Abstract
In urban and degraded areas, ectoparasite abundance can be affected by increasing human population density and habitat fragmentation. This study aimed to characterize the ectoparasitic fly community associated with bats in the urban green areas of Sergipe, Brazil. Campaigns were conducted monthly, for two consecutive nights, between September 2019 and February 2021. To capture the bats, ten mist nets were set up inside and at the edge of the habitat fragments. All ectoparasites found were removed from the bats and stored in 70% alcohol. The specificity index, parasitological rates, and level of parasite aggregation were calculated, and the influence of host sex and seasonality on parasitological rates were verified for the most parasitized bats. The collected ectoparasites corresponded to the families Nycteribiidae (S = 1; n = 26) and Streblidae (S = 13; n = 849), with Trichobius costalimai and Medistopoda aranea being the most abundant species. For some interactions, there was an influence of host sex on the prevalence rates, with the highest number of parasites being found on females, which can be explained by their greater susceptibility to parasitism owing to their long stay in roosts. The seasonality influenced the parasitological rates, and opposing patterns (from what was expected) were observed for some interactions; this influence may be due to the biological differences between parasite species. This study provides relevant data on this interaction, especially for urban areas in northeastern Brazil, expanding the number of studies in the State of Sergipe and promoting future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have suggested that environmental changes, such as urbanization, can significantly influence the interactions between bats and their ectoparasites (Lafferty and Kuris 2005; Pilosof et al. 2012; Frank et al. 2016). However, the complex relationships among the environment, host, and parasite make it difficult to predict how environmental changes will affect their interaction (Lafferty and Kuris 2005). Few studies have used this approach in urban areas; thus, the patterns of parasitism in this type of environment remain unclear (Ramalho et al. 2018, 2021; Urbieta et al. 2014, 2018).
In urban and degraded areas, ectoparasite abundance can be influenced by increased human population density and habitat fragmentation (Bolívar-Cime et al. 2018; Ramalho et al. 2018; Urbieta et al. 2018). According to Urbieta et al. (2018), ectoparasite prevalence rates and average infestation intensities in urban environments may be lower than that in forest environments. This result may be associated with the quality of proximal roosts in the sampled environment, where poor quality shelters lead bats to change shelters more often, reducing the probability of parasite transmission (Barbier and Graciolli 2016). In addition, parasite specificity may also be lower in more urban areas owing to the increased density of bats within a shelter, therefore facilitating parasite transmission (Urbieta et al. 2018).
Bat ectoparasites, as streblids, spend most of their life cycle associated with bats and depend on their roosts to find new hosts; thus, their abundance is highly influenced by the environmental factors that affect bats (Pilosof et al. 2012; Dittmar et al. 2015; Urbieta et al. 2021). The abundance of parasites increases in environmental conditions favorable for their development and decreases when these conditions are harmful to them (Tinsley et al. 2011). According to Rui and Graciolli (2005), during dry seasons (high temperatures and low precipitation), parasitism increases because the reproductive rates of the ectoparasites increases, whereas during the rainy seasons (low temperatures and high precipitation), reproductive rates decrease and mortality rate is higher. Furthermore, it is believed that the geographic distribution, body size, behavior, and sex of the host are also conditions that determine the parasite community structure (Prevedello et al. 2005; Ter Hofstede and Fenton 2005; Krasnov and Poulin 2010). Regarding the sex of bats, female bats may be more parasitized because they are less active, stay longer in the shelters, and form colonies during their reproductive period (Marshall 1982; Komeno and Linhares 1999).
Many populations of bat ectoparasites such as, mites, dipterans, and ticks, feed on their hair follicles, body fluids, or blood (Whitaker et al. 2009). Diptera (of the Streblidae and Nycteribiidae families) are hematophagous and exclusive bat parasites (Dick 2007). In Brazil, 98 species of Streblidae (Graciolli 2022) and 27 species of Nycteribiidae are known (Graciolli and Hrycyna 2022).
In this study, we aimed to characterize the community of ectoparasites associated with bats in urban green areas in northeastern Brazil, describe the specificity indices and parasitological rates, verify parasite aggregation in the hosts, identify infracommunities, and evaluate the influence of host sex, and environmental conditions (precipitation and temperature) on parasitological rates. Our hypotheses were the following: (1) female bats are more parasitized than males owing to the longer time spent in their roosts (Komeno and Linhares 1999); and (2) periods with lower rainfall and higher temperatures lead to an increase in parasitism rates due to the increase in the reproductive rate of the parasites in these conditions (Rui and Graciolli 2005).
Material and methods
Study area
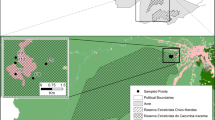
This study was conducted in three urban green areas in Grande Aracaju, Sergipe (Fig. 1). The sampled areas belong to the Campus São Cristóvão da Universidade Federal de Sergipe (UFS; 10° 55′ 34.3″ S; 37° 06′ 09.2″ W), Secretaria de Estado da Fazenda de Sergipe (SEFAZ; 10° 54′ 38.8″ S; 37° 05′ 27.9″ W), and Vila Militar dos Oficiais do Exército (Vila; 10° 55′ 31.6″ S; 37° 03′ 36.7″ W). The minimum distances between the areas are 2.15, 4.17, and 3.5 km from UFS to SEFAZ, UFS to Vila, and Vila to SEFAZ, respectively. The UFS area is a remnant of the Atlantic Forest, encompassing approximately 0.99 ha, and is located next to a fragment of the native forest associated with the Poxim River. This area has dense vegetation, a closed canopy, and intense artificial light surrounding the fragment. The SEFAZ area is equivalent to a fragment of approximately 1.58 ha and is characterized by a banana plantation (Musa sp.) with low vegetation and a dense understory. However, other areas within SEFAZ contain a higher diversity of plant species, less dense understory, and a closed canopy. In the Vila area, the green area is approximately 1.56 ha, characterized by the predominance of widely spaced almond trees (Terminalia catappa L.), which can reach heights of 12 to 15 m; this area lacks an understory and closed canopy (personal observation). During the study period (September 2019 to February 2021), the accumulated precipitation was 1773.4 mm, with an average temperature range of 24.7 to 29.3 °C. December 2019 had the lowest rainfall (8 mm) and April 2020 had the highest (284.6 mm) (INMET 2021).
