Abstract
Aspidogaster limacoides Diesing, 1834 (Aspidogastridae) is redescribed based on light and scanning electron microscopy of specimens from the stomach and intestine of Abramis brama, Rutilus rutilus and Scardinius erythrophthalmus (Actinopterygii: Cyprinidae). The fishes were sampled during 2018 and 2019 at Lake Tollense in Mecklenburg-Western Pomerania, Germany. The prevalence of A. limacoides was highest in R. rutilus (61.7%) followed by Scardinius erythrophthalmus (7.7%) and A. brama (2.9%), while it was absent in Perca fluviatilis from the same lake. The following structures of A. limacoides are described for the first time: a depression on the ventral side of the neck, variations in the number and the arrangement of alveoli, numerous pits scattered all over the body surface, the presence of a few papillae-like structures posterior lateral to the mouth, the number of marginal organs represented by openings of exocrine multicellular glands as shown in histology and the subterminal position of the excretory pore. These characters can be used to distinguish three species of Aspidogaster, namely, A. ijimai, A. conchicola and A. limacoides, suggesting that SEM is a useful and promising tool in differentiating Aspidogaster species. Comparison of molecular data of the ITS1-5.8S-ITS2 regions showed a 94% similarity to A. limacoides from the European part of Russia. Phylogenetic analysis showed that the present specimens clustered in the same clade with A. limacoides sensu stricto, forming a distinct group to the exclusion of congeners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the trematode subclass Aspidogastrea are characterized by a large, alveolated ventral disc and parasitize aquatic ectotherm animals. The subclass Aspidogastrea Faust and Tang, 1936 is an ancient small group of Platyhelminthes which includes only four families (Rohde 2002). Aspidogastreans infect molluscs, fish and reptiles and rarely crustaceans, in marine as well as in freshwater environments with 61 valid species (Alves et al. 2015). This group is sister to the Digenea, together forming the Trematoda, and is thought to retain some primitive features such as a simple life cycle. On the other hand, they have a much more complex nervous system and sensory receptors compared with other groups of Platyhelminthes (Rohde 1994). So far, these parasitic flatworms have not been reported to cause any disease in humans nor have been found to be a significant problem for aquaculture, fisheries and other seafood industries (Lee et al. 2017). However, Aspidogaster conchicola Baer, 1826 and A. limacoides have been reported to cause pathological problems in clams and fish (Rutilus frisii), respectively (Pauley and Becker 1968; Rahanandeh et al. 2016).
In total, 12 species of the genus Aspidogaster Baer, 1826 have been reported so far and, among them, three species in Europe: Aspidogaster antipai Lepsi, 1932, A. conchicola Baer, 1826 and A. limacoides Diesing, 1834 (Alves et al. 2015). Aspidogaster limacoides was reported in Germany from the river Weser by Reimer (2002) from Rutilus rutilus nearly two decades ago. A few years later, this species was recorded from neighbouring countries such as Austria and Poland from Barbus barbus and Rutilus rutilus, respectively (Popiołek et al. 2007; Schludermann et al. 2005). However, the morphological and taxonomical descriptions of this species were solely based on light microscopy, not applying scanning electron microscopy (SEM) combined with DNA sequencing data based phylogenetic techniques. Detailed studies on the members of the genus Aspidogaster Baer, 1826 including SEM are scarce and focused on only two species, namely, A. ijimai Kawamura, 1913 and A. conchicola Baer, 1826 (Gao et al. 2003; Halton and Lyness 1971). Aspidogaster ijimai was recently studied using SEM by Lee et al. (2017).
The objectives of this study are to provide a detailed light and SEM investigation of A. limacoides Diesing, 1834 from a northern location in Germany, including molecular and phylogenetic analyses of the sampled specimens.
