018).
Since the first report of Cryptosporidium cultivation in cell culture (Current and Haynes 1984), great strides have been made in the development and application of tools to both genetically manipulate Cryptosporidium and study its life cycle in vitro (Aldeyarbi and Karanis 2016; Borowski et al. 2010; Cardenas et al. 2020; Edwinson et al. 2016; English et al. 2022; Hashim et al. 2004; Hashim et al. 2006; Heo et al. 2018; Hijjawi et al. 2010; Hijjawi et al. 2001; Huang et al. 2004; Jumani et al. 2019; Mauzy et al. 2012; Miller et al. 2018; Morada et al. 2016; Pawlowic et al. 2019; Petry et al. 2009; Tandel et al. 2019; Tandel et al. 2023; Varughese et al. 2014; Vinayak et al. 2015; Warren et al. 2008; Wilke et al. 2019). The vast majority of these studies have focused on C. parvum due to its wide host range and readily available commercial stocks from animal sources (e.g. from Waterborne Inc, BioPoint Pty Ltd). Comparatively, very few studies on the life cycle and growth characteristics of C. hominis have been reported, despite being the dominant species infecting humans in most countries (Yang et al. 2021). The aim of this study was to acquire comprehensive morphological data using a scanning electron microscopy (SEM) approach to compare the growth characteristics of C. hominis (IdA15G1) and C. parvum (Iowa-IIaA17G2R1 and IIaA18G3R1) in a HCT-8 cell-line based in vitro culture system.
Methods
Collection, purification, and genotyping of Cryptosporidium oocysts
The C. parvum (Iowa-IIaA17G2R1) isolate was obtained commercially from BioPoint Pty Ltd (Sydney, Australia). Both the C. parvum (IIaA18G3R1) and C. hominis (IdA15G1) isolates were obtained from faecal samples from a local public health diagnostic laboratory (Perth, Australia) where Cryptosporidium infection was confirmed via microscopy with Ziehl-Neelsen staining. The C. parvum samples utilised in this study will be referred to as C. parvum (Iowa) and C. parvum (clinical isolate) from this point onwards. Oocysts were purified from faecal matter using a procedure outlined previously (Morgan et al. 1995) with the following modifications: omission of the potassium dichromate incubation step, replacement of ether with ethyl acetate (Chem-Supply), and addition of three phosphate-buffered saline (PBS) washes after PBS-ethyl acetate sedimentation. Samples were further purified using Ficoll-density centrifugation (Lumb et al. 1988) with the following modifications: the 4% and 6% Ficoll 400 (Sigma) in PBS (Gibco) containing 16% sodium diatrizoate (MP Bio) were excluded from the discontinuous step gradient, and the centrifugation step was performed for 20 min at 2000 × g at 4 °C. Following purification, the patient-derived Cryptosporidium oocyst isolates were stored at 4 °C in PBS supplemented with 1% v/v penicillin-streptomycin and 1% v/v amphotericin B (both from Sigma) prior to experiments. The commercially obtained C. parvum (Iowa) isolate was stored at 4 °C in PBS with no further purification required. All oocyst isolates were used for experiments within 12 weeks of purification.
All oocyst isolates were genotyped using a nested PCR targeted to the 60-kDa glycoprotein (gp60) locus, with primer sets described previously (Peng et al. 2001) and shown in Table 1. DNA was amplified in a 20 μL reaction containing 1× GoTaq Master Mix (Promega), 2.5 mM MgCl2, 0.5 mM dNTPs, 0.5 μM forward primer, 0.5 μM reverse primer, and 1 unit GoTaq DNA polymerase (Promega) per reaction. DNA amplification was carried out using a BioRad C1000 Thermal Cycler under the following cycling conditions described previously (Strong et al. 2000): briefly, an initial denaturation cycle was performed at 95 °C for 3 min, followed by 35 cycles of 94 °C for 45 s, 50 °C for 45 s, and 72 °C for 60 s, with a final extension cycle performed at 72 °C for 10 min. Amplicons from the secondary PCR were then run on a 1% agarose gel and subject to Sanger sequencing. The gp60 subtype was determined using NCBI BLAST and Clustal W alignments against an in-house database.
HCT-8 cell culture
A human epithelial cell line (HCT-8, human ileocecal colorectal adenocarcinoma, ATCC CCL-244) was routinely passaged in 75-cm2 culture flasks in RPMI-1640 (Sigma) supplemented with 10% v/v foetal bovine serum (Bovogen), 1% v/v penicillin-streptomycin (Sigma), 2 mM L-glutamine (Sigma), and 15 mM HEPES (Sigma) with pH adjusted to 7.2. The cell line tested negative for mycoplasma prior to experiments, and all experiments were performed between 60 and 100 cell doublings. For infection experiments, cell suspensions were prepared using 0.25% v/v trypsin-EDTA (Sigma), seeded onto 10-mm circular borosilicate glass coverslips at a density of 5.0 ×104 cells per well in 24-well transwell plates, and grown to 70–90% confluence.