Data collection and analysis
In the UFS and SEFAZ areas, campaigns were conducted in September and November 2019; January, June, July, August, September, and November 2020; and January 2021. In the Vila area, campaigns were conducted in September and November 2019, January and December 2020, and January and February 2021. These campaigns were conducted monthly, over two consecutive nights, in each area. It should be noted that the irregularity of the field campaigns occurred because of the quarantine related to the COVID-19 pandemic beginning in March 2020. Therefore, campaigns were suspended in March 2020 and continued in June 2020 in the UFS and SEFAZ areas and only resumed in the Vila area in December 2020, as it is a residential area.
To capture bats, ten mist nets (9 × 3 m, mesh 20 mm) were placed on the inside and edge of the green areas, which remained open between sunset and midnight. These nets were inspected every 30 min, and each captured animal was marked with a numbered ring on the left forearm and released to check for possible recaptures between the areas. The bats were then identified based on the methods described by Díaz et al. (2016) and Reis et al. (2017). Each bat was inspected for ectoparasitic flies, which were collected manually and stored in microtubes containing 70% ethyl alcohol at 92.8° INPM. Ectoparasitic flies species were identified under a stereomicroscope (BEL Engineering; SZT model), according to the morphological characteristics described by Guerrero (1994a, b; 1995).
The specificity index (SI) of the ectoparasites was calculated according to Dick and Gettinger (2005). Prevalence rates (P; %) and mean infestation intensity (MI) were obtained according to Bush et al. (1997) with their respective confidence intervals (CI; 95%). The discrepancy index (D) was calculated to analyze parasite aggregation on the hosts (Poulin 1993). These rates were calculated using the Quantitative Parasitology software (QPweb) (Reiczigel et al. 2019). Primary associations (P > 5%) between two or more ectoparasitic flies species in the same host were identified and characterized as infracommunities.
For statistical analysis, only the most representative bat species (n ≥ 30) and the primary associations were considered. The influence of host sex on the prevalence rates was verified using the chi-square test, and the mean intensity was verified using the t test. A general linear model (GLM) was used to analyze the influence of precipitation and temperature on the prevalence rates and mean infestation intensity. Data normality was tested using the Shapiro–Wilk test, and statistical analyses were performed using R software 4.1 (R Core Team 2021), with a significance level of 5%.
Results
A total of 568 bats were captured, where only 278 (48.94%) were parasitized. The parasitized bats were among ten species that belong to the Phyllostomidae (S = 8) and Vespertilionidae (S = 2) families. A total of 875 bat flies were collected, and they comprised 13 species of the Streblidae family (n = 849) and one species of the Nycteribiidae family (n = 26; Table 1). The most parasitized bats in relation to the total were Artibeus lituratus (Olfers, 1818) (n = 101; 36.3%), Artibeus planirostris (Spix, 1823) (n = 53; 19.1%), Platyrrhinus lineatus (É. Geoffroy, 1810) (n = 35; 12.6%), and Phyllostomus discolor (Wagner, 1843) (n = 32; 11.5%). The single most parasitized host was a representative of P. discolor, on which there were 23 individuals of Trichobius costalimai (Guimarães, 1937) and two individuals of Trichobioides perspicillatus (Pessôa and Galvao, 1937). The most abundant ectoparasite species were T. costalimai (28.8%) and Megistopoda aranea Coquillett, 1899 (13.6%).
Four of the registered associations were considered non-primary (P < 5%; Table 1). Considering only the primary associations, the highest prevalence values were obtained for the interactions between Myotis lavali Moratelli, Peracchi, Dias and Oliveira, 2011 and Basilia travassosi Guimarães, 1938 (85.7%), Myotis riparius Handley, 1960 and B. travassosi, P. discolor and T. costalimai, and Phyllostomus hastatus (Pallas, 1767) and Trichobius longipes (Rudow, 1871) (all 100%). The highest mean intensity values were found for P. discolor and T. costalimai (8.5), and P. hastatus and T. longipes (13.0). Regarding the specificity index, 11 associations (36.3%) had values greater than 90%. For the discrepancy index, 62.5% of the parasite-host associations presented a value greater than 0.70 (Table 1). Infracommunities were recorded on 18.3% of the parasitized bats, all of which were composed of individuals from different genera. The most recorded infracommunities were Paratrichobius longicrus (Miranda-Ribeiro, 1907) and Trichobius sp. (dugesii complex) parasitizing A. lituratus, and T. perspicillatus and T. costalimai parasitizing P. discolor (Table 2).
