Abstract
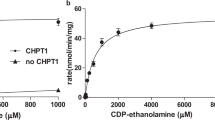
The de novo biosynthesis of phosphatidylcholine and phosphatidylethanolamine in Entamoeba histolytica is largely dependent on the CDP-choline and CDP-ethanolamine pathways. Although the first enzymes of these pathways, EhCK1 and EhCK2, have been previously characterized, their enzymatic activity was found to be low and undetectable, respectively. This study aimed to identify the unusual characteristics of these enzymes in this deadly parasite. The discovery that EhCKs prefer Mn2+ over the typical Mg2+ as a metal ion cofactor is intriguing for CK/EK family of enzymes. In the presence of Mn2+, the activity of EhCK1 increased by approximately 108-fold compared to that in Mg2+. Specifically, in Mg2+, EhCK1 exhibited a Vmax and K0.5 of 3.5 ± 0.1 U/mg and 13.9 ± 0.2 mM, respectively. However, in Mn2+, it displayed a Vmax of 149.1 ± 2.5 U/mg and a K0.5 of 9.5 ± 0.1 mM. Moreover, when Mg2+ was present at a constant concentration of 12 mM, the K0.5 value for Mn2+ was ~ 2.4-fold lower than that in Mn2+ alone, without affecting its Vmax. Although the enzyme efficiency of EhCK1 was significantly improved by about 25-fold in Mn2+, it is worth noting that its Km for choline and ATP were higher than in equimolar of Mg2+ in a previous study. In contrast, EhCK2 showed specific activity towards ethanolamine in Mn2+, exhibiting Michaelis–Menten kinetic with ethanolamine (Km = 312 ± 27 µM) and cooperativity with ATP (K0.5 = 2.1 ± 0.2 mM). Additionally, we investigated the effect of metal ions on the substrate recognition of human choline and ethanolamine kinase isoforms. Human choline kinase α2 was found to absolutely require Mg2+, while choline kinase β differentially recognized choline and ethanolamine in Mg2+ and Mn2+, respectively. Finally, mutagenesis studies revealed that EhCK1 Tyr129 was critical for Mn2+ binding, while Lys233 was essential for substrate catalysis but not metal ion binding. Overall, these findings provide insight into the unique characteristics of the EhCKs and highlight the potential for new approaches to treating amoebiasis. Amoebiasis is a challenging disease for clinicians to diagnose and treat, as many patients are asymptomatic. However, by studying the enzymes involved in the CDP-choline and CDP-ethanolamine pathways, which are crucial for de novo biosynthesis of phosphatidylcholine and phosphatidylethanolamine in Entamoeba histolytica, there is great potential to discover new therapeutic approaches to combat this disease.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ADP :
-
Adenosine diphosphate
- ATP :
-
Adenosine triphosphate
- BSA :
-
Bovine serum albumin
- CK :
-
Choline kinase
- CDP-Choline :
-
Cytidine 5’-diphosphocholine
- CDP-Etn :
-
Cytidine 5’-diphosphoethanolamine
- EDTA :
-
Ethylenediaminetetraacetic acid
- EhCK :
-
E. histolytica Choline kinase
- EhEK :
-
E. histolytica Ethanolamine kinase
- EK :
-
Ethanolamine kinase
- GST :
-
Glutathione-S-transferase
- hCK :
-
Human choline kinase
- hEK :
-
Human ethanolamine kinase
- His- :
-
6X histidine tag
- IPTG :
-
Isopropyl β-D-1-thiogalactopyranoside
- LB :
-
Luria-Bertani (medium)
- MBP :
-
Maltose binding protein
- NADH :
-
Reduced nicotinamide adenine dinucleotide
- ORF :
-
Open reading frame
- PCho :
-
Phosphocholine
- PCR :
-
Polymerase chain reaction
- PEtn :
-
Phosphoethanolamine
- PSD :
-
Phosphatidylserine decarboxylase
- PtdCho :
-
Phosphatidylcholine
- PtdEtn :
-
Phosphatidylethanolamine
References
Adams JA (2001) Kinetic and catalytic mechanisms of protein kinases. Chem Rev 101(8):2271–2290. https://doi.org/10.1021/cr000230w
Aguilar-Díaz H, Díaz-Gallardo M, Laclette JP, Carrero JC (2010) In vitro induction of Entamoeba histolytica cyst-like structures from trophozoites. PLoS Negl Trop Dis 4(2):e607. https://doi.org/10.1371/journal.pntd.0000607
Alberge B, Gannoun-Zaki L, Bascunana C, Tran van Ba C, Vial H, Cerdan R (2009) Comparison of the cellular and biochemical properties of Plasmodium falciparum choline and ethanolamine kinases. Biochem J 425(1):149–158. https://doi.org/10.1042/BJ20091119