Materials and methods
Host examination and parasite collection
In total, 181 specimens of roach Rutilus rutilus (n = 47), rudd Scardinius erythrophthalmus (n = 13), bream Abramis brama (n = 35) and perch Perca fluviatilis (n = 86) were sampled from Lake Tollense in the northern part of Germany during 2018–2019. The fishes were caught by local fishermen with gillnets, killed on site, locally deep frozen and kept on ice during transportation until arrival at the University of Rostock, Germany, where they were transferred to a freezer (− 18 °C) for storage until subsequent examination for parasites. According to standard protocols, the stomach and intestines of the fish were examined for parasites after recording the total and standard length and slaughter weight. The parasites were counted to obtain prevalence, intensity and abundance data as defined by Bush et al. (1997). Selected parasites were fixed with 70% EtOH and 4% formalin for microscopic examination; others were fixed and stored in 99.6% EtOH for further DNA analyses.
Parasite preparation for examination
Light microscopy and histology
Selected specimens fixed with 70% EtOH were stained with acetic carmine for light microscopic examination. Specimens were dehydrated in an ascending series of ethanol (70%, 90% and twice in 100% ethanol), cleared in 50% eugenol followed by 100% eugenol and mounted in Canada balsam. The body, organs and taxonomically relevant characters were measured with measurements given in micrometres. Voucher specimens were deposited in the Natural History Museum Berlin (Museum für Naturkunde Berlin (ZMB), Germany).
Additional specimens were preserved in 4% formalin for histology. Following dehydration of the parasites in an ascending series of ethanol [70%, 80%, 90% and 100% (two times)], samples were placed into xylol (three times for 1 h) and then in paraffin at 59–60 °C for 1 h. For paraffin embedding, the samples were transferred into standard cassettes with paraffin. The paraffin slices containing parasites were cut into different sizes (5 µm, 10 µm and 20 µm) by using a rotation microtome (Leica RM 2255) at room temperature and placed in a water bath (Leica HI 1210). The paraffin slices were transferred onto slides and dried on a heating plate (Medax 14,800) at 37 °C, and the slides were placed in a rack and incubated overnight at 37–38 °C. For staining, the slides were dipped into xylol (three times for 10 min) for deparaffinizing, rehydrated in alcohol (100% two times for 1 min; 90%, 80%, 70% and 50% for one time for 1 min), deionized with water (three times for 2 min each time) and stained with haematoxylin and eosin and alcian blue (1% in 3% acetic acid) according to the standard staining protocols of the Institute for Anatomy, University Medicine, University of Rostock, dehydrated and cleared with an ethanol series and xylol. The slides were covered with mounting medium and a cover glass.
Scanning electron microscopy
For scanning electron microscopy, formalin-fixed specimens were dehydrated in an ascending ethanol series and transferred to 100% acetone (twice for 10 min each), critical point dried (Emitech K850, Co. Quorum Technologies LTD, East Sussex), mounted on SEM-carrier with adhesive conductive carbon tape (Co. PLANO, Wetzlar), coated with gold under vacuum (EM SCD 004, Co. BALTEC, Balzers) and analysed by a field emission scanning electron microscope (FE-SEM, MERLIN® VP Compact, Co. Zeiss, Oberkochen) at the Electron Microscopy Centre, University Medicine Rostock.
DNA isolation, amplification and sequencing
Genomic DNA was extracted from mature worms stored in 99.6% EtOH following the standard protocol of the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). The ribosomal DNA (rDNA) region comprising ITS-1, 5.8S, ITS-2 and flanking sequences (= ITS +) was amplified with a polymerase chain reaction (PCR) with the universal primers BD1 (5′-GTCGTAACAAGGTTTCCGTA-3′) and BD2 (5′-TATGCTTAA(G/A)TTCAGCGGGT-3′) (Luton et al. 1992). The PCR reaction was performed in a total volume of 50 µl consisting of 2.5 µl of each primer (10 pmol µl−1), 5 µl extracted DNA, 5 µl H2O and 25 µl ready-to-use master mix (Qiagen, Hilden, Germany). The amplification cycle consisted of the following conditions: initial denaturation at 94 °C for 3 min, followed by 40 cycles of 30-s denaturation at 94 °C, 30-s annealing at 54 °C and 2-min elongation at 72 °C; and a final extension hold at 72 °C for 7 min (Atopkin et al. 2017).
