Abstract
Free-living amoebae (FLA) are protozoa which have been reported in different countries worldwide from diverse sources (water, soil, dust, air), contributing to the environmental microbiological contamination. Most of the FLA species present a life cycle with two different phases: an active vegetative and physiologically form named trophozoite, and an extremely resistant phase called cyst. Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, Sapinia pedata, Vahlkampfia spp., Paravahlkampfia spp. and Vermamoeba vermiformis have been reported not only as causal agents of several opportunistic diseases including fatal encephalitis or epithelial disorders, but also as capable to favour the intracellular survival of common pathogenic bacteria, which could avoid the typical water disinfection systems, non-effective against FLAs cysts. Even though Santiago Island possesses high levels of humidity compared to the rest of the archipelago of Cape Verde, the water resources are scarce. Therefore, it is important to carry out proper microbiological quality controls, which currently do not contemplate the FLA presence in most of the countries. In the present work, we have reported the presence of Acanthamoeba spp. (69.2%); Vannella spp. (15.4%); Vermamoeba vermiformis (7.7%) and the recently discovered Stenamoeba dejonckheerei (7.7%) in different water sources of Santiago Island.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cabo Verde Archipelago is located on the west coast of Africa, and consists in 10 islands divided in two groups: the Barlavento group (Santo Antão, São Vicente, Santa Luzia, São Nicolau, Sal and Boavista islands) and the Sotavento group (Maio, Santiago, Fogo and Brava islands). Nevertheless, each island presents topographic and climatic differences, promoting historically the active movement of population and goods (Leal et al. 2020). Cape Verde’s climate is milder than the African mainland, due to the surrounding sea which moderates temperatures of the islands and the cold Atlantic currents which produce an arid atmosphere around the archipelago. However, the islands do not receive the upwellings (cold streams) that affect the West African coast, so the air temperature is cooler than in Senegal. Nevertheless, the sea is warmer, because the orographic relief of some islands, such as Santiago with steep mountains, covers it with rich woods and luxuriant vegetation where the humid air condenses and soaks the environment (Coverdell 2009). Due to this water availability, one of the main industries in the island is agriculture, followed by tourism, fishing and others, alongside some manufacturing. However, even though Santiago Island possesses high levels of humidity compared to the rest of the archipelago, the water resources are scarce. Consequently, it is important to highlight the importance of proper microbiological quality controls.
The widely reported protozoa free-living amoebae (FLA) have been reported in different water sources, soils or air among others (Schuster and Visvesvara 2004). Moreover, their contribution to the environmental microbiological contamination has been demonstrated (Guimaraes et al. 2016). Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, Sapinia pedata, Vahlkampfia spp., Paravahlkampfia spp. and Vermamoeba vermiformis have been reported as causal agents of several opportunistic diseases including fatal encephalitis or epithelial disorders (Scheid et al. 2019; Schuster and Visvesvara 2004; Siddiqui et al. 2021). The life cycle of these pathogenic microorganisms is formed by a physiologically active vegetative trophozoite and an extremely resistant and persistent stage called cyst (Siddiqui and Khan 2012). Furthermore, this resistant stage can favour the intracellular survival of common pathogenic bacteria, avoiding the typical water disinfection systems, non-effective against FLAs cysts (Lorenzo-Morales et al. 2015).
Regarding the quality of water on the island of Santiago, there is a regulatory decree number 5/2017 of 6 November that establishes the criteria and standards that define the essential requirements for the quality of water intended for human consumption, as well as the control systems, the sanctioning regime and protective measures, with a view to protecting human health from the adverse effects resulting from possible water contamination. However, for irrigation systems where the water comes from springs and boreholes used in this study, there is still no quality control to evaluate the physical, chemical and microbiological parameters weekly or quarterly. Therefore, the aim of our study was to evaluate the presence of FLA from water samples of different water sources along Santiago Island.