Ancelin ML, Vial HJ (1986) Choline kinase activity in Plasmodium-infected erythrocytes: characterization and utilization as a parasite-specific marker in malarial fractionation studies. Biochem Biophys Acta 875(1):52–58. https://doi.org/10.1016/0005-2760(86)90010-x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brundiers R, Lavie A, Veit T, Reinstein J, Schlichting I, Ostermann N et al (1999) Modifying human thymidylate kinase to potentiate azidothymidine activation. J Biol Chem 274(50):35289–35292. https://doi.org/10.1074/jbc.274.50.35289
Chang CH (2015) Biochemical Characterization of Entamoeba histolytica Choline/Ethanolamine Kinases. Dissertation, Universiti Sains Malaysia
Diamant S, Azem A, Weiss C, Goloubinoff P (1995) Increased efficiency of GroE-assisted protein folding by manganese ions. J Biol Chem 270(47):28387–28391. https://doi.org/10.1074/jbc.270.47.28387
Eichinger L, Noegel AA (2005) Comparative genomics of Dictyosteliumdiscoideum and Entamoeba histolytica. Curr Opin Microbiol 8(5):606–611. https://doi.org/10.1016/j.mib.2005.08.009
Gallego-Ortega D, Ramirez de Molina A, Ramos MA, Valdes-Mora F, Barderas MG, Sarmentero-Estrada J, Lacal JC (2009) Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PloS One 4(11):7819. https://doi.org/10.1371/journal.pone.0007819
Gardberg A, Shuvalova L, Monnerjahn C, Konrad M, Lavie A (2003) Structural basis for the dual thymidine and thymidylate kinase activity of herpes thymidine kinases. Structure (London, England : 1993) 11(10):1265–1277. https://doi.org/10.1016/j.str.2003.09.003
Garfinkel L, Altschuld RA, Garfinkel D (1986) Magnesium in cardiac energy metabolism. J Mol Cell Cardiol 18(10):1003–1013. https://doi.org/10.1016/s0022-2828(86)80289-9
Gee P, Kent C (2003) Multiple isoforms of choline kinase from Caenorhabditis elegans: cloning, expression, purification, and characterization. Biochem Biophys Acta 1648(1–2):33–42. https://doi.org/10.1016/s1570-9639(03)00106-7
Gibellini F, Hunter WN, Smith TK (2008) Biochemical characterization of the initial steps of the Kennedy pathway in Trypanosoma brucei: the ethanolamine and choline kinases. Biochem J 415(1):135–144. https://doi.org/10.1042/BJ20080435
Gómez-Pérez V, McSorley T, See Too WC, Konrad M, Campos JM (2012) Novel 4-amino bis-pyridinium and bis-quinolinium derivatives as choline kinase inhibitors with antiproliferative activity against the human breast cancer SKBR-3 cell line. ChemMedChem 7(4):663–669. https://doi.org/10.1002/cmdc.201100505
Gong D, Gong Z, Guo Y, Zhu J-K (2002) Expression, activation, and biochemical properties of a novel arabidopsis protein kinase. Plant Physiol 129(1):225–234. https://doi.org/10.1104/pp.010776
Gruber J, See Too WC, Wong MT, Lavie A, McSorley T, Konrad M (2012) Balance of human choline kinase isoforms is critical for cell cycle regulation: implications for the development of choline kinase-targeted cancer therapy. FEBS J 279(11):1915–1928. https://doi.org/10.1111/j.1742-4658.2012.08573.x
Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9(8):576–596
Jha AK, Jha P, Chaudhary M, Purkayastha S, Jha SK, Ranjan R et al (2019) Evaluation of factors associated with complications in amoebic liver abscess in a predominantly toddy-drinking population: a retrospective study of 198 cases. JGH Open 3(6):474–479. https://doi.org/10.1002/jgh3.12183
Kantor M, Abrantes A, Estevez A, Schiller A, Torrent J, Gascon J et al (2018) Entamoeba Histolytica: updates in clinical manifestation, pathogenesis, and vaccine development. Can J Gastroenterol Hepatol 2018:4601420. https://doi.org/10.1155/2018/4601420
Khalifa M, Few LL, See Too WC (2020) ChoK-ing the pathogenic bacteria: potential of human choline kinase inhibitors as antimicrobial agents. Biomed Res Int 2020:1823485. https://doi.org/10.1155/2020/1823485
Kim KH, Voelker DR, Flocco MT, Carman GM (1998) Expression, purification, and characterization of choline kinase, product of the CKI gene from Saccharomyces cerevisiae. J Biol Chem 273(12):6844–6852. https://doi.org/10.1074/jbc.273.12.6844
Kim K, Kim KH, Storey MK, Voelker DR, Carman GM (1999) Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J Biol Chem 274(21):14857–14866. https://doi.org/10.1074/jbc.274.21.14857
Lovitt B, VanderPorten EC, Sheng Z, Zhu H, Drummond J, Liu Y (2010) Differential effects of divalent manganese and magnesium on the kinase activity of leucine-rich repeat kinase 2 (LRRK2). Biochemistry 49(14):3092–3100. https://doi.org/10.1021/bi901726c