Pre-treatment of oocysts and infection of HCT-8 cells
Cryptosporidium oocyst suspensions were quantified via haemocytometer count, pelleted at 1800 × g for 10 min, then resuspended in 0.25% v/v sodium hypochlorite and incubated at 4 °C for 30 min. Excystation pre-treatment of the oocysts was performed as described previously (King et al. 2015), omitting the infection medium washing steps after centrifugation. Oocysts were resuspended at a concentration of 1.0 × 105 oocysts mL−1 in an infection medium composed of RPMI-1640 (Sigma) supplemented with 2 mM L-glutamine, 5.6 mM glucose, 0.02% w/v bovine bile, 15 mM HEPES, 0.6 μM folic acid, 7.3 μM 4-aminobenzoic acid, 2.1 μM calcium pantothenate, 50 μM L-ascorbic acid, 2.5 μg mL−1 amphotericin B, 1% v/v penicillin-streptomycin (all from Sigma), and 1% v/v foetal bovine serum (Bovogen), with pH adjusted to 7.2. The oocyst suspension was immediately applied to HCT-8 cell monolayers at a volume of 500 μL, which corresponded to 5.0 × 104 oocysts per well. Four wells containing HCT-8 monolayers were infected per Cryptosporidium subtype for each time-point, and separate plates were used for each time-point to facilitate downstream sample processing. Immediately following oocyst application to monolayers, the plates were centrifuged at 410 × g for 5 min at room temperature. Each plate was then incubated at 37 °C and 5% CO2 in a humidified cell culture incubator for 5 h, before cell monolayers were gently rinsed with pre-warmed PBS and the infection medium was replaced. The plates were incubated for a further 19 h (for 24-h infections) and 43 h (for 48-h infections), with infection medium refreshed at 24-h post-infection for plates designated for 48-h infections. These two time-points were selected specifically to assess the transition from asexual to sexual development which has been documented to occur in this time-frame (English et al. 2022; Hijjawi et al. 2001; Tandel et al. 2019).
Sample preparation for scanning electron microscopy
At 24-h and 48-h post-infection, the infection medium was aspirated from each well, and monolayers were rinsed three times with PBS (Gibco). Samples were then chemically fixed in 2.5% v/v Grade I glutaraldehyde (Sigma) in PBS pH 7.4 through overnight incubation at 4 °C. The next morning, the glutaraldehyde was aspirated from each well, and monolayers were rinsed three times with PBS. Samples were dehydrated with analytical reagent grade ethanol (Chem-Supply) at concentrations of 30% v/v, 50% v/v, 70% v/v, and 90% v/v, all diluted with deionised water, with two 5-min incubation periods for each dilution. Three subsequent 7.5-min incubation periods with 100% v/v ethanol immediately followed. Samples underwent further chemical dehydration with hexamethyldisilazane (Sigma) diluted with ethanol at concentrations of 30% v/v, 50% v/v, and 70% v/v, with one 7.5-min incubation period per dilution. Three subsequent 7.5-min incubation periods with 100% v/v hexamethyldisilazane immediately followed, with residual hexamethyldisilazane from the final incubation period allowed to evaporate overnight in the fume hood. Borosilicate glass coverslips containing the sample were then mounted onto 12.5-mm diameter aluminium specimen stubs (Emgrid Australia) using carbon tape, and the edges were sealed with carbon paint. Samples were then sputter coated with 3 nm platinum.
Scanning electron microscopy image acquisition and data analysis
High resolution images were acquired using a Zeiss 55VP field emission SEM using the in-lens secondary electron detector and an accelerating voltage set to 5 kV. Large-area automated image acquisition was also undertaken using FEI Maps 2.0 software (Thermo Fisher Scientific) on a FEI Verios 460 XHR field emission SEM using the Everhart-Thornley detector with the accelerating voltage set to 5 kV. The FEI Maps 2.0 software was used to set up unsupervised batch data collection from all samples at a magnification of 5000 ×. Data from both electron microscopes was collected from a minimum of three separate infected wells per time-point per subtype. In the case of automated image collection, data was acquired from each sample from a minimum of eight 10 × 10 grids of images (i.e. 100 images), with each image accounting for a 1050 μm2 area of the sample. The data acquired from large areas was manually translated into numerical counts of Cryptosporidium life cycle stages. Each life cycle stage was identified based on criteria previously described in detail (Borowski et al. 2010). Ambiguous life cycle stages were further evaluated using additional sources (Koh et al. 2014; Pinto et al. 2022). Boxplots of numerical data were generated using ggplot2 v3.4.0 (Wickham 2016).
Statistical analysis
Statistical analyses were performed using R (v4.1.1) (R Core Team 2021). Data was assessed for normality using a Shapiro-Wilk test and homogeneity of variance using a Levene’s test. Given that these assumptions for parametric testing were not met, a Kruskal-Wallis test with a Dunn’s multiple comparisons test was performed for each life cycle stage at each time-point to assess whether there were any statistically significant differences in these variables between the three subtypes of Cryptosporidium that were utilised in this study.
Results
This study utilised morphological data to compare the in vitro life cycle progression of C. parvum (Iowa-IIaA17G2R1 and IIaA18G3R1) and C. hominis (IdA15G1) at 24-h and 48-h post-infection. Overall, in vitro life cycle stages were primarily asexual at the 24-h time-point across all three subtypes, and both isolates of C. parvum (Iowa and clinical isolate) still predominantly exhibited asexual developmental stages at the 48-h time-point. Notably, sexual life cycle stages were frequently observed at 48-h post-infection in C. hominis. At 24-h post-infection, a total of 915 individuals (at different life cycle stages) were counted for C. hominis, 828 for C. parvum (clinical isolate), and 836 for C. parvum (Iowa). At 48-h post-infection, a total of 1281 individuals (at different life cycle stages) were counted for C. hominis, 2356 for C. parvum (clinical isolate), and 3710 were counted for C. parvum (Iowa).
No oocysts or sporozoites were directly observed at either time-point across any of the three subtypes of Cryptosporidium that were utilised in this study. Trophozoites were observed in all three Cryptosporidium subtypes at both time-points and accounted for the majority of the life cycle stages identified in all cases (Figs. 1A and 2A; Table 2). In C. hominis cultures, immature trophozoites were observed to be spherical in shape, with a diameter of approximately 1 μm and a smooth surface (Fig. 1B). Mature trophozoites were observed to be more oblong in shape, 2–4 μm × 2 μm in size and distinctly located extracellularly. Attachment to the host cell through feeder organelles was clearly visible in the mature trophozoites from an extracellular location (Fig. 1C–D). Trophozoites were also observed in end-to-end formation in the C. hominis sample at both time-points, although no connecting discs between the two parasites were visible, and observations of this phenomenon were rare (Fig. 1E).