Materials and methods
Sampling and FLA culture
In order to reveal the presence of potentially pathogenic FLA in water samples of Santiago Island in Cape Verde (15°04′40″N 23°37′29″O), a total of 31 samples were collected from different towns across the island (Fig. 1). The evaluated samples correspond to tap (3/31), depurated (3/31), lake (2/31), swimming pool (4/31), conditioned air (1/31), sea (6/31) and irrigation waters (12/31), and they were collected in sterile bottles and maintained at 4 °C until further processing in the laboratory. As the samples were collected in Cape Verde and processed in Tenerife, Canary Islands (Spain), the processing time oscillated between 48 and 72 h after the sample collection. The samples collected from lakes, swimming pools, sea and depurated or irrigation systems were taken from the surface of each water body. The processing protocol consisted in filtering the water samples using a vacuum multiple system and 0.45 μm nitrocellulose filters (Pall, Madrid, Spain), and the filters were cultured inverted onto 2% non-nutrient agar (NNA) plates also seeded with heat-killed Escherichia coli. These plates were incubated at room temperature (~ 26 °C) and monitored daily. Those plates suspicious for FLA growth following morphological features (Page 1969) were cloned by dilution in new NNA plates until a monoxenic culture was obtained (Lorenzo-Morales et al. 2005; Reyes-Batlle et al. 2015).

adapted from Sousa-Ramos et al. 2021)
Cape Verde archipelago geographical situation and Santiago Island relative location (
DNA extraction
For molecular characterization, DNA from positive samples was extracted from 1 to 2 ml of amoebic culture suspension. To obtain this amoeba suspension, 4 ml of Page’s Amoeba Solution (PAS) was added to the plate with the monoxenic amoeba culture. The plate was scraped and this suspension was centrifuged and the concentrated amoeba culture was directly placed into the Maxwell® 16 tissue DNA purification kit sample cartridge (Promega, Madrid, Spain) following the manufacturer’s instructions and as it has been previously described (Reyes-Batlle et al. 2019). The protozoa genomic DNA yield and purity were determined using the DS-11 Spectro-hotometer (DeNovix®, USA).
PCR and molecular characterization of the obtained isolates
The amplification by PCR of the 18S rRNA gene from the obtained DNA was carried out using two universal primers for FLAf/r (Tsvetkova et al., 2004) and Ame f977/r1534 (Liang et al. 2010). On the other hand, to amplify the 18S rRNA DF3 fragment to distinguish Acanthamoeba genotypes, we have used the JDP-1f/JDP-2r (Schroeder et al. 2001).
Amplification reactions were performed with a total of 50 μl of mixture, containing 80 ng DNA, and the PCRs were carried out in 40 cycles with denaturation (95 °C, 30 s), annealing (FLAf/r 55 °C and Ame f977/r1534 62 °C, 30 s) and primer extension (72 °C, 30 s) for FLA universal primers (FLA and Ame). Nevertheless, for Acanthamoeba spp. primers, the 50 μl PCR mixture contains 40 ng of DNA yield and 35 cycles with denaturation (95 °C, 30 s), annealing (50 °C, 30 s) and primer extension (72 °C, 30 s). A primer extension of 7 min at 72 °C was maintained after the last cycle. PCRs products were analysed by electrophoresis through a 2% agarose gel and positive PCR products were sequenced by Macrogen Spain service (C/Martínez Villergas S2, Planta Baja, Of. 1, izda., Madrid, Spain). Different species were identified based on sequence homology analysis by comparison to the DNA sequences present in the Genbank database.
Phylogenetic analyses
To proceed to sequence alignment, we have used the Mega X software program (Kumar et al. 2018; Tamura et al. 2004). The evolutionary history was inferred using the maximum likelihood method based on the Tamura-Nei model (Tamura and Nei 1993). The current analysis has involved 21 nucleotide sequences: 13 sample sequences and 8 GenBank standard sequences. The total of the ambiguous positions has been removed for each sequence pair.
Results
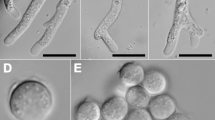
From the total of the 31 analysed waters, 12 of them were positive for FLA growth (38.7%). However, we were able to genotype 13 FLA strains, being Acanthamoeba the most common identified genus (9/13; 69.2%), followed by Vannella spp. (2/13; 15.4%) and Vermamoeba vermiformis and Stenamoeba dejonckheerei (1/13; 7.7%).
The obtained sequences in the present study have been deposited in the GenBank database under the following accession numbers: MW757016- MW757028. All of them present ˃95% of homology with the available DNA sequences in this database (Table 1).
The phylogenetic relationship of the FLA strains isolated in the present study is represented in Fig. 2, where the Discosea species (Stenamoeba spp. and Vannella spp.) and the Lobosea species (Acanthamoeba spp. and Vermamoeba spp.) are well differentiated. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura-Nei model (Tamura and Nei 1993). The initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then selecting the topology with a superior log-likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. There were a total of 2328 positions in the final dataset and the evolutionary analyses were conducted in MEGA X (Kumar et al. 2018).