Lykidis A (2007) Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Prog Lipid Res 46(3–4):171–199. https://doi.org/10.1016/j.plipres.2007.03.003
Madeira F, Pearce M, Tivey ARN et al (2022) Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 50(W1):W276–W279. https://doi.org/10.1093/nar/gkac240
Malito E, Sekulic N, See Too WC, Konrad M, Lavie A (2006) Elucidation of human choline kinase crystal structures in complex with the products ADP or phosphocholine. J Mol Biol 364(2):136–151. https://doi.org/10.1016/j.jmb.2006.08.084
Maloy SR, Stewart VJ, Taylor RK (1995) Genetic analysis of pathogenic bacteria: a laboratory manual, 1st edn. Cold Spring Harbor Laboratory Press, New York
Martínez-Castillo M, Serrano-Luna J, Coronado-Velázquez D, Pacheco-Yépez J, de la Garza M, Shibayama M (2021) Molecular mechanisms of host immune responses to Entamoeba. In: Liu D (ed) Molecular Food Microbiology, 1st edn. CRC Press, pp 461–477
Picazarri K, Nakada-Tsukui K, Sato D, Nozaki T (2008) Analysis of autophagy in the enteric protozoan parasite Entamoeba. Methods Enzymol 451:359–371. https://doi.org/10.1016/S0076-6879(08)03224-2
Ravindranath SD, Fridovich I (1975) Isolation and characterization of a manganese-containing superoxide dismutase from yeast. J Biol Chem 250(15):6107–6112
Reddy MM, Rajasekharan R (2006) Role of threonine residues in the regulation of manganese-dependent arabidopsis serine/threonine/tyrosine protein kinase activity. Arch Biochem Biophys 455(2):99–109. https://doi.org/10.1016/j.abb.2006.09.009
Romani A, Scarpa A (1992) Regulation of cell magnesium. Arch Biochem Biophys 298(1):1–12. https://doi.org/10.1016/0003-9861(92)90086-c
Romani AM, Scarpa A (2000) Regulation of cellular magnesium. Front Biosci 5:D720–D734. https://doi.org/10.2741/romani
Saidin S, Othman N, Noordin R (2019) Update on laboratory diagnosis of amoebiasis. Eur J Clin Microbiol Infect Dis 38(1):15–38. https://doi.org/10.1007/s10096-018-3379-3
Salit IE, Khairnar K, Gough K, Pillai DR (2009) A possible cluster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clin Infect Dis 49(3):346–353. https://doi.org/10.1086/600298
See Too WC, Wong MT, Few LL, Konrad M (2010) Highly specific antibodies for co-detection of human choline kinase α1 and α2 isoforms. PloS One 5(9):e12999. https://doi.org/10.1371/journal.pone.0012999
Stanley SLJ (2005) The Entamoeba histolytica genome: something old, something new, something borrowed and sex too? Trends Parasitol 21(10):451–453. https://doi.org/10.1016/j.pt.2005.08.006
Uchida T, Yamashita S (1990) Purification and properties of choline kinase from rat brain. Biochem Biophys Acta 1043(3):281–288. https://doi.org/10.1016/0005-2760(90)90028-v
Yu J, Yu X, Liu J (2004) A thermostable manganese-containing superoxide dismutase from pathogen Chlamydia pneumoniae. FEBS Lett 562(1–3):22–26. https://doi.org/10.1016/S0014-5793(04)00170-X
Yuan C, Kent C (2004) Identification of critical residues of choline kinase A2 from Caenorhabditis elegans. J Biol Chem 279(17):17801–17809. https://doi.org/10.1074/jbc.M401382200
Yuan CJ, Huang CY, Graves DJ (1993) Phosphorylase kinase, a metal ion-dependent dual specificity kinase. J Biol Chem 268(24):17683–17686
Acknowledgements
We would like to thank the laboratory staff of the School of Health Sciences for their technical assistance.
Funding
This work was supported by the Malaysia Ministry of Higher Education Fundamental Research Grant Scheme (FRGS), FRGS/1/2021/SKK0/USM/02/34 (203/PPSK/6171299).
Author information
Authors and Affiliations
Contributions
Ling Ling Few, Boon Huat Lim, Get Bee Yvonne-Tee, Ai Lan Chew, and Wei Cun See Too contributed to the study conception and design. Material preparation, data collection, and analyses were performed by Chiat Han Chang and Wei Cun See Too. The first draft of the manuscript was written by Chiat Han Chang and Ling Ling Few. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Dietmar Steverding.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, C.H., Few, L.L., Lim, B.H. et al. Unusual metal ion cofactor requirement of Entamoeba histolytica choline and ethanolamine kinase isoforms. Parasitol Res 122, 1651–1661 (2023). https://doi.org/10.1007/s00436-023-07869-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07869-5