The influence of host sex on parasitological rates is shown in Table 3. A difference was observed only in the prevalence of the associations between A. lituratus and Trichobius sp. (complexo dugesii) (χ2 = 4.48; p = 0.049), Artibeus obscurus (Schinz, 1821) and M. aranea (χ2 = 7.72; p = 0.007), and Artibeus planirostris (Spix, 1823) and M. aranea (χ2 = 4.03; p = 0.049) in which the highest parasite numbers were obtained from females. The influence of temperature on the prevalence (F = 5.35; p = 0.04) and mean intensity (F = 5.02; p = 0.04) of the interaction between A. lituratus and P. longicrus, showed a decrease in parasitological rates during periods with higher temperatures. For the interaction between Platyrrhinus lineatus and Trichobius angulatus Wenzel, 1976 (F = 7.70; p = 0.02), temperature only influenced the prevalence rate, with the same interaction pattern observed between A. lituratus and P. longicrus. The mean intensity obtained for the interaction between P. discolor and T. costalimai was influenced by both precipitation (F = 9.04; p = 0.02) and temperature (F = 24.58; p = 0.002), indicating that the mean intensity decreased with increasing temperature, and prevalence rate increased with increasing precipitation (Fig. 2).
Relationship between temperature (°C), precipitation (mm), and prevalence rates (%) and mean intensity for interactions between (a) Artibeus lituratus and Paratrichobius longicrus, (b) Platyrrhinus lineatus and Trichobius angulatus, and (c) Phyllostomus discolor and Trichobius costalimai observed in urban green areas of the Grande Aracaju, Sergipe
Discussion
All ectoparasites recorded in this study have been previously reported in the State of Sergipe (Bezerra et al. 2016; Soares et al. 2017; Bezerra and Bocchiglieri 2018; Barbier et al. 2019). The most representative family in this study is Streblidae (97%); this family of ectoparasites is commonly found in association with phyllostomid bats (Graciolli and Aguiar 2002; Prevedello et al. 2005); thus, the high representation of Streblidae in this study may be related to the high capture rate of bats belonging to the Phyllostomidae family. Several species of this family often inhabit disturbed and fragmented habitats and are well-adapted to landscapes dominated by plantations, secondary vegetation, and urban environments (Bertola et al. 2005; Reis et al. 2007; Esbérard et al. 2014; Herr et al. 2015; Nunes et al. 2017). In addition, the plant species used in urban afforestation is a potential food source for these bats (Müller and Reis 1992; Zortéa and Chiarello 1994; Reis et al. 2011), contributing to their occurrence in these types of environments.
The richness of bat flies found in this study (S = 14) was lower than that recorded by Bezerra et al. (2016), Soares et al. (2017), Bezerra and Bocchiglieri (2018), and Barbier et al. (2019) whose studies were conducted in the preserved areas of the Atlantic Forest of Sergipe. Compared to urban areas, the richness found in this study was equivalent to that recorded by Urbieta et al. (2018) (S = 14) in Cerrado and lower than that recorded by Palheta et al. (2020) (S = 21) in Amazon. These variations in the richness of ectoparasites may be associated with different sampling efforts and the environmental conditions of the areas (Pilosof et al. 2012). Furthermore, Bertola et al. (2005) and Dick and Gettinger (2005) reported that a greater richness of ectoparasites may be directly related to a greater richness of hosts. Thus, the highest richness of ectoparasites recorded by Palheta et al. (2020) might be associated with the greater richness of bats captured at the study site in area of Amazon remnants. Despite the lower sampling effort, the similarity in the richness of ectoparasitic flies in relation to the study conducted by Urbieta et al. (2018) may be associated with the greater abundance of bats recorded in the sampled area, e.g., Artibeus lituratus, Artibeus planirostris, and Platyrrhinus lineatus. This may have allowed for a better sampling of the parasites, since the more bats examined, the greater the chance of associations being recorded.
The most parasitized bats were A. lituratus, A. planirostris, P. lineatus, and P. discolor; this may be associated with the high number of captures of these species in the sampled areas. These species have been frequently reported in urban environments, and their occurrence may be related to the availability of food resources (insects attracted by the lighting in urban areas and planting of fruit trees) (Barros et al. 2006; Ferreira et al. 2010; Nunes et al. 2017; Leal et al. 2019). The highest parasite load was found on P. discolor, which use more durable and less exposed roosts (caves, tree hollows, and human dwellings), and may present a greater number of ectoparasites, as the increased durability and decreased exposure of their roosts increase the probability of the hosts being present at the time of the parasite outbreak (Ter Hofstede and Fenton 2005; Willig et al. 2007; Reis et al. 2017). Additionally, the most abundant ectoparasites were T. costalimai and M. aranea; this may be associated with the high abundance of their hosts, P. discolor and Artibeus spp., respectively. Such associations have also been commonly reported in other studies in different ecoregions (Graciolli and Rui 2001; Bezerra et al. 2016; Hrycyna et al. 2019; Barbier et al. 2021). The species P. discolor has a generalist diet, which may favor its occurrence in urban areas (Kwiecinski 2006); whereas species of the genus Artibeus are considered opportunistic, with varied feeding habits, and may benefit from fruit plants used in local trees and public roads in Aracaju, accounting for their high occurrence in these environments (Zortéa and Chiarello 1994; Barros et al. 2006; Santos et al. 2015).