Discussion
Cape Verde has high levels of poverty and unemployment, partly attributable to a lack of obvious economic growth opportunities and a scarcity of resources, particularly water (Paul D. Coverdell 2009). The largest island, both in size and population, is Santiago, where Praia, the capital, is located. These islands with a volcanic origin count with some of the windiest beaches in the world, and they vary widely in terrain (Paul D. Coverdell 2009). Water shortages and successive droughts have greatly weakened crop production capacity over the last century (Paul D. Coverdell 2009). Santiago has vegetation-clad (cloud forests) where the dense moisture condenses and soaks the plants and soil and favours agriculture (Paul D. Coverdell 2009). Therefore, taking into account the importance of having appropriate quality water sources, it is important to go deep into the possible microbial contamination, such as bacteria, viruses or protozoa.
FLA have been considered amphizoic protozoa, which means that these protozoa do not require a host organism to be able to survive (Cateau et al. 2014). Consequently, they are considered an emerging group of opportunistic pathogens (Lorenzo-Morales et al. 2015) since they represent a health risk not only because they are capable of causing several diseases in humans and other animals, but also because they act as vehicles for potentially pathogenic bacteria (Siddiqui and Khan 2012). In the present work, we have isolated Acanthamoeba spp. (69.2%); Vannella spp. (15.4%); Vermamoeba vermiformis (7.7%) and Stenamoeba dejonckheerei (7.7%) in different water sources of Santiago Island. Up to now, in Europe and Africa, there is no legislation related to the presence of FLA in water bodies. However, Australia possesses a Drinking Water Guideline where they gather the National Water Quality Management Strategies (Australian drinking water guidelines 62011 national water quality management strategy). In this document, even though there is no guideline value for Acanthamoeba, Vannella, V. vermiformis and Stenamoeba species in drinking water, they named Acanthamoeba and Vannella in a different manner.
Most of the Acanthamoeba species are capable to produce cerebral infections known as granulomatous amoebic encephalitis (GAE), corneal infection, Acanthamoeba keratitis (AK) or both (Lorenzo-Morales et al. 2015). In the referred guideline, they remark that the relative importance of water as a source of Acanthamoeba infection is unknown. Moreover, they highlight the wide distribution of Acanthamoeba in a natural environment such as soil, airborne dust and water and how the delays in the diagnosis of GAE and AK cases have made it difficult to investigate possible sources of infection, while the lack of a stable classification of Acanthamoeba makes difficult the identification of individual isolates, including the matching of amoebae from infections with organisms from the environment (Australian drinking water guidelines 62011 national water quality management strategy). Even though the regular monitoring for Acanthamoeba is not appropriate in this guideline, they enhance that these organisms need to be considered when planning the maintenance of eyewash stations that use main water. On the other hand, Vannella spp. is only contemplated as a microorganism which could cause taste and odour problems. So far, there is no evidence related to the pathogenicity of Vannella spp. However, it is well known that this genus can facilitate the growth of bacteria (Loret et al. 2008; Schulz et al. 2015) such as Legionella (Kuroki et al. 1998) or other human pathogenic organisms (Scheid 2007).
In contrast, V. vermiformis has been recently reported as one of the most prevalent and thermotolerant FLA (Reyes-Batlle et al. 2016; Siddiqui and Khan 2021). V. vermiformis pathogenicity is not only due to its capability to produce corneal, encephalitic or epithelial infections, but also to its numerous reported relationships with pathogenic bacteria (Scheid 2019; Sousa-Ramos et al. 2021). On the other hand, until now, no pathogenicity has been associated to Stenamoeba genus, which was finally defined in 2007 as a member of Thecamoebida (Discosea) (Adl et al. 2019). However, this new FLA species was isolated from fresh-water reservoirs at 37 °C, a temperature which could indicate a potentially pathogenic risk. This is the third report of the new species Stenamoeba dejonckheerei worldwide (Borquez-Román et al. 2020; Lares-Jiménez et al. 2018; Sousa-Ramos et al. 2021) and the second time in Cape Verde Archipelago (Sousa-Ramos et al. 2021).