For electrophoresis, a 1% agarose (in TAE 1X-buffer) gel was used. Five µl of DNA PCR products were mixed with 1 µl of GelPilot® DNA Loading Dye (Qiagen, Hilden, Germany), premixed according to the manufacturer’s protocol and loaded for each sample. As reference, 6 µl of GelPilot® 1 kb Ladder (100) (Qiagen, Hilden, Germany), premixed according to manufacturer’s protocol, was added to the first well of the gel. Samples and ladder were dyed with GelRed® Nucleic Acid Gel Stain (Biotium, Inc. Fremont, California), according to the manufacturer’s protocol. Electrophoresis was run at 100 V, 130 mA and 50 W for an hour. Then, the gel was placed under ultraviolet transillumination and photographed. The samples showing bands were purified with a Qiagen QIAqick® PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol.
For sequencing, we used the 3S (5′-GGTACCGGTGGATCACGTGGCTAGTG-3′) primer as mentioned by Atopkin et al. (2017), but the primer did not work even though the gel showed bands. Therefore, we used the PCR primers BD1 (5′-GTCGTAACAAGGTTTCCGTA-3′) and BD2 (5′-TATGCTTAA(G/A)TTCAGCGGGT-3′) for sequencing with a mixture of 10 µl of purified PCR product and 4 µl primer in a new labelled Eppendorf tube. Then, purified PCR products were sent to Microsynth Seqlab (Göttingen, Germany) for sequencing using the same amplification primers.
Alignment and phylogenetic analysis
Contiguous sequences were edited manually and assembled using BioEdit 7.2. The resulting sequences were subjected to nucleotide BLAST searches to find the highest matching sequences available in the GenBank (http://www.ncbi.nlm.nih.gov/blast). A total of 22 sequences of ITS regions of other Aspidogaster spp. were downloaded from NCBI to infer the phylogeny of the worms obtained in the present study (Table 1). Phylogenetic analysis of the nucleotide sequences was performed using maximum likelihood (ML) methods. The model TIM2 + I was estimated as the best fitting for the data set using jModeltest v.2.1.10 software (Darriba et al., 2012) according to the Akaike information criterion (AIC). Phylogenetic relationship significance was estimated using a bootstrap analysis (Felsenstein 1985) with 100 replications. Phylogenetic trees were reconstructed with PhyML 3.1 software (Guindon and Gascuel 2003).
Results
Aspidogaster limacoides was found in the stomach and intestine of Abramis brama, Rutilus rutilus and Scardinius erythrophthalmus. This is the first study which confirms A. brama as a host of A. limacoides in Germany. The highest prevalence was recorded in R. rutilus (61.7%), while the lowest was in A. brama (2.9%). Aspidogaster limacoides was absent in Perca fluviatilis.
Order: Aspidogastrida Dollfus, 1958
Family: Aspidogastridae Poche, 1907
Genus: Aspidogaster Baer, 1827
Species: Aspidogaster limacoides Diesing, 1834
Synonym: A. donicum Popoff, 1926
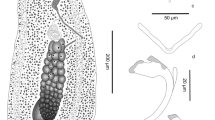
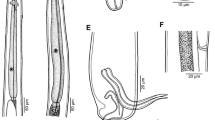
Description and measurements (in µm, Figs. 1–4): Body elongated, bluntly rounded at the anterior and tapering towards the posterior end; widest at the level of the cirrus sac region (Fig. 1A, B). Body length (n = 13) 1,399–3,069 and width (n = 13) 470–1,082. Oral and ventral suckers absent. Mouth at anterior extremity, aperture circular or elongate (Fig. 1B). Oral aperture muscular. Buccal funnel surrounds mouth. Mouth cup-shaped (Fig. 2A, B). A few papillae-like structure posterolateral to the mouth (Fig. 2C, D). Ventral disc oval, bears 4 longitudinal rows each with 13–14 alveoli; 4 alveoli on each transverse row with individual alveolus at the anterior and posterior extremities; alveoli in median the rows transversely elongate, other alveoli more or less square or oval (Fig. 2B). Alveoli number varies from 54 to 58. Median alveoli more regularly arranged than the peripheral alveoli especially on the extreme ends of the periphery of the disc. Ventral disc covers most of body, often including mouth. Ventral disc length (n = 13) 1,261–1,923 and width (n = 13) 920–1,369. Marginal organs, as openings of exocrine, multicellular glands, pyriform, regular arranged on margins of both sides at junction of interalveolar septa of ventral adhesive disc (Figs. 2E and F, 3A, 4D–F). Opening of the terminal ducts of marginal organs at junction between transverse and longitudinal ridges on the rim of the ventral disc (Figs. 2E and F, 3A, 4D–F). Mouth connected to the pharynx via duct which gives funnel-shaped appearance to the mouth. Prepharynx length (n = 10) 183–367. Pharynx large, globular, strongly muscular. Pharynx length (n = 13) 203–320 and width (n = 13) 187–283. The intestine is simple, with single caecum, which reaches to the posterior end of the body (Fig. 1A). Caecum length (n = 1) 852. Presence of numerous pori (small openings) all over the body including inside and around the mouth, on neck, on fold between neck and ventral disc (including alveoli and on septa); dorsal, ventral, both lateral sides and posterior region (Figs. 2D, 3B-D). Excretory pore subterminal, towards more on the dorsal side (Fig. 3E).