Regarding parasitological rates, apart from the interaction between P. discolor and T. costalimai, other interactions may have overestimated values because of the low number of captured hosts. For the specificity index, only 41.6% of the associations presented a value greater than 90%, indicating low specificity; this was also reported by Santos et al. (2013), Bezerra and Bocchiglieri (2018), and Palheta et al. (2020). The parasitological rates and specificity indices showed variations when compared to other studies (P % = 0.6 to 100; MI = 1.0 to 20.0) (Santos et al. 2013; França et al. 2013; Palheta et al. 2020; Biz et al. 2021), including areas of the Atlantic Forest in the State of Sergipe, which may be lower or higher than those previously reported (Bezerra et al. 2016; Soares et al. 2017; Bezerra and Bocchiglieri 2018; Barbier et al. 2019).
Low specificity and may be a result of habitat degradation, where very fragmented areas with low shelter availability can cause multiple species to share a single roost (Cottontail et al. 2009). This could result in an increased contact between different bat species, favoring the transmission of ectoparasites (Cottontail et al. 2009). Overall, the degree of specificity can present great variation (1.0 to 100). This is due to the adaptation processes of the host and the phylogeny of the parasite and host (Balashov 1984), since the presence of the same species of parasite on more than one host species host can occur because of the inheritance of genetic characteristics from the ancestor (Poulin and Rohde 1997). The physical approach of the hosts is necessary for parasitism to occur, and it is believed that most of the parasites are highly specific (Marshall 1982), with high specificity being a frequent feature in the relationship between ectoparasites and bats (Fritz 1983; Giorgi et al. 2004). However, previous studies in urban areas have reported that lower parasite specificity is possible, as the low quality and availability of shelters would favor contact between different bat species and increase the transmission of ectoparasitic flies between them (Barbier and Graciolli 2016; Urbieta et al. 2018). In addition, the non-primary associations can be the result of contamination or occasional infestation, which could have occurred during the capture of the animals or through the sharing of shelters between different host species (Graciolli and Carvalho 2001; Dick 2007; Aguiar and Antonini 2016), thus, may can explain the registered associations considered non-primary 33.3% (n = 8).
Differences in parasitological rates may also be due to variations in the richness and abundance of the bat community, the biogeographic history of the area, to host size, and roost characteristics (Rui and Graciolli 2005; Hiller et al. 2018). In addition, environmental differences between areas, such as temperature and precipitation, directly affect bat flies (Eriksson et al. 2020), where their rate of development may be favored in places with higher temperatures and lower rainfall (Gray et al. 2009; Pilosof et al. 2012). This can influence the survival of pupae, reducing the abundance of parasites on hosts that use exposed shelters (Dittmar et al. 2009; Pilosof et al. 2012).
Previous studies suggest that a decrease in prevalence rates and average intensities could be due to poor roost quality and availability in urban environments (Urbieta et al. 2018), which would cause bats to have less fidelity to the shelter, making parasite infestation difficult (Barbier and Graciolli 2016; Urbieta et al. 2018). However, some bat species may be favored in places with a higher degree of anthropization (Willig et al. 2007) while other species may be unable to explore the urban matrix, and are therefore restricted to vegetation fragments (Nunes et al. 2017), thus, consequently increasing their local density (Medellín et al. 2000; Willig et al. 2007). Therefore, an increase in density promotes greater aggregation of individuals, facilitating the transmission of flies and resulting in greater infestation (Pilosof et al. 2012).
Regarding the discrepancy index, most interactions (> 60%) presented high aggregation values, corroborating the findings of Barbier and Graciolli (2016) and Barbier et al. (2019). These high values indicate a disproportionate distribution of parasites on the hosts, where most of the parasites occur in groups and are found in a minority of hosts, i.e., most hosts are parasitized by a few parasites, and only a few hosts are parasitized by many parasites. This form of distribution is known as an aggregate (Dajoz 2005; Barbier and Graciolli 2016; Barbier et al. 2019).
All infracommunities identified and the associations found in this study have been previously recorded (Bezerra et al. 2016; Dornelles and Graciolli 2017; Bezerra and Bocchiglieri 2018), and were all composed of parasite species of different genera. Infracommunities can be molded by limiting the similarity or environmental filtering (Ingram and Shurin 2009; Krasnov et al. 2014). Limiting similarity is characterized by the association of unrelated parasites that reduce competition among themselves because there is less niche overlap. This allows a few species to adapt and exploit a single host, which could result in associations between different species of the same genus that share ecological and adaptive characteristics (Ingram and Shurin 2009; Krasnov et al. 2014). Thus, the infracommunities observed in this study may be shaped by the limiting similarity. In addition, some studies have demonstrated that ectoparasites exhibit spatial segregation on the host body, where different parasite species show preferences for certain parts of the host, facilitating the coexistence of different species (Bittencourt and Rocha 2002; Almeida et al. 2015).
Regarding the influence of host sex on parasitism, the higher prevalence rates in females found in this study corroborated the findings of Bertola et al. (2005) and Bezerra and Bocchiglieri (2018), who found higher rates of parasitism in females of Sturnira lilium (E. Geoffroy, 1810), Carollia perspicillata (Linnaeus, 1758), and A. lituratus. The greater parasitism in some females may be related to them staying longer in the shelter, owing to their more gregarious behavior during the reproductive stage and the care of their offspring. Additionally, during reproduction, females may become less active and reduce their grooming intensity, which is one of the main causes of parasite mortality (Marshall 1982; Komeno and Linhares 1999; Schaik and Kerth 2017).