A Proportion of trophozoites observed at 24-h post-infection across all three isolates. B Scanning electron micrograph of immature C. hominis trophozoites at 24-h post-infection (scale bar: 2 μm). C–D Scanning electron micrographs of mature C. hominis trophozoites at 24-h post-infection with feeder organelles indicated by arrows (scale bars: C = 2 μm, D = 1 μm). E Scanning electron micrograph of C. hominis trophozoites in lateral pairing observed at 48-h post-infection (scale bar: 200 nm)
A Proportion of trophozoites observed at 48-h post-infection across all three isolates. B–D Scanning electron micrographs of C. parvum (Iowa) trophozoites at 48-h post-infection (scale bars: B =1 μm, C–D = 2 μm). E–G Scanning electron micrographs of C. parvum (clinical isolate) trophozoites at 48-h post-infection (scale bars: 2 μm)
The C. parvum (clinical isolate and Iowa) trophozoites were frequently observed in aggregates of two or more at both time-points (Fig. 2B–G). The C. parvum (Iowa) trophozoites were spherical in shape, approximately 2 μm in diameter and were relatively uniform in shape and size. In contrast to C. parvum (Iowa) trophozoites, the C. parvum (clinical isolate) trophozoites were heterogenous in shape and size. They were observed in some instances to be spherical in shape and approximately 2 μm in diameter (Fig. 2E, G), and in other occurrences, they were observed to be oblong in shape and approximately 2–3 μm × 1–2 μm in size (Fig. 2F).
By 48-h post-infection, C. hominis trophozoites accounted for a lower proportion of total individuals observed in comparison to the C. parvum subtypes (Fig. 2A), H(2) = 6.3, p = 0.043. Post hoc two-tailed multiple comparison tests indicated that this difference was statistically significant when compared to C. parvum (Iowa, difference = 6.00) where the critical difference (α = 0.05 corrected for number of tests) was 5.54. This difference was not statistically significant when compared to C. parvum (clinical isolate, difference = 4.25) where the critical difference (α = 0.05 corrected for number of tests) was 5.18.
Meronts were infrequently observed in all subtypes at 24-h post-infection (Fig. 3A), and by 48-h post-infection, they were observed in C. hominis and C. parvum (clinical isolate) only (Fig. 4A). No statistically significant differences in the proportion of meronts between each subtype were observed at 24-h post-infection, H(2) = 3.35, p = 0.187, or 48-h post-infection, H(2) = 2.16, p = 0.340. In C. hominis cultures, meronts were 2.0–3.0 μm × 2.0–2.5 μm in size and distinct in appearance, with the borders of each internal merozoite clearly visible. The number of internal merozoites visible in each electron micrograph varied between six and eight. The meronts were often observed in close proximity to one another (Fig. 3B, F, G). In the case of C. parvum (clinical isolate and Iowa), meronts were approximately 2.0–2.5 × 2.5–3.0 μm in size with between four and seven internal merozoites visible from the host cell surface. These meronts were commonly found in close proximity to each other, as well as to clusters of trophozoites (Fig. 4B–C).
Merozoites were identified in C. hominis, C. parvum (Iowa), and C. parvum (clinical isolate) cultures at 24-h post-infection (Fig. 5) and 48-h post-infection (Fig. 6). There was no significant differences in the proportion of merozoites between subtypes at 24-h post-infection, H(2) = 3.00, p = 0.351, or at 48-h post-infection, H(2) = 3.00, p = 0.223. The merozoites observed were approximately 3.0 μm in length × 0.5 μm in width, with each merozoite displaying well-defined apical regions (Figs. 5B and 6B).
Macrogamont-like structures were infrequently observed in C. parvum (clinical isolate) and C. parvum (Iowa) at 48-h post-infection, but observations of this life cycle stage were relatively more frequent in C. hominis cultures at the same time-point (Fig. 7). The difference in proportion of macrogamonts between samples was significant, H(2) = 6.75, p = 0.034. Post-hoc two-tailed multiple comparison tests indicated that this difference was only statistically significant when compared to C. parvum (Iowa, difference = 6.33) where the critical difference (α = 0.05 corrected for number of tests) was 5.54. This difference was not statistically significant when compared to C. parvum (clinical isolate, difference = 4.00) where the critical difference (α = 0.05 corrected for number of tests) was 5.18.
The morphology of each observed macrogamont was highly heterogenous even within cultures of the same isolate. Trophozoite-like structures were occasionally observed attached to the surface of the macrogamont (Fig. 7C–D). While clear attachment zones to the host monolayer were observed, a distinct feeder organelle was not evident among many of the macrogamonts. The size of each macrogamont varied between 8 and 12 μm × 4 and 6 μm (Fig. 7B–E).
Microgamonts were rare but present at 48-h post-infection across the three subtypes of Cryptosporidium utilised in this study (Fig. 8A). No significant differences were observed in proportion of microgamonts observed between the different Cryptosporidium subtypes, H(2) = 0.612, p = 0.737. The microgamont shown in Fig. 8B measured approximately 10 μm in length × 8 μm in width, and was observed to be hanging from a stalk with microgametes budding from the surface.
Discussion
The present study aimed to examine and compare the in vitro growth characteristics of C. parvum (Iowa-IIaA17G2R1), C. parvum (IIaA18G3R1), and C. hominis (IdA15G1) using data acquired via SEM to quantify life cycle stages based on parasite morphology. At 24-h post-infection, predominantly asexual development was observed across all three subtypes of Cryptosporidium utilised in this study. The key finding of this study was the observation that C. hominis displayed a higher number of sexual life cycle stages at 48-h post-infection when compared to C. parvum (Iowa) and C. parvum (clinical isolate) at the same time point, and that this was statistically significant when compared to C. parvum (Iowa).