Aspidogaster limcoides, SEM: A dorsal view (scale bar = 100 µm); B ventral view with ventral disc (scale bar = 100 µm); C neck region, arrow showing depression of neck (scale bar = 20 µm), square D showing papillae-like structures posterior lateral to mouth; D posterior lateral papillae (arrow) (scale bar = 2 µm); E ventral disc, arrow showing marginal organ (scale bar = 20 µm); F ventral rim with marginal organ with terminal duct (arrow) (scale bar = 10 µm)
Aspidogaster limcoides, SEM: A marginal organ with terminal duct (scale bar = 2 µm); B and B 2: Arrow showing pits inside the mouth (scale bar = 20 µm); C Ventral disc showing pits on alveoli and septa (scale bar = 10 µm); D dorsal view, posterior body with pits (scale bar = 2 µm); E excretory pore (scale bar = 20 µm)
Cross-section of Aspidogaster limacoides, histology, from the level of the anterior edge of the ventral disc: A and D arrows (black and blue) showing marginal organs on ventral disc; B, E and F diagram of marginal organ (black and red arrows); C dorsoventral and longitudinal muscles (green arrows). Note: A–C H&E staining and D–F Alcian blue staining
Testis single, elongate, large and post-ovarian. Testis length (n = 5) 272–340 and width (n = 5) 170–239. Cirrus sac claviform, sinistral to pharynx, with doubled layered very thick muscular wall at proximal part, which becomes thinner at distal part. Cirrus sac length (n = 5) 402–537 and width (n = 5) 194–236. Internal seminal vesicle present, connected to pars prostatica, which opens into the ejaculatory duct. Genital pore not clearly visible, apparently opens at anterior level of pharynx.
Ovary single, globular and pretesticular. Ovary length (n = 2) 208–245 and width (n = 3) 141–170. The uterus is very long, fills posterior half of the body. Eggs are numerous, oval, often with opened operculum in distal part of the uterus. Egg length (n = 9) 72–93 and width (n = 9) 40–52.
Vitelline follicular, follicles globular, lateral fields beginning at equator, present on either side and confluent or nearly so at posterior end (Fig. 1A, B).
Histology of the ventral disc
The ventral disc consists of four longitudinal rows of alveoli. In cross-section, the ventral disc consists of longitudinal muscles, marginal gland cells interspersed with dorsoventral muscles and a layer with more tightly arranged nuclei of the marginal gland cells (Fig. 4A–C). The marginal organ consists of the duct connected with the marginal gland cells, the ampulla with the secretion, a muscular papilla and the terminal duct and opening (Fig. 4D–F).
Taxonomic summary
Type hosts: Not designated. First mentioned by Diesing. Squalius cephalus (L.) and Leuciscus idus (L.) (Diesing, 1834 (short version), 1835 (extended version)).
Type locality: South of Vienna, Austria (see Reimer 2002).
Other hosts: Rutilus rutilus (L.), Scardinius erythrophthalmus (L.) and Abramis brama (L.)