In this study, for some interactions, the opposite of what was expected was observed; specifically, a decrease in prevalence rates and average intensity with increasing temperature, and an increase in average intensity with increasing precipitation. This result may be associated with low-temperature range (24.7 to 29.3 °C) and precipitation (8.2 to 274 mm) in the region during the period of this study. In addition, the influence of seasonality may be related to the behavior of each species; this may harm the survival of some species and favor the development of others (Pilosof et al. 2012; Zarazúa-Carbajal et al. 2016). According to Marshall (1982), the temperature can directly affect the reproductive rate of some parasites, in addition to copulation, oviposition, egg survival, and adult longevity. Considering the importance of parasitism in the control and maintenance of the host community, analyzing the influence of host characteristics on the abundance and distribution of ectoparasites is important to provide information on the aspects that shape this interaction. Thus, this study provides relevant data on this interaction, especially for urban areas in northeastern Brazil, increasing the number of studies in Sergipe and promoting future studies.
Data availability
Not applicable.
References
Aguiar LMS, Antonini Y (2016) Prevalence and intensity of Streblidae in bats from a Neotropical savanna region in Brazil. Folia Parasitol 63:1–8. https://doi.org/10.14411/fp.2016.024
Almeida J, Serra-Freire N, Peracchi A (2015) Anatomical location of Periglischrus iheringi (Acari: Spinturnicidae) associated with the great fruit-eating bat (Chiroptera: Phyllostomidae). Rev Bras Parasitol Vet 24:361–364. https://doi.org/10.1590/S1984-29612015022
Balashov YS (1984) Interaction between blood-sucking arthropods and their hosts, and its influence on vector potential. Annu Rev Entomol 29:137–150
Barbier E, Graciolli G (2016) Community of bat flies (Streblidae and Nycteribiidae) on bats in the Cerrado of Central-West Brazil: hosts, aggregation, prevalence, infestation intensity, and infracommunities. Stud Neotrop Fauna Environ 51:176–187. https://doi.org/10.1080/01650521.2016.1215042
Barbier E, Urbieta GL, Nunes H, Bomfim SS, Da Rocha PA (2019) High specificity and aggregation, but the low prevalence in bat-fly interactions in an environmental protection area in Brazil. Acta Chiropt 21:443–452. https://doi.org/10.3161/15081109ACC2019.21.2.018
Barbier E, Falcão F, Bernard E (2021) Bat-ectoparasitic fly relationships in a seasonally dry tropical forest in Brazil. Parasitol Res 120:3507–3517. https://doi.org/10.1007/s00436-021-07301-w
Barros RD, Bisaggio EL, Borges RC (2006) Morcegos (Mammalia, Chiroptera) em fragmentos florestais urbanos no município de Juiz de Fora, Minas Gerais, sudeste do Brasil. Biota Neotrop 6:1–6
Bertola PB, Aires CC, Favorito SE, Graciolli G, Amaku M, Rocha RP (2005) Bat flies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: Chiroptera) at Parque Estadual da Cantareira, São Paulo, Brazil: parasitism rates and host-parasite associations. Mem Inst Oswaldo Cruz 100:25–32
Bezerra RHS, Bocchiglieri A (2018) Association of ectoparasites (Diptera and Acari) on bats (Mammalia) in a resting habitat in northeastern Brazil. Parasitol Res 117:3413–3420. https://doi.org/10.1007/s00436-018-6034-0
Bezerra RHS, Vasconcelos PF, Bocchiglieri A (2016) Ectoparasites of bats (Mammalia: Chiroptera) in Atlantic forest fragments in northeastern Brazil. Parasitol Res 115:3759–3765. https://doi.org/10.1007/s00436-016-5137-8
Bittencourt EB, Rocha CD (2002) Spatial use of rodents (Rodentia: Mammalia) host body surface by ectoparasites. Braz J Biol 62:419–425
Biz LS, Cascaes MF, Luciano BFL, Preuss G, Bôlla DAS, Graciolli G, Carvalho F (2021) Parasitic interactions between bats (Mammalia: Chiroptera) and flies (Insecta: Diptera) in the intersection area of temperate and tropical climates in Brazil. Stud Neotrop Fauna Environ 1-10. https://doi.org/10.1080/01650521.2020.1869490
Bolívar-Cime B, Cuzim-koyoc A, Reyes-Novelo E, Morales-Malacara JB, Laborde J, Flores-Peredo R (2018) Habitat fragmentation and the prevalence of parasites (Diptera, Streblidae) on three phyllostomid bat species. Biotropica 50:90–97. https://doi.org/10.1111/btp.12489
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its terms: Margolis et al revisited. J Parasitol 83:575–583
Cottontail VM, Wellinghausen N, Kalko EKV (2009) Habitat fragmentation and haemoparasites in the common fruit bat, Artibeus jamaicensis (Phyllostomidae) in a tropical lowland forest in Panamá. Parasitol 136:1133–1145. https://doi.org/10.1017/S0031182009990485