Cryptosporidium hominis and C. parvum are morphologically indistinguishable by light microscopy (Morgan-Ryan et al. 2002), and share a high level of genomic sequence similarity (~97%) (Arias-Agudelo et al. 2020; Guo et al. 2015). Earlier in vitro studies have reported species-specific differences in the in vitro growth characteristics between C. parvum and C. hominis, such as the ability for C. parvum to infect both bovine and human-derived primary intestinal epithelial cells, in contrast to C. hominis producing infection in the human-derived primary intestinal epithelial cells only (Hashim et al. 2004). The same study also reported that C. hominis (5942 and TU502) infected HCT-8 cell lines less efficiently and less uniformly than C. parvum (Iowa) (Hashim et al. 2004). In contrast, other studies have reported minimal differences in the life cycle of C. parvum and C. hominis in HCT-8 cells, with the exception that C. hominis completed its life cycle more rapidly than C. parvum (72 h vs. 5 days) (Hijjawi et al. 2001). In the present study, there were no significant differences in the monolayer infection patterns between C. hominis and C. parvum, but clear differences in life cycle progression were observed.
Sporozoites
In the present study, sporozoites were not visible in the C. parvum or C. hominis cultures at either 24-h or 48-h post-infection; an observation which was unsurprising due to the rapid sequence of events in which sporozoite invasion occurs. Sporozoite invasion of the host cell begins within seconds of oocyst excystation (Forney et al. 1999; Guérin et al. 2021; Wetzel et al. 2005). Upon exit from the oocyst, sporozoites exhibit an actin and myosin-dependent gliding motility (Forney et al. 1998; Wetzel et al. 2005), making helical movements prior to productive host cell invasion (Wetzel et al. 2005).
Trophozoites
Invasive sporozoites transform into replicative trophozoites, and in the in vitro setting, this has been shown to be triggered by proteins in foetal bovine serum, the secretome of HCT-8 cells, and by Gal-GalNAc (Edwinson et al. 2016). The MEDLE-2 secreted protein is exported from the trophozoite into the host cell cytoplasm, where it has been demonstrated to induce an endoplasmic reticulum stress response in the host cell (Dumaine et al. 2021). In the present study, C. parvum and C. hominis trophozoites were identified at 24-h and 48-h post-infection and represented the most frequently observed life cycle stage at both time-points across all Cryptosporidium subtypes. Trophozoites were variable in size ranging from ~1 μm in diameter to larger trophozoites (>2 μm in diameter). Larger C. parvum (Iowa) trophozoites were observed to be spherical and relatively uniform in shape and size, whereas C. parvum (clinical isolate) and C. hominis trophozoites exhibited more heterogeneity in appearance. Smaller trophozoites were often found to have smooth surfaces, which indicate that they were located within the parasitophorous vacuole. Borowski et al. (2010) consistently described C. parvum trophozoites to be spherical in shape, regardless of size and relative maturity, which is in line with our observations of C. parvum (Iowa). Species-specific differences in Cryptosporidium trophozoite maturation have been described previously, where the HCT-8 secretome stimulated C. parvum (Iowa) trophozoite development but had minimal effect on C. hominis (TU502) development (Edwinson et al. 2016). In the present study, larger C. hominis trophozoites were observed to be attached to the host cell via a feeder organelle, which is consistent with morphological and ultrastructural studies on C. parvum (Borowski et al. 2010; Huang et al. 2004). Additionally, trophozoites from all Cryptosporidium isolates utilised in this study were frequently observed in aggregates of two or more, in line with observations where abundant extracellular accumulations of trophozoites were described (Borowski et al. 2010; Hijjawi et al. 2010; Hijjawi et al. 2004; Rosales et al. 2005). The broader biological significance of why some trophozoites appear located within the parasitophorous vacuole, while others appear extracellular, is currently unknown.
Meronts and merozoites
In the present study, C. parvum and C. hominis trophozoites were identified at 24-h and 48-h post-infection and represented the highest proportion of life cycle stages at both time-points across all Cryptosporidium subtypes. The proportion of trophozoites observed across the two C. parvum subtypes remained the same between the 24-h and 48-h time-points, whereas in the case of C. hominis, the proportion of trophozoites observed had dropped considerably by 48-h post-infection. Across both species and all subtypes, each meront was found attached to the apical surface of the host cell membrane, which contrasted with previous observations where C. parvum meronts were described to be engulfed by the host cell apical membrane (Borowski et al. 2010). In agreement with previous work (Borowski et al. 2010), internal C. hominis merozoites occurred in numbers of six or eight, and meronts were never observed to appear perforated to facilitate merozoite release. There appeared to be more diversity in the number of internal merozoites observed within C. parvum (clinical isolate) meronts, with numbers as varied as four and seven. The merozoites inside C. parvum meronts have been observed to be aligned solely in parallel orientation (Borowski et al. 2010); however, this was not exclusively the case with the meronts observed across all subtypes of Cryptosporidium in the present study, which were infrequently observed to be aligned perpendicularly.
Meronts release motile merozoites that infect new HCT-8 cells and repeat the asexual replication cycle. Studies on C. parvum have reported merozoite release to occur between 12 and 18-h post-infection (English et al. 2022; Guérin et al. 2021; Jumani et al. 2019). In the present study, free merozoites were identified across both species and all subtypes at 24-h and 48-h post-infection and were observed to have a tubular shape and well-defined apical regions. Rod-shaped merozoites with pointed apical ends were reported previously at 24-h post-infection in C. parvum and were observed to adhere to host cells along their full body length and increase in width throughout invasion (Borowski et al. 2010), which was also the case in the current study.
