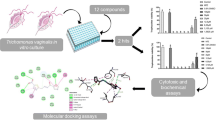
Abstract
Trichomoniasis is a great public health burden worldwide and the increase in treatment failures has led to a need for finding alternative molecules to treat this disease. In this study, we present in vitro and in silico analyses of two 2,8-bis(trifluoromethyl) quinolines (QDA-1 and QDA-2) against Trichomonas vaginalis. For in vitro trichomonacidal activity, up to seven different concentrations of these drugs were tested. Molecular docking, biochemical, and cytotoxicity analyses were performed to evaluate the selectivity profile. QDA-1 displayed a significant effect, completely reducing trophozoites viability at 160 µM, with an IC50 of 113.8 µM, while QDA-2 at the highest concentration reduced viability by 76.9%. QDA-1 completely inhibited T. vaginalis growth and increased reactive oxygen species production and lipid peroxidation after 24 h of treatment, but nitric oxide accumulation was not observed. In addition, molecular docking studies showed that QDA-1 has a favorable binding mode in the active site of the T. vaginalis enzymes purine nucleoside phosphorylase, lactate dehydrogenase, triosephosphate isomerase, and thioredoxin reductase. Moreover, QDA-1 presented a level of cytotoxicity by reducing 36.7% of Vero cells’ viability at 200 µM with a CC50 of 247.4 µM and a modest selectivity index. In summary, the results revealed that QDA-1 had a significant anti-T. vaginalis activity. Although QDA-1 had detectable cytotoxicity, the concentration needed to eliminate T. vaginalis trophozoites is lower than the CC50 encouraging further studies of this compound as a trichomonacidal agent.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Allsworth JE, Ratner JA, Peipert JF (2009) Trichomoniasis and other sexually transmitted infections: results from the 2001–2004 national health and nutrition examination surveys. Sex Transm Dis 36:738–744. https://doi.org/10.1097/OLQ.0b013e3181b38a4b
Alves MSD, dasNeves RN, Sena-Lopes A et al (2020) Antiparasitic activity of furanyl N-acylhydrazone derivatives against Trichomonas vaginalis: in vitro and in silico analyses. Parasit Vectors 13:59. https://doi.org/10.1186/s13071-020-3923-8
Ambrozio CL, Nagel AS, Jesk S et al (2016) Trichomonas vaginalis prevalence and risk factors for women in southern Brazil. Rev Inst Med Trop Sao Paulo 58:1–5. https://doi.org/10.1590/S1678-9946201658061
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:1–31
Barbosa-Lima G, Moraes AM, A da Araújo S et al (2017) 2,8-bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine. Eur J Med Chem 127:334–340. https://doi.org/10.1016/j.ejmech.2016.12.058
Bezerra-Souza A, Fernandez-Garcia R, Rodrigues GF et al (2019) Repurposing butenafine as an oral nanomedicine for visceral leishmaniasis. Pharmaceutics 11:353. https://doi.org/10.3390/pharmaceutics11070353
Birmann PT, Casaril AM, Hartwig D et al (2020) A novel pyrazole-containing selenium compound modulates the oxidative and nitrergic pathways to reverse the depression-pain syndrome in mice. Brain Res 1741:146880. https://doi.org/10.1016/j.brainres.2020.146880
Bosserman EA, Helms DJ, Mosure DJ et al (2011) Utility of antimicrobial susceptibility testing in Trichomonas vaginalis-infected women with clinical treatment failure. Sex Transm Dis 38:983–987. https://doi.org/10.1097/OLQ.0b013e318224db39
Briguglio I, Loddo R, Laurini E et al (2015) Synthesis, cytotoxicity and antiviral evaluation of new series of imidazo[4,5-g]quinoline and pyrido[2,3-g]quinoxalinone derivatives. Eur J Med Chem 105:63–79. https://doi.org/10.1016/J.EJMECH.2015.10.002
Brunet LR (2001) Nitric oxide in parasitic infections. Int Immunopharmacol 1:1457–1467. https://doi.org/10.1016/S1567-5769(01)00090-X