Dajoz R (2005) As populações, as comunidades e os fatores ecológicos. In: Murrad F, Oliveira PL (eds) Princípios de ecologia, 7rd edn. Porto Alegre, Artmed 113–132
Díaz MM, Solari S, Aguirre LF, Aguiar LMS, Barquez RM (2016) Clave de identificação de los murciélagos de Sudamérica. Publicación especial no. 2, Programa de Conservación de los murciélagos de Argentina
Dick CW (2007) High host specificity of obligate ectoparasites. Ecol Entomol 32:446–450
Dick CW, Gettinger D (2005) A faunal survey of streblidae flies (Diptera: Streblidae) associated with bats in Paraguay. J Parasitol 91:1015–1024. https://doi.org/10.1645/GE-536R.1
Dittmar K, Dick CW, Patterson BD, Whiting MF, Gruwell ME (2009) Pupal deposition and ecology of bat flies (Diptera: Streblidae): Trichobius sp. (caecus group) in a Mexican cave habitat. J Parasitol 95:308–314. https://doi.org/10.1645/GE-1664.1
Dittmar K, Morse SF, Dick CW, Patterson BD (2015) Bat flies evolution from the Eocene to the present (Hippoboscoidea, Streblidae and Nycteribiidae). In: Morand S, Krasnov BR, Littlewood DTJ (eds) Parasite diversity and diversification: evolutionary ecology meets phylogenetics. Cambridge University Press, Cambridge, pp 246–264
Dornelles GDP, Graciolli G (2017) Streblid bat flies on phyllostomid bats from an island off the coast of Sao Paulo Brazil. Pap Avulsos Zool 57:31–36. https://doi.org/10.11606/0031-1049.2017.57.04
Dos Santos CLC, Pereira ACN, Bastos VJC, Graciolli G, Rebêlo JMM (2013) Parasitism of ectoparasitic flies on bats in the northern Brazilian Cerrado. Acta Parasitol 58:207–214. https://doi.org/10.2478/s11686-013-0135-9
Eriksson A, Doherty JF, Fischer E, Graciolli G, Poulin R (2020) Hospedeiros e ambiente ofuscam a distância espacial como condutores da composição de espécies de morcegos nos Neotrópicos. J Biogeogr 47:736–747. https://doi.org/10.1111/jbi.13757
Esbérard CEL, Biavatti TC, Carvalho WD, Costa LDM, Godoy MDSA, Gomes LAC, Luz JL, Pol A, Silva EP, Tato GK, Graciolli G (2014) Trichobius longipes (Diptera, Streblidae) as a parasite of Phyllostomus hastatus (Chiroptera, Phyllostomidae). Rev Bras Parasitol Vet 23:315–319. https://doi.org/10.1590/S1984-29612014066
Ferreira CMM, Fischer E, Pulchério-Leite A (2010) Bat fauna in urban remnants of Cerrado in Campo Grande, Mato Grosso do Sul. Biota Neotrop 10:155–160
França DS, Pereira SN, Maas ACS, Martins MA, Bolzan DP, Lima IP, Dias D, Peracchi AL (2013) Ectoparasitic flies (Diptera, Streblidae) of bats (Chiroptera, Phyllostomidae) in an Atlantic Forest area, southeastern Brazil. Braz J Biol 73:847–854. https://doi.org/10.1590/S1519-69842013000400022
Frank HK, Mendenhall CD, Judson SD, Daily GC, Hadly EA (2016) Anthropogenic impacts on Costa Rican bat parasitism are sex specific. Ecol Evol 6:4898–4909. https://doi.org/10.1002/ece3.2245
Fritz GN (1983) Biology and ecology of bat flies (Diptera: Streblidae) on bats in the genus Carollia. J Med Entomol 20:1–10
Giorgi MS, Arlettaz R, Guillaume F, Nusslé S, Ossola C, Vogel P, Christe P (2004) Causal mechanisms underlying host specificity in bat ectoparasites. Oecologia 138:648–654
Graciolli G (2022) Streblidae in Catálogo Taxonômico da Fauna do Brasil. PNUD. http://fauna.jbrj.gov.br/fauna/faunadobrasil/2624. Accessed 21 september 2022
Graciolli G, Aguiar LS (2002) Ocorrência de moscas ectoparasitas (Diptera, Streblidae e Nycteribiidae) de morcegos (Mammalia, Chiroptera) no Cerrado de Brasília, Distrito Federal, Brasil. Rev Bras Zool 19:177–181
Graciolli G, Carvalho CJB (2001) Moscas ectoparasitas (Diptera, Hippoboscoidea, Nycteribiidae) de morcegos (Mammalia, Chiroptera) do Estado do Paraná. II. Streblidae. Chave pictórica para gêneros e espécies. Rev Bras Zool 18:907–960
Graciolli G, Rui AM (2001) Streblidae (Diptera, Hippoboscoidea) em morcegos (Chiroptera, Phyllostomidae) no nordeste do Rio Grande do Sul, Brasil. Iheringia, Sér Zool 90:85–92
Graciolli G, Hrycyna G (2022) Nycteribiidae in Catálogo Taxonômico da Fauna do Brasil. PNUD. http://fauna.jbrj.gov.br/faana/faunadobrasi l/1145. Accesse 26 june 2022
Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E (2009) Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infec Dis 2009:1–12. https://doi.org/10.1155/2009/593232
Guerrero R (1994a) Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. II. Los grupos: pallidus, caecus, major, uniformis, y longipes del gênero Trichobius Gervais, 1844. Acta Biol Venez 15:1–18
Guerrero R (1994b) Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. IV. Trichobiinae com alas desarrolladas. Acta Biol Venez 9:161–192
Guerrero R (1995) Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. V. Trichobiinae con alas reducidas o ausentes y miscelaneos. Bol Entomol Venez 10:135–160