Conclusions
In the present work, according to a previous study which analysed soil samples from Santiago Island (Sousa-Ramos et al. 2021), we have reported the presence of FLA in environmental sources, specifically in water samples. The presence of Acanthamoeba species, most of them belonging to the virulent T4 genotype (7 isolates), and V. vermiformis, enhances the importance to control the protozoa contamination in human-related water sources. In fact, we should take care about not only this pathogenic potential, but also the capability of these protozoa species to transport other human pathogens, also shared by Vannella spp. Finally, as S. dejonckheerei is a recently discovered species, we consider it important to notify its presence in human-related environments.
References
Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov A, . . . Zhang Q (2019) Revisions to the classification, nomenclature, and diversity of eukaryotes. J Eukaryot Microbio 66(1), 4-119https://doi.org/10.1111/jeu.12691
Australian drinking water guidelines 6 2011 national water quality management strategy
Borquez-Román MA, Lares-Jiménez LF, Rodriguez-Anaya LZ, Gonzalez-Galaviz JR, Fuerst PA, Ibarra-Gámez JC, . . . Lares-Villa F (2020) Stenamoeba dejonckheerei sp. nov., a free-living amoeba isolated from a thermal spring. Pathogens (Basel, Switzerland) 9(7) https://doi.org/10.3390/pathogens9070586
Cateau E, Delafont V, Hechard Y, Rodier MH (2014) Free-living amoebae: what part do they play in healthcare-associated infections? J Hosp Infect 87(3):131–140. https://doi.org/10.1016/j.jhin.2014.05.001
Coverdell PD (2009) A piece corps. Washington DC
Guimaraes AJ, Gomes KX, Cortines JR, Peralta JM, Peralta R H (2016) Acanthamoeba spp. as a universal host for pathogenic microorganisms: one bridge from environment to host virulence. Microbiol Res 193, 30–38. S0944–5013(16)30518–3 [pii]
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kuroki T, Yagita K, Yabuuchi E, Agata K, Ishima T, Katsube Y, Endo T (1998) Isolation of Legionella and free-living amoebae at hot spring spas in Kanagawa, Japan. Kansenshogaku Zasshi the Journal of the Japanese Association for Infectious Diseases 72(10):1050–1055. https://doi.org/10.11150/kansenshogakuzasshi1970.72.1050
Lares-Jiménez LF, Borquez-Román MA, Lares-García C, Otero-Ruiz A, Gonzalez-Galaviz JR, Ibarra-Gámez JC, Lares-Villa F (2018) Potentially pathogenic genera of free-living amoebae coexisting in a thermal spring. Exp Parasitol 195:54–58. https://doi.org/10.1016/j.exppara.2018.10.006
Leal SDV, Fernandes Varela IB, Lopes Goncalves AAL, Sousa Monteiro DD, de Ramos Sousa CM, Lima Mendonca MDL, . . . Osorio HC (2020) Abundance and updated distribution of Aedes aegypti (Diptera: Culicidae) in Cabo Verde archipelago: a neglected threat to public health. Int J Environ Res Public Health, 17(4), https://doi.org/10.3390/ijerph17041291. E1291 [pii]
Liang SY, Ji DR, Hsia KT, Hung CC, Sheng WH, Hsu BM, . . . Ji DD (2010). Isolation and identification of Acanthamoeba species related to amoebic encephalitis and nonpathogenic free-living amoeba species from the rice field. J Appl Microbio 109(4), 1422-1429https://doi.org/10.1111/j.1365-2672.2010.04779.x
Lorenzo-Morales J, Khan NA, Walochnik J (2015) An update on acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite (paris, France) 22:10. https://doi.org/10.1051/parasite/2015010
Lorenzo-Morales J, Ortega-Rivas A, Foronda P, Martinez E, Valladares B (2005) Isolation and identification of pathogenic acanthamoeba strains in Tenerife, Canary Islands, Spain from water sources. Parasitol Res 95(4):273–277. https://doi.org/10.1007/s00436-005-1301-2
Loret JF, Jousset M, Robert S, Saucedo G, Ribas F, Thomas V, Greub G (2008) Amoebae-resisting bacteria in drinking water: risk assessment and management. Water Sci Technol: A J Int Assoc Water Pollut Res 58(3):571–577. https://doi.org/10.2166/wst.2008.423
Page FC (1969) Mitosis and pseudopod formation in Vexillifera bacillipedes n. sp., a mayorellid amoeba. Trans Am Microsc Soc 88(3):394–400