Macrogamonts and microgamonts
Notable differences in the abundance of macrogamonts at 48-h post-infection between the two species and three subtypes were evident, where C. hominis macrogamonts were frequently identified but were uncommonly observed in either C. parvum isolate. Despite the numerical difference between all species as demonstrated in Fig. 7A, this difference was statistically significant when compared to C. parvum (Iowa), but not in C. parvum (clinical isolate), which was likely due to insufficient number of independent biological replicates to form a statistical model. The size range of the C. hominis macrogamonts in the present study was between 8-12 μm × 4–6 μm, which raised the question as to whether some of these larger macrogamont-like structures were actually extracellular gamont-like stages (Hijjawi et al. 2004; Hijjawi et al. 2002). In the case of C. parvum, macrogamonts have been described to have less contact with the host cell than the asexual life cycle stages (Borowski et al. 2010), which is in agreement with our findings.
Microgamonts were observed at 48-h post-infection in all three subtypes but they occurred very rarely (summed to nine observations out of 9926 in total across all three subtypes). In C. parvum, microgamonts have been described to be smaller than macrogamonts, full of microgametes, and frequently attached to the host cell monolayer via a stalk. Microgamonts had not previously been observed in C. parvum cultures until four days post-infection (Borowski et al. 2010). The C. parvum (clinical isolate) microgamont shown in our data was morphologically similar to microgamonts already documented in the literature (Koh et al. 2014; Pinto et al. 2022).
Using a genetically engineered C. parvum Iowa II strain, a recent study conducted live imaging of the C. parvum life cycle in HCT-8 cultures and reported three rounds of asexual meront production, followed by a single generation of gametes (Tandel et al. 2019). In that study, sexual C. parvum stages were observed at 36 h and represented approximately 28% of all stages by 48 h and 80% of all stages by 72 h (Tandel et al. 2019). Had sampling continued to 72 h in the present study, it is highly likely more C. parvum sexual stages would have been observed. In the study by Tandel et al. (2019), direct development of both macrogamonts and microgamonts was observed from type I meronts containing eight merozoites, and no evidence of type II meronts containing four merozoites was observed, which is consistent with Tyzzer’s original description (Tyzzer 1910). In the present study, we observed variable numbers of merozoites within meronts, but given that we were only able to visualise the apical surface of the infected cell monolayer, the total number of merozoites within the meront may have been higher.
Oocysts
In the present study, no excysted oocyst shells were observed on the host cell monolayer at either 24-h or 48-h post-infection for C. parvum and C. hominis. This finding was expected as Cryptosporidium oocysts have low attachment efficiency due to the presence of a thick layer of acidic glycoproteins on the outer surface of the oocysts (Kuznar and Elimelech 2006; Liu et al. 2010) and were therefore unlikely to be detected. The binding of Cryptosporidium oocysts to surfaces is highly variable between different species and influenced by oocyst age (Sarkhosh et al. 2019) but excysted C. parvum oocyst shells have been reported to adhere to the host cell surface at 24-h post-infection in a similar in vitro culturing system (Borowski et al. 2010), suggesting that the detection of oocyst shells on the host cell surface was unlikely but still possible. Tandel et al. (2019) reported that fertilisation of C. parvum gametes in HCT-8 cell culture is limited or absent with no new oocysts produced. Future studies on the C. hominis life cycle in vitro will confirm if fertilisation is also absent or minimal in this species in static culture.
Conclusions
This study provided a comparative analysis of the in vitro growth characteristics of C. hominis (IdA15G1), C. parvum (IIaA18G3R1), and C. parvum (Iowa-IIaA17G2R1). Our systematic SEM observations showed that C. hominis yielded a notably higher proportion of macrogamonts at 48-h post-infection, indicating higher levels of sexual development at this time-point when compared to both subtypes of C. parvum that were utilised in this study. This difference was statistically significant when compared to C. parvum (Iowa) but was not significant in the case of C. parvum (clinical isolate). This was also reflected in statistically significant differences in proportion of trophozoites between C. hominis and C. parvum (Iowa) at 48-h post-infection. No significant differences in the proportion of meronts or merozoites were measured at either time-point, nor was the frequency of microgamont observations at 48-h post-infection significantly different between any of the three subtypes of Cryptosporidium that were utilised in this study.
A limitation of the present study is that the findings and conclusions are based only on the HCT-8 transwell system, given that the in vitro development of Cryptosporidium may differ under alternative culture conditions using different cell types. Additionally, samples were only analysed at 24 h and 48 h. A more complete comparison of the life cycle characteristics of C. parvum and C. hominis would have been achieved by analysis at a greater number of time-points and continuing the evaluation until at least 72 h. Furthermore, while oocysts no older than 12 weeks were used in the experiments described herein, the data collected in this study did not incorporate the relative oocyst age of each isolate into the analyses and the impact this may have had on Cryptosporidium development. The findings of this study justify further investigation of differences of in vitro growth characteristics between C. parvum and C. hominis and should focus on an increased number of time-points over an extended period. Further avenues for future studies may include assessment of the growth characteristics of a larger number of C. parvum and C. hominis subtypes with a greater number of independent biological replicates in HCT-8 cultures and examination of the supernatant for oocysts. This study has advanced our understanding of the in vitro development of C. hominis and provided insights into how it may contrast with C. parvum.
Data availability
Further information and requests for materials should be directed to the corresponding author.