Other locality: Lake Tollensesee, Mecklenburg-Western Pomerania, Germany (53°30′26″N 13°12′41″E).
Prevalence: Rutilus rutilus 61.7%, Scardinius erythrophthalmus 7.7%, Abramis brama 2.9%
Intensity (mean): Rutilus rutilus: 1–53 (9.3), Scardinius erythrophthalmus: 3 (3) and Abramis brama: 18 (18.0).
Site of infection: Mainly stomach and few specimens in intestine, post-mortem migration possible.
Deposition of voucher specimens: Natural History Museum Berlin (Museum für Naturkunde Berlin (ZMB), Germany); E.7645-E7647.
Phylogenetic analyses
Two contiguous sequences, 1,454 (accession number: MT951619) and 1,469 (accession number: MT951620) base pairs (bp) long, of the ITS1-5.8S-ITS2 region of rDNA were generated from adult worms isolated from Rutilus rutilus and Abramis brama. The newly obtained sequences varied by a single base. Our new sequence data showed 98.35% to 98.78% identity with ITS1-5.8S-ITS2 rDNA sequences of A. limacoides derived from R. rutilus sampled from Russia (accession codes: HE863971, HE863970, HE863969 and HE863966) using BLAST service. A. ijimai clade was subdivided according to geographical origin. This cluster formed a sister clade to A. chongqingensis with strong nodal support. Specimens of A. conchicola formed a distinct third clade. The fourth clade contained specimens of A. limacoides from German waters (present study) and the European part of Russia (Fig. 5).
Phylogenetic tree based on analyses of ITS1-5.8S-ITS2 sequences of species belonging to the genus Aspidogaster using the maximum likelihood method of phylogenetic reconstruction with TIM2 + I model according to jModelTest software v 2.1.10. Nodal numbers give bootstrap statistical support for the analyses. AN, Amur River, Nikolaevsk-na-Amure; AK, Amur River, Khabarovsk; Kh, Khanka Lake; Chi, China; ER, European part of Russia; Ger, Germany; JPN, Japan. *Misidentified A. chongqingensis
Discussion
The present specimens belong to the genus Aspidogaster Baer, 1827 due to the presence of a ventral disc with 4 longitudinal rows of alveoli, absence of head lobes, a single testis and a single blind caecum as described by Rohde (2002). Aspidogaster limacoides was firstly reported and described by Diesing from Leuciscus cephalus (L.) (now Squalius cephalus) and L. idus (L.) (Diesing 1834) and described in more detail with figures by Diesing (1835). Subsequently, Voeltzkow (1888) provided a further morphological description of Diesing’s type material. Additionally, this species was described by Bychowsky and Bychowsky (1934) from the Caspian Sea. Alves et al. (2015) summarized all host and locality records for this species. However, even though these authors recognized the publication of Reimer (2002) in a German magazine, they did not consider the record of A. limacoides from R. rutilus in the Weser River in North Rhine-Westphalia, Germany. Therefore, the present record is the second for A. brama worldwide (already known from Georgia, border between Europe and Asia) and the first in Germany, where only R. rutilus has been recognized as a host so far.
The prevalence and mean intensity vary greatly between these fish species. The highest prevalence of A. limacoides was reported in R. rutilus followed by S. erythrophthalmus and A. brama. However, the highest mean intensity was found in A. brama, suggesting also this cyprinid as a regular host. Rutilus rutilus and S. erythrophthalmus can be considered as the most common hosts for A. limacoides, and Zhokhov (2001) considered that the significant variations in the occurrence of A. limacoides in these fish species were mainly due to their different feeding behaviours. The author suggested that A. limacoides could be used as a feeding indicator in four different cyprinid fish species.