Bruni MP, Lopes ÂS, Stauffert D et al (2015) Trichomonas vaginalis infection among women attending frequency, risk factors and clinical signs. DST - J Bras De Doenças Sexualmente Transmissíveis 27:86–91. https://doi.org/10.5533/DST-2177-8264-2015273404
Casaril AM, Domingues M, Lourenço DDA et al (2020) 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole ameliorates long-lasting depression- and anxiogenic-like behaviors and cognitive impairment in post-septic mice: Involvement of neuroimmune and oxidative hallmarks. Chem Biol Interact 331:109278. https://doi.org/10.1016/j.cbi.2020.109278
Cheng WH, Huang KY, Huang PJ et al (2015) Nitric oxide maintains cell survival of Trichomonas vaginalis upon iron depletion. Parasit Vectors 8:393. https://doi.org/10.1186/s13071-015-1000-5
Cheng WH, Huang KY, Ong SC et al (2020) Protein cysteine S-nitrosylation provides reducing power by enhancing lactate dehydrogenase activity in Trichomonas vaginalis under iron deficiency. Parasit Vectors 13:477. https://doi.org/10.1186/s13071-020-04355-0
Choi CW, Kim SC, Hwang SS et al (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163:1161–1168
Colín-Lozano B, León-Rivera I, Chan-Bacab MJ et al (2017) Synthesis, in vitro and in vivo giardicidal activity of nitrothiazole-NSAID chimeras displaying broad antiprotozoal spectrum. Bioorg Med Chem Lett 27:3490–3494. https://doi.org/10.1016/j.bmcl.2017.05.071
Cudmore SL, Delgaty KL, Shannon F et al (2004) Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev 17:783–793. https://doi.org/10.1128/CMR.17.4.783
Das S, Huengsberg M, Shahmanesh M (2005) Treatment failure of vaginal trichomoniasis in clinical practice. Int J STD AIDS 16:284–286. https://doi.org/10.1258/0956462053654258
Diamond LS (1957) The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 43:488–490
dosGatti FAA, Ceolan E, Greco FSR et al (2017) The prevalence of trichomoniasis and associated factors among women treated at a university hospital in southern Brazil. PLoS ONE 12:e0173604. https://doi.org/10.1371/journal.pone.0173604
Esfandiari F, Motazedian MH, Asgari Q et al (2019) Erratum: Paromomycin-loaded mannosylated chitosan nanoparticles: synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop 197:105072. https://doi.org/10.1016/j.actatropica.2019.105072
Figueroa-Angulo EE, Estrella-Hernández P, Salgado-Lugo H et al (2012) Cellular and biochemical characterization of two closely related triosephosphate isomerases from Trichomonas vaginalis. Parasitology 139:1729–1738. https://doi.org/10.1017/S003118201200114X
Flagg EW, Meites E, Phillips C et al (2019) Prevalence of Trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis 46:e93. https://doi.org/10.1097/OLQ.0000000000001013
Frank LA, Contri RV, Beck RCR et al (2015) Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 7:623–639. https://doi.org/10.1002/wnan.1334
FDA (2021) FDA approved drug products. In: Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 10 Mar 2022
Grotto D, Maria LS, Valentini J et al (2009) Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova 32:169–174. https://doi.org/10.1590/S0100-40422009000100032
Hamann AR, de Kock C, Smith PJ et al (2014) Synthesis of novel triazole-linked mefloquine derivatives: biological evaluation against Plasmodium falciparum. Bioorg Med Chem Lett 24:5466–5469. https://doi.org/10.1016/j.bmcl.2014.10.015
Hanwell MD, Curtis DE, Lonie DC et al (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17. https://doi.org/10.1186/1758-2946-4-17
Harmych SE, Sidawy E, Komuniecki R (1996) Lactate dehydrogenase from the protozoan parasite, Trichomonas vaginalis. Comp Biochem Physiol b: Biochem Mol Biol 115:405–409. https://doi.org/10.1016/S0305-0491(96)00164-2