References
Aldeyarbi HM, Karanis P (2016) The fine structure of sexual stage development and sporogony of Cryptosporidium parvum in cell-free culture. Parasitol 143(6):749–761. https://doi.org/10.1017/S0031182016000275
Arias-Agudelo LM, Garcia-Montoya G, Cabarcas F, Galvan-Diaz AL, Alzate JF (2020) Comparative genomic analysis of the principal Cryptosporidium species that infect humans. PeerJ 8:e10478. https://doi.org/10.7717/peerj.10478
Borowski H, Thompson RCA, Armstrong T, Clode PL (2010) Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitol 137(1):13–26. https://doi.org/10.1017/S0031182009990837
Cardenas D, Bhalchandra S, Lamisere H, Chen Y, Zeng X-L, Ramani S, Karandikar UC, Kaplan DL, Estes MK, Ward HD (2020) Two- and three-dimensional bioengineered human intestinal tissue models for Cryptosporidium. In: Mead JR, Arrowood MJ (eds) Cryptosporidium: methods and protocols. Springer, New York NY, pp 373–402
Chalmers RM, Robinson G, Elwin K, Elson R (2019) Analysis of the Cryptosporidium spp. and gp60 subtypes linked to human outbreaks of cryptosporidiosis in England and Wales 2009 to 2017. Parasit Vect 12(1):95. https://doi.org/10.1186/s13071-019-3354-6
Current WL, Haynes TB (1984) Complete development of Cryptosporidium in cell culture. Science 224(4649):603–605. https://doi.org/10.1126/science.6710159
Diptyanusa A, Sari IP (2021) Treatment of human intestinal cryptosporidiosis: a review of published clinical trials. Int J Parasitol: Drugs Drug Resist 17:128–138. https://doi.org/10.1016/j.ijpddr.2021.09.001
Dumaine JE, Sateriale A, Gibson AR, Reddy AG, Gullicksrud JA, Hunter EN, Clark JT, Striepen B (2021) The enteric pathogen Cryptosporidium parvum exports proteins into the cytosol of the infected host cell. eLife 10. https://doi.org/10.7554/eLife.70451
Edwinson A, Widmer G, McEvoy J (2016) Glycoproteins and Gal-GalNAc cause Cryptosporidium to switch from an invasive sporozoite to a replicative trophozoite. Int J Parasitol 46(1):67–74. https://doi.org/10.1016/j.ijpara.2015.09.001
English ED, Guérin A, Tandel J, Striepen B (2022) Live imaging of the Cryptosporidium parvum life cycle reveals direct development of male and female gametes from type I meronts. PLoS Biol 20(4). https://doi.org/10.1371/journal.pbio.3001604
Feng Y, Ryan UM, Xiao L (2018) Genetic diversity and population structure of Cryptosporidium. Trends Parasitol 34(11):997–1011. https://doi.org/10.1016/j.pt.2018.07.009
Forney JR, Dewald DB, Shiguang Y, Speer CA, Healey MC (1999) A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect Immun 67(2):844–852. https://doi.org/10.1128/IAI.67.2.844-852.1999
Forney JR, Vaughan DK, Yang S, Healey MC (1998) Actin-dependent motility in Cryptosporidium parvum sporozoites. J Parasitol 84(5):908–913. https://doi.org/10.2307/3284619
Gharpure R, Perez A, Miller AD, Wikswo ME, Silver R, Hlavsa MC (2019) Cryptosporidiosis outbreaks-United States, 2009–2017. MMWR 68:568–572. https://doi.org/10.15585/mmwr.mm6825a3
Guérin A, Roy NH, Kugler EM, Berry L, Burkhardt JK, Shin J-B, Striepen B (2021) Cryptosporidium rhoptry effector protein ROP1 injected during invasion targets the host cytoskeletal modulator LMO7. Cell Host Microbe 29(9):1407–1420.e5. https://doi.org/10.1016/j.chom.2021.07.002
Guo Y, Tang K, Rowe LA, Li N, Roellig DM, Knipe K, Frace M, Yang C, Feng Y, Xiao L (2015) Comparative genomic analysis reveals occurrence of genetic recombination in virulent Cryptosporidium hominis subtypes and telomeric gene duplications in Cryptosporidium parvum. BMC Genom 16(1):320. https://doi.org/10.1186/s12864-015-1517-1
Hashim A, Clyne M, Mulcahy G, Akiyoshi D, Chalmers R, Bourke B (2004) Host cell tropism underlies species restriction of human and bovine Cryptosporidium parvum genotypes. Infect Immun 72(10):6125–6131. https://doi.org/10.1128/IAI.72.10.6125-6131.2004
Hashim A, Mulcahy G, Bourke B, Clyne M (2006) Interaction of Cryptosporidium hominis and Cryptosporidium parvum with primary human and bovine intestinal cells. Infect Immun 74(1):99–107. https://doi.org/10.1128/IAI.74.1.99-107.2006
Heo I, Dutta D, Schaefer DA, Iakobachvili N, Artegiani B, Sachs N, Boonekamp KE, Bowden G, Hendrickx APA, Willems RJL, Peters PJ, Riggs MW, O’Connor R, Clevers H (2018) Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol 3(7):814–823. https://doi.org/10.1038/s41564-018-0177-8
Hijjawi N, Estcourt A, Yang R, Monis P, Ryan U (2010) Complete development and multiplication of Cryptosporidium hominis in cell-free culture. Vet Parasitol 169(1):29–36. https://doi.org/10.1016/j.vetpar.2009.12.021
Hijjawi N, Meloni BP, Morgan UM, Thompson RCA (2001) Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int J Parasitol 31(10):1048–1055. https://doi.org/10.1016/S0020-7519(01)00212-0
Hijjawi N, Meloni BP, Ng'anzo M, Ryan UM, Olson ME, Cox PT, Monis PT, Thompson RCA (2004) Complete development of Cryptosporidium parvum in host cell-free culture. Int J Parasitol 34(7):769–777. https://doi.org/10.1016/j.ijpara.2004.04.001
Hijjawi N, Meloni BP, Ryan UM, Olson ME, Thompson RCA (2002) Successful in vitro cultivation of Cryptosporidium andersoni: evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. Int J Parasitol 32(14):1719–1726. https://doi.org/10.1016/S0020-7519(02)00199-6