Heer K, Helbig-Bonitz M, Fernandes RG, Mello MA, Kalko EK (2015) Effects of land use on bat diversity in a complex plantation–forest landscape in northeastern Brazil. J Mammal 96:720–731. https://doi.org/10.1093/jmammal/gyv068
Hiller T, Honner B, Page RA, Tschapka M (2018) Leg structure explains host site preference in bat flies (Diptera: Streblidae) parasitizing neotropical bats (Chiroptera: Phyllostomidae). Parasitol 145:1475–1482. https://doi.org/10.1017/S0031182018000318
Hrycyna G, Martins ACM, Graciolli G (2019) Infracomunidades de moscas ectoparasitas (Diptera: Streblidae e Nycteribiidae) de morcegos (Mammalia: Chiroptera) em três unidades de conservação no Estado do Amapá, Brasil. Biota Neotrop 19:1–9. https://doi.org/10.1590/1676-0611-BN-2018-0715
Ingram T, Shurin JB (2009) Trait-based assembly and phylogenetic structure in northeast Pacific rockfish assemblages. Ecol 90:2444–2453. https://doi.org/10.1890/08-1841.1
INMET (2021) Dados históricos anuais. Instituto Nacional de Meteorologia. https://portal.inmet.gov.br/dadoshistoricos. Accessed 1 june 2022
Komeno CA, Linhares AX (1999) Bat flies parasitic on some phyllostomid bats in southeastern Brazil: parasitism rates and host-parasite relationships. Mem Inst Oswaldo Cruz 94:151–156
Krasnov BR, Poulin R (2010) Ecological properties of a parasite: species-specific stability and geographical variation. In: Krasnov BR, Morand S (eds) The biogeography of host–parasite interactions. Oxford University Press, Oxford, pp 99–113
Krasnov BR, Pilosof S, Stanko M, Morand S, Korallo-Vinarskaya NP, Vinarski MV, Poulin R (2014) Co-occurrence and phylogenetic distance in communities of mammalian ectoparasites: limiting similarity versus environmental filtering. Oikos 123:63–70. https://doi.org/10.1111/j.1600-0706.2013.00646.x
Kwiecinski GG (2006) Phyllostomus Discolor. Mamm Species 801:1–11. https://doi.org/10.1644/801.1
Lafferty KD, Kuris AM (2005) Parasitism and environmental disturbances. In: Thomas F, Renaud F, Guegan J (eds) Parasitism and ecosystems. Oxford University Press, New York, pp 113–124
Leal ESB, Guerra-Filho DQ, Ramalho DF, Silva JM, Bandeira RS, Silva LAM, Oliveira MAB (2019) Bat Fauna (Chiroptera) in an urban environment in the Atlantic Forest, northeastern Brazil. Neotrop Biol Conserv 14:55–82. https://doi.org/10.3897/neotropical.14.e34837
Marshall AG (1982) Ecology of insects ectoparasitic on bats. In: Kunz TH (ed) Ecology of bats. Plenum Press, New York, USA, pp 369–401
Medellín RA, Equihua M, Amin MA (2000) Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conserv Biol 14:1666–1675
Müller MF, Reis NR (1992) Partição de recursos alimentares entre quatro espécies de morcegos frugívoros (Chiroptera, Phyllostomidae). Rev Bras Zool 9:345–355
Nunes H, Rocha FL, Cordeiro-Estrela P (2017) Bats in urban areas of Brazil: roosts, food resources and parasites in disturbed environments. Urban Ecosyst 20:953–969. https://doi.org/10.1007/s11252-016-0632-3
Palheta LR, Urbieta GL, Brasil LS, Dias-Silva K, Da Silva JB, Graciolli G, Aguiar LMS, Vieira TB (2020) The effect of urbanization on bats and communities of bat flies (Diptera: Nycteribiidae and Streblidae) in the Amazon, northern Brazil. Acta Chiropt 22:403–416. https://doi.org/10.3161/15081109ACC2020.22.2.014
Pilosof S, Dick CW, Korine C, Patterson BD, Krasnov BR (2012) Effects of anthropogenic disturbance and climate on patterns of bat fly parasitism. PlosOne 7:1–7. https://doi.org/10.1371/journal.pone.0041487
Poulin R (1993) The disparity between observed and uniform distributions: a new look at parasite aggregation. Int J Parasitol 23:937–944
Poulin R, Rohde K (1997) Comparing the richness of metazoan ectoparasite communities of marine fishes: controlling for host phylogeny. Oecologia 110:278–283
Prevedello JA, Graciolli G, Carvalho CJB (2005) A fauna de dípteros (Streblidae e Nycteribiidae) ectoparasitos de morcegos (Chiroptera) do Estado do Paraná, Brasil: composição e distribuição e áreas prioritárias para novos estudos. Biociências 13:193–209
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 15 june 2022
Ramalho DF, Graciolli G, Aguiar LMS (2018) Bat fly (Diptera: Streblidae) parasitism in degraded and preserved areas in a neotropical savanna. Mastozool Neotrop 25:245–250. https://doi.org/10.31687/saremMN.18.25.1.0.21
Ramalho DF, Diniz UM, Aguiar L (2021) Anthropization affects the assembly of bat-bat fly interaction networks. Front Environ Sci 9:1–8
Reiczigel J, Marozzi M, Fábián I, Rózsa L (2019) Biostatistics for parasitologists: a primer to quantitative parasitology. Trends Parasitol 34:277–281. https://doi.org/10.1016/j.pt.2019.01.003