Reyes-Batlle, Niyyati M, Martín-Navarro CM, López-ArencibiaA, Valladares B, Martínez-Carretero E, . . . Lorenzo-Morales J (2015) Unusual Vermamoeba vermiformis strain isolated from snow in Mount Teide, Tenerife, Canary Islands, spain. Novelty Biomed 3(4), 189–192. Retrieved from https://doaj.org/article/11234cd60b1647adbe911a6eafc3e38c
Reyes-Batlle M, Hernandez-Pinero I, Rizo-Liendo A, Lopez-Arencibia A, Sifaoui I, Bethencourt-Estrella CJ, . . . Lorenzo-Morales J (2019) Isolation and molecular identification of free-living amoebae from dishcloths in Tenerife, Canary Islands, Spain. Parasitol Res 118(3), 927-933https://doi.org/10.1007/s00436-018-06193-7
Reyes-Batlle M, Wagner C, Zamora-Herrera J, Vargas-Mesa A, Sifaoui I, Gonzalez AC, . . . Lorenzo-Morales J (2016) Isolation of thermotolerant Vermamoeba vermiformis strains from water sources in Lanzarote Island, Canary Islands, Spain. Acta Parasitologica, 61(3), 650-653https://doi.org/10.1515/ap-2016-0088
Scheid PL, Lam TT, Sinsch U, Balczun C (2019) Vermamoeba vermiformis as etiological agent of a painful ulcer close to the eye. Parasitol Res 118(6):1999–2004. https://doi.org/10.1007/s00436-019-06312-y
Scheid P (2019) Vermamoeba vermiformis - a free-living amoeba with public health and environmental health significance. Open Parasitol J 7(1):40–47. https://doi.org/10.2174/1874421401907010040
Scheid P (2007) Mechanism of intrusion of a microspordian-like organism into the nucleus of host amoebae (Vannella sp.) isolated from a keratitis patient. Parasitol Res 101(4):1097–1102. https://doi.org/10.1007/s00436-007-0590-z
Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, . . . Byers TJ (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol 39(5), 1903-1911https://doi.org/10.1128/JCM.39.5.1903-1911.2001
Schulz F, Tyml T, Pizzetti I, Dyková I, Fazi S, Kostka M, Horn M (2015) Marine amoebae with cytoplasmic and perinuclear symbionts deeply branching in the gammaproteobacteria. Sci Rep 5:13381. https://doi.org/10.1038/srep13381
Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34(9):1001–1027. https://doi.org/10.1016/j.ijpara.2004.06.004
Siddiqui R, Khan NA (2012) Biology and pathogenesis of acanthamoeba. Parasit Vectors 5:6–6. https://doi.org/10.1186/1756-3305-5-6
Siddiqui R, Makhlouf Z, Khan NA (2021) The increasing importance of Vermamoeba vermiformis. J Eukaryot Microbiol e12857. https://doi.org/10.1111/jeu.12857
Sousa-Ramos D, Reyes-Batlle M, Bellini NK, Rodríguez-Expósito RL, Piñero JE, Lorenzo-Morales J (2021) Free-living amoebae in soil samples from Santiago Island, Cape Verde. Microorganisms, 9(7) https://doi.org/10.3390/microorganisms9071460
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101(30):11030–11035. https://doi.org/10.1073/pnas.0404206101
Tsvetkova N, Schild M, Panaiotov S, Kurdova-Mintcheva R, Gottstein B, Walochnik J, . . . Muller N (2004) The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res 92(5), 405-413https://doi.org/10.1007/s00436-003-1052-x
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by the RICET (project no. RD16/0027/0001 of the programme of Redes Temáticas de Investigación Cooperativa, FIS), CIBER: Consorcio Centro de Enfermedades Infecciosas (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea – NextGenerationEU and Cabildo de Tenerife 21/0587 cofunded by FDCAN and MEDI (Tenerife Innova Programme) and Alumni ULL. R.L.R.-E. was funded by a grant from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI) cofunded by Fondo Social Europeo (FSE) y FEDER (TESIS2020010117).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Sutherland Maciver
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sousa-Ramos, D., Reyes-Batlle, M., Bellini, N.K. et al. Pathogenic free-living amoebae from water sources in Cape Verde. Parasitol Res 121, 2399–2404 (2022). https://doi.org/10.1007/s00436-022-07563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07563-y