Huang BQ, Chen X-M, LaRusso NF (2004) Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: a morphologic study. J Parasitol 90(2):212–22110
Jumani RS, Hasan MM, Stebbins EE, Donnelly L, Miller P, Klopfer C, Kovi B, Teixeira JE, Love MS, McNamara CW, Huston CD (2019) A suite of phenotypic assays to ensure pipeline diversity when prioritizing drug-like Cryptosporidium growth inhibitors. Nat Commun 10(1). https://doi.org/10.1038/s41467-019-09880-w
Khan SM, Witola WH (2023) Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: a comprehensive review. Front Cell Infect Microbiol 13. https://doi.org/10.3389/fcimb.2023.1115522
King BJ, Fanok S, Phillips R, Swaffer B, Monis P (2015) Integrated Cryptosporidium assay to determine oocyst density, infectivity, and genotype for risk assessment of source and reuse water. Appl Environ Microbiol 81(10):3471–3481. https://doi.org/10.1128/aem.00163-15
Koh W, Thompson A, Edwards H, Monis P, Clode PL (2014) Extracellular excystation and development of Cryptosporidium: tracing the fate of oocysts within Pseudomonas aquatic biofilm systems. BMC Microbiol 14(1):281. https://doi.org/10.1186/s12866-014-0281-8
Kuznar ZA, Elimelech M (2006) Cryptosporidium oocyst surface macromolecules significantly hinder oocyst attachment. Environ Sci Technol 40(6):1837–1842. https://doi.org/10.1021/es051859p
Liu Y, Kuhlenschmidt MS, Kuhlenschmidt TB, Nguyen T, H. (2010) Composition and conformation of Cryptosporidium parvum oocyst wall surface macromolecules and their effect on adhesion kinetics of oocysts on quartz surface. Biomacromole 11(8):2109–2115. https://doi.org/10.1021/bm100477j
Lumb R, Lanser JA, O’Donoghue PJ (1988) Electrophoretic and immunoblot analysis of Cryptosporidium oocysts. Immunol Cell Biol 66(5-6):369–376. https://doi.org/10.1038/icb.1988.48
Mauzy MJ, Enomoto S, Lancto CA, Abrahamsen MS, Rutherford MS (2012) The Cryptosporidium parvum transcriptome during in vitro development. PloS One 7(3):e31715. https://doi.org/10.1371/journal.pone.0031715
Miller CN, Jossé L, Brown I, Blakeman B, Povey J, Yiangou L, Price M, Cinatl J, Xue W-F, Michaelis M, Tsaousis AD (2018) A cell culture platform for Cryptosporidium that enables long-term cultivation and new tools for the systematic investigation of its biology. Int J Parasitol 48(3):197–201. https://doi.org/10.1016/j.ijpara.2017.10.001
Morada M, Lee S, Gunther-Cummins L, Weiss LM, Widmer G, Tzipori S, Yarlett N (2016) Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46(1):21–29. https://doi.org/10.1016/j.ijpara.2015.07.006
Morgan UM, Constantine CC, O’Donoghue P, Meloni BP, O’Brien PA, Thompson RCA (1995) Molecular characterization of Cryptosporidium isolates from humans and other animals using random amplified polymorphic DNA analysis. Am J Trop Med Hyg 52(6):559–564. https://doi.org/10.4269/ajtmh.1995.52.559
Morgan-Ryan UM, Fall A, Ward LA, Hijjawi N, Sulaiman I, Fayer R, Thompson RCA, Olson M, Lal A, Xiao L (2002) Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol 49(6):433–440. https://doi.org/10.1111/j.1550-7408.2002.tb00224.x
Pawlowic MC, Somepalli M, Sateriale A, Herbert GT, Gibson AR, Cuny GD, Hedstrom L, Striepen B (2019) Genetic ablation of purine salvage in Cryptosporidium parvum reveals nucleotide uptake from the host cell. PNAS 116(42):21160–21165. https://doi.org/10.1073/pnas.1908239116
Peng MM, Matos O, Gatei W, Das P, Stantic-Pavlinic M, Bern C, Sulaiman IM, Glaberman S, Lal AA, Xiao L (2001) A comparison of Cryptosporidium subgenotypes from several geographic regions. J Eukaryot Microbiol 48(s1):28s–31s. https://doi.org/10.1111/j.1550-7408.2001.tb00442.x
Petry F, Kneib I, Harris JR (2009) Morphology and in vitro infectivity of sporozoites of Cryptosporidium parvum. J Parasitol 95(5):1243–1246. https://doi.org/10.1645/ge-2021.1
Pinto P, Ribeiro CA, Kváč M, Tsaousis AD (2022) Cryptosporidium. In: de Souza W (ed) Lifecycles of pathogenic protists in humans. Springer International Publishing, Cham, pp 331–389
Prediger J, Ježková J, Holubová N, Sak B, Konečný R, Rost M, McEvoy J, Rajský D, Kváč M (2021) Cryptosporidium sciurinum n. sp. (Apicomplexa: Cryptosporidiidae) in Eurasian red squirrels (Sciurus vulgaris)) Microorgan 9(10):2050. https://doi.org/10.3390/microorganisms9102050
R Core Team (2021) R: a language and environment for statistical computing, Austria
Rahman SU, Mi R, Zhou S, Gong H, Ullah M, Huang Y, Han X, Chen Z (2022) Advances in therapeutic and vaccine targets for Cryptosporidium: challenges and possible mitigation strategies. Acta Trop 226:106273. https://doi.org/10.1016/j.actatropica.2021.106273
Rosales MJ, Cordón GP, Moreno MS, Sánchez CM, Mascaró C (2005) Extracellular like-gregarine stages of Cryptosporidium parvum. Acta Trop 95(1):74–78. https://doi.org/10.1016/j.actatropica.2005.03.009
Ryan U, Feng Y, Fayer R, Xiao L (2021) Taxonomy and molecular epidemiology of Cryptosporidium and Giardia – a 50 year perspective (1971–2021). Int J Parasitol 51(13):1099–1119. https://doi.org/10.1016/j.ijpara.2021.08.007