Reis NR, Shibatta OA, Peracchi AL, Pedro WA, Lima IP (2007) Sobre os morcegos brasileiros. Reis NR, Peracchi AL, Pedro WA, Lima IP (orgs) Morcegos do Brasil. Editora da Universidade Estadual de Londrina, Londrina, pp 17–25
Reis NR, Peracchi AL, Batista CB, Lima IP, Pereira AD, (org), (2017) História Natural dos Morcegos Brasileiros. Technical Books Editora, Rio de Janeiro
Reis NR, Shibatta OA, Peracchi AL, Pedro WA, Lima IP (2011) Sobre os mamíferos do Brasil. In: Reis NR, Peracchi AL, Pedro WA, Lima IP (eds) Mamíferos do Brasil, 2rd edn. Londrina, Nelio R. dos Reis 23–29
Rui AM, Graciolli G (2005) Moscas ectoparasitas (Diptera, Streblidae) de morcegos (Chiroptera, Phyllostomidae) no sul do Brasil: associações hospedeiros-parasitos e taxas de infestação. Rev Bras Zool 22:438–445
Santos CZA, Ferreira RA, Santos LR, Santos LI, Gomes SH, Graça DAS (2015) Análise qualitativa da arborização urbana de 25 vias públicas da cidade de Aracaju-SE. Ciênc Florest 25:751–763
Schaik JV, Kerth G (2017) Host social organization and mating system shape parasite transmission opportunities in three European bat species. Parasito Res 116:589–599. https://doi.org/10.1007/s00436-016-5323-8
Soares FAM, Rocha PA, Mikalauskas JS, Graciolli G, Ferrari SF (2017) Ectoparasitic bat flies (Diptera, Streblidae) of bats (Chiroptera, Mammalia) from Mata do Junco Wildlife Refuge, Sergipe, northeastern Brazil. Oecologia Aust 12:385–395. https://doi.org/10.4257/oeco.2017.2104.03
Ter Hofstede HM, Fenton MB (2005) Relationships between roost preferences, ectoparasite density, and grooming behavior of neotropical bats. J Zool 266:333–340. https://doi.org/10.1017/S095283690500693X
Tinsley RC, York JE, Everard ALE, Stott LC, Chapple SJ, Tinsley MC (2011) Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on egg development. Parasitol 138:1029–1038
Urbieta GL, Torres JM, Almeida LBM, Shinohara A, Anjos EC (2014) Infestação de morcegos (Mammalia, Chiroptera) por moscas do gênero Megistopoda (Diptera, Streblidae) em um fragmento urbano de Cerrado de Campo Grande, Mato Grosso do Sul. Bol Soc Bras Mastozool 69:10–13
Urbieta GL, Torres JM, Carvalho Dos Anjos EA, Espinola Carvalho CM, Graciolli G (2018) Parasitism of bat flies (Nycteribiidae and Streblidae) on bats in the urban environments: lower prevalence, infracommunities, and specificity. Acta Chiropt 20:511–518. https://doi.org/10.3161/15081109ACC2018.20.2.021
Urbieta GL, Graciolli G, Vizentin-Bugoni J (2021) Modularity and specialization in bat-fly interaction networks are remarkably consistent across patches within urbanized landscapes and spatial scales. Curr Zool 67:403–410. https://doi.org/10.1093/cz/zoaa072
Whitaker JO Jr, Ritzi CM, Dick CW (2009) Collecting and preserving bat ectoparasites for ecological study. In: Kunz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats, 2nd edn. The Johns Hopkins University Press, Baltimore, pp 806–827
Willig MR, Presley SJ, Bloch CP, Hice CL, Yanoviak SP, Díaz MM, Chauca LA, Pacheco V, Weaver SC (2007) Phyllostomid bats of lowland Amazonia: effects of habitat alteration on abundance. Biotropica 39:737–746. https://doi.org/10.1111/j.1744-7429.2007.00322.x
Zarazúa-Carbajal M, Saldaña-Vázquez RA, Sandoval-Ruiz CA, Stoner KE, Benitez-Malvido J (2016) The specificity of host-bat fly interaction networks across vegetation and seasonal variation. Parasitol Res 115:4037–4044. https://doi.org/10.1007/s00436-016-5176-1
Zortéa M, Chiarello AG (1994) Observations on the big fruit-eating bat Artibeus lituratus, in an urban reserve of southeast Brazil. Mammalia 58:665–670
Acknowledgements
We are grateful to the Universidade Federal de Sergipe (UFS), Secretaria de Estado da Fazenda de Sergipe, and Vila Militar dos Oficiais do Exército for logistical support.
Funding
This study is funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001 and AUXPE 2417/2013, 1941/2017, and process 88881.157961/2017–01.
Author information
Authors and Affiliations
Contributions
R.H.S.B.: methodology, validation, formal analysis, investigation, writing—original draft. A.B.: conceptualization, methodology, formal analysis, resources, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animals were captured, tagged, and when necessary collected, with permission granted by the Biodiversity Information and Authorization System/ICMBio (SISBIO # 71378–1 and 71378–5).
Consent to participate
Not applicable.
Consent for publication
All authors approve the final version and submission of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bezerra, R.H.S., Bocchiglieri, A. Ectoparasitic flies of bats (Mammalia: Chiroptera) in urban green areas of northeastern Brazil. Parasitol Res 122, 117–126 (2023). https://doi.org/10.1007/s00436-022-07703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07703-4