So far, within the genus, scanning electron microscopy has been used for only A. ijimai and A. conchicola (Gao et al. 2003). This is the first study which provides SEM pictures of A. limacoides. Topographical features such as the number and arrangement of alveoli and pits and the presence of papillae-like structures as well as the position of the excretory pore are unique for each of these species (Table 2). Yamaguti (1963) suggested that the number of alveoli is useful for the differentiation of Aspidogaster species, with A. ijimai having 42 alveoli, A. limacoides 50–74 and A. conchicola 60–174. This observation was later confirmed by several authors. Popiołek et al. (2007) quoted authors including Skrjabin (1952), Bauer (1987) and Bykhovskaya−Pavlovskaya et al. (1962) that the number of alveoli in A. conchicola and in A. limacoides is higher than 110 and fewer than 70, respectively. Based on the figure provide by Bychowsky and Bychowsky (1934), the number of alveoli ranged from 50 to 74 for A. liacoides. Similarly, Reimer (2002) observed 50 to 74 alveoli in A. limacoides from the river Weser, Northwest Germany, if considering 4 alveoli on each transverse row and two terminal alveoli at each end, but Reimer did not provided pictures. In the present study, the number of alveoli in A. limacoides varied from 54 to 58 based on SEM, and 58 and 62 alveoli have been reported for this species by Atopkin et al. (2017) and Popiołek et al. (2007), respectively. The number of alveoli in A. ijimai was 46 based on the figure provided by Lee et al. (2017) and Sokolov et al. (2019), whereas in A. conchicola, 114 alveoli were counted based on the figure provided by Atopkin et al. (2017) and 110 as reported by Bakker and Diegenbach (1974).
In addition to the number of alveoli, the arrangement of the alveoli in transverse rows on the ventral disc also differs between the Aspidogaster species, e.g. A. conchicola has one alveolus at each end, two transverse rows with two alveoli (one anterior and one posterior) and 4 alveoli on each remaining transverse row (Atopkin et al. 2017; Bakker and Diegenbach 1974). A similar pattern was observed in A. ijimai (Atopkin et al. 2017; Lee et al. 2017). However, the present study reveals that A. limacoides has 1 alveolus on each end and 4 alveoli in the remaining transverse rows, without 2 alveoli in transverse rows, in agreement with Atopkin et al. (2017) and Bychowsky and Bychowsky (1934). This suggests that also the arrangement of the alveoli on the ventral disc is a useful character to distinguish the species of Aspidogaster.
Numerous pits were found in A. ijimai, A. conchicola and A. limacoides, but microridges were found only on the neck region in the trough of folds in A. ijimai while uniciliated sensory structures were observed only in A. conchicola (Gao et al. 2003). In this study, few papillae-like structures were found only in the posterior lateral to the mouth. However, they are scattered all over the surface in A. ijimai and A. conchicola (Gao et al. 2003). There is also a considerable difference on the posterior end of A. ijimai, A. conchicola and A. limacoides. The excretory pore is terminal in the former two species but subterminal (dorsoterminal) in the latter species. On the other hand, a couple of morphological features are common to these three congeners, e.g. the presence of marginal organs with their terminal ducts on the rim of the ventral disc at the junction of the longitudinal and transverse ridges and a depression in the neck region (see Table 2). According to Huehner et al. (1989), these marginal organs are probably used to store and release secretions for extracorporeal digestion. The histology of the marginal organs in the present study demonstrates the secretory nature of these organs. They represent openings of the exocrine multicellular glands, with a duct connected with the marginal gland cells, an ampulla with secretion, a muscular papilla and a terminal duct with an opening. This morphology corresponds to the marginal organ described for other aspidogastreans, e.g. for Lobatostoma manteri Rohde, 1973 by Rohde (1973, 1994).
This study is the first SEM examination of A. limacoides and a first detailed study of this species from a northern location in Germany. In comparison to earlier SEM examination, it is evident that this technique provides additional significant insights into relevant topographical features of Aspidogaster species, especially the bulbous papillae, distribution of the pori and clear discrimination and arrangement of the alveoli, which are of taxonomic and systematic importance in this interesting group of parasites. Therefore, further SEM studies are needed for better species description and to differentiate between the species not only inside Aspidogaster but also between other aspidogastrean taxa.
Code availability
Not applicable.
Data availability
All data published within this text.