Hopper M, Yunfil J, Zhou B et al (2016) Auranofin inactivates Trichomonas vaginalis thioredoxin reductase and is effective against trichomonads in vitro and in vivo. Int J Antimicrob Agents 48:690–694. https://doi.org/10.1016/j.ijantimicag.2016.09.020
Katerji M, Filippova M, Duerksen-Hughes P (2019) Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev 2019:1–29
Kirkcaldy RD, Augostini P, Asbel LE et al (2012) Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD surveillance network, 2009–2010. Emerg Infect Dis 18:939–943. https://doi.org/10.3201/eid1806.111590
Leitsch D, Kolarich D, Binder M et al (2009) Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol Microbiol 72:518–536. https://doi.org/10.1111/j.1365-2958.2009.06675.x
Leitsch D, Kolarich D, Duchêne M (2010) The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol Biochem Parasitol 171:17–24. https://doi.org/10.1016/j.molbiopara.2010.01.001
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Mallo N, Lamas J, Sueiro RA, Leiro JM (2020) Molecular targets implicated in the antiparasitic and anti-inflammatory activity of the phytochemical curcumin in trichomoniasis. Molecules 25:5321. https://doi.org/10.3390/molecules25225321
Mavedzenge SN, van der Pol B, Cheng H et al (2010) Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis 37:460–466. https://doi.org/10.1097/OLQ.0b013e3181cfcc4b
McClelland RS, Sangaré L, Hassan WM et al (2007) Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 195:698–702. https://doi.org/10.1086/511278
Meites E, Gaydos CA, Hobbs MM et al (2015) A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 61:S837–S848. https://doi.org/10.1093/cid/civ738
Menezes CB, Frasson AP, Tasca T (2016) Trichomoniasis – are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microbial Cell 3:404–418. https://doi.org/10.15698/mic2016.09.526
Meyer Y, Buchanan BB, Vignols F, Reichheld J-P (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu Rev Genet 43:335–367. https://doi.org/10.1146/annurev-genet-102108-134201
Miranda-Ozuna JFT, Hernández-García MS, Brieba LG et al (2016) The glycolytic enzyme triosephosphate isomerase of Trichomonas vaginalis is a surface-associated protein induced by glucose that functions as a laminin- and fibronectin-binding protein. Infect Immun 84:2878–2894. https://doi.org/10.1128/IAI.00538-16
Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385:113–142. https://doi.org/10.1016/j.ijpharm.2009.10.018
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues thiobarbituric acid reaction. Anal Biochem 95:351–358
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera? A visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Rinaldo-Matthis A, Wing C, Ghanem M et al (2007) Inhibition and structure of Trichomonas vaginalis purine nucleoside phosphorylase with picomolar transition state analogues †. Biochemistry 46:659–668. https://doi.org/10.1021/bi061515r
Rocha-Garduño G, Hernández-Martínez NA, Colín-Lozano B et al (2020) Metronidazole and secnidazole carbamates: synthesis, antiprotozoal activity, and molecular dynamics studies. Molecules 25:793. https://doi.org/10.3390/molecules25040793
Sattar A, Chen D, Jiang L et al (2017) Preparation, characterization and pharmacokinetics of cyadox nanosuspension. Sci Rep 7:2289. https://doi.org/10.1038/s41598-017-02523-4
Schwebke JR, Barrientes FJ (2006) Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother 50:4209–4210. https://doi.org/10.1128/AAC.00814-06
Seña AC, Bachmann LH, Hobbs MM (2014) Persistent and recurrent Trichomonas vaginalis infections: epidemiology, treatment and management considerations. Expert Rev Anti Infect Ther 12:673–685. https://doi.org/10.1586/14787210.2014.887440