Ryan U, Hill K, Deere D (2022) Review of generic screening level assumptions for quantitative microbial risk assessment (QMRA) for estimating public health risks from Australian drinking water sources contaminated with Cryptosporidium by recreational activities. Water Res 220:118659. https://doi.org/10.1016/j.watres.2022.118659
Sarkhosh T, Zhang XF, Jellison KL, Jedlicka SS (2019) Calcium-mediated biophysical binding of Cryptosporidium parvum oocysts to surfaces is sensitive to oocyst age. Appl Environ Microbiol 85(17):e00816–e00819. https://doi.org/10.1128/AEM.00816-19
Strong WB, Gut J, Nelson RG (2000) Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect Immun 68(7):4117–4134. https://doi.org/10.1128/IAI.68.7.4117-4134.2000
Tandel J, English ED, Sateriale A, Gullicksrud JA, Beiting DP, Sullivan MC, Pinkston B, Striepen B (2019) Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat Microbiol 4:2226–2236. https://doi.org/10.1038/s41564-019-0539-x
Tandel J, Walzer KA, Byerly JH, Pinkston B, Beiting DP, Striepen B (2023) Genetic ablation of a female-specific Apetala 2 transcription factor blocks oocyst shedding in Cryptosporidium parvum. mBio 0(0):e03261-22. https://doi.org/10.1128/mbio.03261-22
Tyzzer EE (1910) An extracellular Coccidium, Cryptosporidium Muris (Gen. Et Sp. Nov.), of the gastric glands of the common mouse. J Med Res 23(3):487–510
Varughese EA, Bennett-Stamper CL, Wymer LJ, Yadav JS (2014) A new in vitro model using small intestinal epithelial cells to enhance infection of Cryptosporidium parvum. J Microbiol Methods 106:47–54. https://doi.org/10.1016/j.mimet.2014.07.017
Vinayak S, Pawlowic MC, Sateriale A, Brooks CF, Studstill CJ, Bar-Peled Y, Cipriano MJ, Striepen B (2015) Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523(7561):477–480. https://doi.org/10.1038/nature14651
Warren CA, Destura RV, Sevilleja JEAD, Barroso LF, Carvalho H, Barrett LJ, O’Brien AD, Guerrant RL (2008) Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis. J Infect Dis 198(1):143–149. https://doi.org/10.1086/588819
Wetzel DM, Schmidt J, Kuhlenschmidt MS, Dubey JP, Sibley LD (2005) Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infect Immun 73(9):5379–5387. https://doi.org/10.1128/IAI.73.9.5379-5387.2005
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Widmer G, Köster PC, Carmena D (2020) Cryptosporidium hominis infections in non-human animal species: revisiting the concept of host specificity. Int J Parasitol 50(4):253–262. https://doi.org/10.1016/j.ijpara.2020.01.005
Wilke G, Funkhouser-Jones LJ, Wang Y, Ravindran S, Wang Q, Beatty WL, Baldridge MT, VanDussen KL, Shen B, Kuhlenschmidt MS, Kuhlenschmidt TB, Witola WH, Stappenbeck TS, Sibley LD (2019) A stem-cell-derived platform enables complete Cryptosporidium development in vitro and genetic tractability. Cell Host Microbe 26(1):123–134. https://doi.org/10.1016/j.chom.2019.05.007
Yang X, Guo Y, Xiao L, Feng Y (2021) Molecular epidemiology of human cryptosporidiosis in low- and middle-income countries. Clin Microbiol Rev 34(2):e00087–e00019. https://doi.org/10.1128/CMR.00087-19
Acknowledgements
We thank Dr Andrew Ball from Water New South Wales, Dr Nicholas Crosbie from Melbourne Water, Dr Paul Fisher from Seqwater, and Dr Leon van der Linden from South Australian Water Corporation for their role as industry partners and contribution to funding. We thank Ms Frances Brigg for performing Sanger sequencing. The authors acknowledge use of the facilities of Microscopy Australia at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments. Ms Samantha Gunasekera undertook this research with the support of a Murdoch Strategic Scholarship.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was funded by the Australian Research Council, grant number LP170100096.
Author information
Authors and Affiliations
Contributions
Samantha Gunasekera and Una Ryan wrote the manuscript, and Peta L. Clode, Brendon King, Pal Monis, Benjamin Thierry, Jill Carr, Abha Chopra, Mark Watson, Mark O’Dea, and Nawal Hijjawi reviewed and edited the manuscript. Samantha Gunasekera performed data acquisition, and Samantha Gunasekera, Una Ryan, Peta L. Clode, and Nawal Hijjawi interpreted morphological data. Samantha Gunasekera performed statistical analyses and final figure preparation. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
An exemption from Human Ethics approval was granted on the understanding that research was conducted according the standards of the Australian Code for the Responsible Conduct of Research (2007) and Murdoch University policies, and as the relevant National Statement on Ethical Conduct in Human Research 2007 (updated 2018) at all times.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors Dr Brendon King and Dr Paul Monis are research scientists and employees of South Australian Water Corporation that has part-funded this project.
Additional information
Handling Editor: Julia Walochnik
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunasekera, S., Clode, P.L., King, B. et al. Comparison of in vitro growth characteristics of Cryptosporidium hominis (IdA15G1) and Cryptosporidium parvum (Iowa-IIaA17G2R1 and IIaA18G3R1). Parasitol Res 122, 2891–2905 (2023). https://doi.org/10.1007/s00436-023-07979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07979-0