Change history
14 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Alves PV, Vieira FM, Santos CP, Scholz T, Luque JL (2015) A checklist of the Aspidogastrea (Platyhelminthes: Trematoda) of the world. Zootaxa 3918(3): 339–396. https://doi.org/10.11646/zootaxa.3918.3.2
Atopkin DM, Shedko MB, Sokolov SG, Zhokhov AE (2017) Phylogenetic relationships among European and Asian representatives of the genus Aspidogaster Baer, 1827 (Trematoda: Aspidogastrea) inferred from molecular data. J Helminthol 92(3):343–352. https://doi.org/10.1017/S0022149X17000505
Bakker KE, Diegenbach PC (1974) The structure of the opisthaptor of Aspidogaster conchicola Baer, 1826 (Aspidogastridae, Trematoda). Netherlands J Zool 24(2):162–170. https://doi.org/10.1163/002829674X00020
Bauer ON (1987) Parazitologičeskie mnogokle-točnye. In: Opredelitel' parazitov presnowodnyh rybfauny SSSR. Vol. 3. (Ed. ON Bauer). Nauka, Leningrad
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4): 575–583. https://doi.org/10.2307/3284227
Bychowsky I, Bychowsky B (1934) Über die Morphologie und die Systematik des Aspidogaster limacoides Diesing. Z Parasitenkd 7(2):125–137. https://doi.org/10.1007/BF02121888
Bykhovskaya−Pavlovskaya IE, Gusev AV, Dubinina MN, Izyumova NA, Smirnova TS, Sokolovskaya IK, Sthein GA, Shul'man SS, Epsthein VM (1962) Opriedelitiel parazitov pres-nowodnyh ryb SSSR. Izd. AN SSSR, Moskva, Leningrad
Chen M, Zhang L, Wen C, Sun J, Gao Q (2010) Phylogenetic relationship of species in the genus Aspidogaster (Aspidogastridae, Aspidogastrinae) in China as inferred from its rDNA sequences. Acta Hydrobiol Sin 34(2):312–316. https://doi.org/10.3724/SP.J.1035.2009.00312
Darriba D, Taboda GL, Doallao R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772
Diesing KM (1834) Aspidogaster limacoides 1834. Eine neue Art Binnenwurm. Isis von Oken 27:1231
Diesing KM (1835) Aspidogaster limacoides, eine neue Art Binnenwurm, beschrieben und durch eine Abbildung erläutert. Medicinishe Jahrbücher des K.-K. österreichischen Staates. Wien 16:420–430
Felsenstein J (1985) Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783–791
Gao Q, Nie P, Yao WJ (2003) Scanning electron microscopy of Aspidogaster ijimai Kawamura, 1913 and A. conchicola Baer, 1827 (Aspidogastrea, Aspidogastridae) with reference to their fish definitive-host specificity. Parasitol Res 91(6):439-443. https://doi.org/10.1007/s00436-003-1002-7
Guindon S, Gascuel O (2003) PhyML: a simple fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Halton DW, Lyness RAW (1971) Ultrastructure of the tegument and associated structures of Aspidogaster conchicola (Trematoda: Aspidogastrea). J Parasitol 57(6):1198–1210. https://doi.org/10.2307/3277967
Huehner MK, Hannan K, Garvin M (1989) Feeding habits and marginal organ histochemistry of Aspidogaster conchicola (Trematoda: Aspidogastrea). J Parasitol 75(6):848–852. https://doi.org/10.2307/3282862
Lee D, Park H, Choe S, Kang Y, Jeon HK, Eom KS (2017) New record of Aspidogaster ijimai Kawamura, 1913 (Trematoda: Aspidogastridae) from Cyprinus carpio in Korea. Korean J Parasitol 55(5):575. https://doi.org/10.3347/kjp.2017.55.5.575
Luton K, Walker D, Blair D (1992) Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol Biochem Parasitol 56(2):323–327. https://doi.org/10.1016/0166-6851(92)90181-I
Pauley GB, Becker CD (1968) Aspidogaster conchicola in molluscs of the Columbia River system with comments on the host’s pathological response. J Parasitol 54:917–920. https://doi.org/10.2307/3277119
Popiołek M, Łuczyński T, Jarnecki H (2007) The first record of Aspidogaster limacoides Diesing, 1834 (Aspidogastridae: Aspidogastrea) in Poland. Wiadomości Parazytologiczne 53(2):139–141