Sena-Lopes Â, das Neves RN, Bezerra FSB et al (2017) Antiparasitic activity of 1,3-dioxolanes containing tellurium in Trichomonas vaginalis. Biomed Pharmacother 89:284–287. https://doi.org/10.1016/j.biopha.2017.01.173
Setzer M, Byler K, Ogungbe I, Setzer W (2017) Natural products as new treatment options for trichomoniasis: a molecular docking investigation. Sci Pharm 85:5. https://doi.org/10.3390/scipharm85010005
Shin JW, Jung KH, Lee ST et al (2014) Mefloquine improved progressive multifocal leukoencephalopathy in a patient with immunoglobulin A nephropathy. J Clin Neurosci 21:1661–1664
Sosa AM, Álvarez AM, Bracamonte E et al (2020) Efficacy of topical treatment with (-)-epigallocatechin gallate, a green tea catechin, in mice with cutaneous leishmaniasis. Molecules 25:1–9. https://doi.org/10.3390/molecules25071741
Sumangala V, Poojary B, Chidananda N et al (2010) Synthesis and antimicrobial activity of 1,2,3-triazoles containing quinoline moiety. Arch Pharmacal Res 33:1911–1918. https://doi.org/10.1007/s12272-010-1204-3
Stanzione F, Giangreco I, Cole JC 2021 Use of molecular docking computational tools in drug discovery. In: Progress in Medicinal Chemistry. Elsevier B.V., pp 273–343. https://doi.org/10.1016/bs.pmch.2021.01.004.
Tiuman TS, Ueda-Nakamura T, Garcia Cortez DA et al (2005) Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother 49:176–182. https://doi.org/10.1128/AAC.49.11.176-182.2005
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
van der Pol B, Kwok C, Pierre-Louis B et al (2008) Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis 197:548–554. https://doi.org/10.1086/526496
Vique-Sánchez JL, Caro-Gómez LA, Brieba LG, Benítez-Cardoza CG (2020) Developing a new drug against trichomoniasis, new inhibitory compounds of the protein triosephosphate isomerase. Parasitol Int 76:102086. https://doi.org/10.1016/j.parint.2020.102086
Waterhouse A, Bertoni M, Bienert S et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
WHO (2012) Global incidence and prevalence of selected curable sexually transmitted infections - 2008. World Health Organization, Geneva
WHO (2018) Report on global sexually transmitted infection surveillance, 2018. World Health Organization, Geneva
Wierenga RK, Kapetaniou EG, Venkatesan R (2010) Triosephosphate isomerase: a highly evolved biocatalyst. Cell Mol Life Sci 67:3961–3982
Workowski KA, Bolan GA (2015) Sexually Transmitted Diseases Treatment Guidelines, 2015. Centers for Disease Control and Prevention (2015) MMWR 64 (RR03):1–137
Funding
This study was partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001—Ph.D. Scholarship (MSDA, grant number 88882.346971/2019–01).
Author information
Authors and Affiliations
Contributions
Conceptualization: Mirna Samara Dié Alves, Ângela Sena-Lopes, Sibele Borsuk; Methodology: Mirna Samara Dié Alves, Ângela Sena-Lopes, Angela Maria Casaril; Investigation: Mirna Samara Dié Alves, Raquel Nascimento das Neves, Emerson Teixeira da Silva, Angela Maria Casaril, Micaela Domingues, Paloma Taborda Birmann; Formal analysis: Mirna Samara Dié Alves; Writing – original draft: Mirna Samara Dié Alves; Writing – review & editing: All authors; Project administration: Mirna Samara Dié Alves, Ângela Sena-Lopes, Sibele Borsuk; Supervision: Ângela Sena-Lopes, Sibele Borsuk; and Resources: Emerson Teixeira da Silva, Marcus Vinicius Nora de Souza, Lucielli Savegnago, Sibele Borsuk.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alves, M.S.D., Sena-Lopes, Â., das Neves, R.N. et al. In vitro and in silico trichomonacidal activity of 2,8-bis(trifluoromethyl) quinoline analogs against Trichomonas vaginalis. Parasitol Res 121, 2697–2711 (2022). https://doi.org/10.1007/s00436-022-07598-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07598-1