Rahanandeh M, Alinezhad S, Moghadam MK, Hallajian A (2016) The pathological study of gastro intestinal Aspidogaster limacoides Diesing, of the Caspian Sea kutum fish Rutilus frisii kutum (Kamenskii, 1901). International Journal of Fisheries and Aquatic Studies 4(6):397-399
Reimer LW (2002) Aspidogaster limacoides – ein Neozoe aus einer Plötze der mittleren Weser. Fischer Und Teichwirt 1:10–11
Rohde K (1973) Structure and development of Lobatostoma manteri sp. nov. (Trematoda: Aspidogastrea) from the Great Barrier Reef. Australia Parasitology 66:63–83. https://doi.org/10.1017/S0031182000044450
Rohde K (1994) The minor groups of parasitic Platyhelminthes. Adv Parasitol 33:145–234. https://doi.org/10.1016/s0065-308x(08)60413-3
Rohde K (2002) Aspidogastrea. In: Gibson DI, Jones A, Bray RA (eds) Keys to the trematoda, vol 1. CAB International and Natural History Museum, London, p 521
Schludermann C, Laimgruber S, Konecny R, Schabuss M (2005) Aspidogaster limacoides Diesing, 1835 (Trematoda, Aspidogastridae): a new parasite of Barbus barbus (L.) (Pisces, Cyprinidae) in Austria. Annalen des Naturhistorischen Museums in Wien Serie B für Botanik und Zoologie:141–144
Skrjabin KI (1952) Podklasa Aspidogastrea Faust et Tang, 1936. In: Trematody zhivotnykh i cheloveka (Ed. KI Skrjabin) T. 6. Izd. AN SSSR, Moskva
Sokolov SG, Atopkin DM, Urabe M (2019) Redescription and supplementary molecular characteristics of Aspidogaster ijimai Kawamura, 1915 (Trematoda, Aspidogastrea, Aspidogastridae), a parasite of Cyprinus carpio Linnaeus, 1758 s. lato (Actinopterygii) and freshwater bivalves in East Asia. Parasitolohy International 71:167–176. https://doi.org/10.1016/j.parint.2019.04.017
Voeltzkow A (1888) Aspidogaster limacoides. Arbeiten Aus Dem Zoology Zootom Institut in Würzburg 8:290–292
Yamaguti S (1963) Systema helminthum. Vol. IV. Monogenea and Aspidocotylea. New York. London: Interscience Publishers, John Wiley & Sons Ltd., Glen House, Stag Place, S.W.I. p 700
Zhokhov AE (2001) The study of the transition of Cyprinidae fish to feeding on mollusk Dreissena polymorpha (Bivalvia, Dreissenidae) in the Rybinsk Reservoir using parasite Aspidogaster (Aspidogastrea, Aspidogastridae). J Ichthyol 41(8):620–624
Acknowledgements
The authors are deeply grateful to Dr. Armin Springer and to Ms Karoline Schulz and Ms Ute Schulz from the Electron Microscopy Centre, University Medicine, Rostock, for their help with the electron microscopy. We also thank Prof. Dr. Marcus Kipp and Ms. Frauke Winzer from the Institute for Anatomy, University Medicine, University of Rostock, for their help with histology (preparation, staining and discussion).
Funding
Open Access funding enabled and organized by Projekt DEAL. The present study was funded by the European Fisheries Fund and the Ministry of Agriculture and Environment, Mecklenburg-Western Pomerania, as a part of the Project Hygiene management and health concept for surface water-dependent partial circulation systems in M.V. (MV-II. 12-LM-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All fishes were captured by local fishermen according to the international, national and/or institutional guidelines. Fish species is not listed in CITES or CMS and listed under Least Concern in IUCN Red List Status.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Shokoofeh Shamsi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suthar, J., Al-Jufaili, S., Bray, R.A. et al. Redescription of Aspidogaster limacoides Diesing, 1834 (Aspidogastrea: Aspidogastridae) from freshwater fishes of northern Germany. Parasitol Res 120, 3405–3416 (2021). https://doi.org/10.1007/s00436-021-07253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07253-1