Abstract
Fasciola hepatica (liver fluke), a significant threat to food security, causes global economic loss for the livestock industry and is re-emerging as a foodborne disease of humans. In the absence of vaccines, treatment control is by anthelmintics; with only triclabendazole (TCBZ) currently effective against all stages of F. hepatica in livestock and humans. There is widespread resistance to TCBZ and its detoxification by flukes might contribute to the mechanism. However, there is limited phase I capacity in adult parasitic helminths with the phase II detoxification system dominated by the soluble glutathione transferase (GST) superfamily. Previous proteomic studies have demonstrated that the levels of Mu class GST from pooled F. hepatica parasites respond under TCBZ-sulphoxide (TCBZ-SO) challenge during in vitro culture ex-host. We have extended this finding by exploiting a sub-proteomic lead strategy to measure the change in the total soluble GST profile (GST-ome) of individual TCBZ-susceptible F. hepatica on TCBZ-SO-exposure in vitro culture. TCBZ-SO exposure demonstrated differential abundance of FhGST-Mu29 and FhGST-Mu26 following affinity purification using both GSH and S-hexyl GSH affinity. Furthermore, a low or weak affinity matrix interacting Mu class GST (FhGST-Mu5) has been identified and recombinantly expressed and represents a new low-affinity Mu class GST. Low-affinity GST isoforms within the GST-ome was not restricted to FhGST-Mu5 with a second likely low-affinity sigma class GST (FhGST-S2) uncovered. This study represents the most complete Fasciola GST-ome generated to date and has supported the potential of subproteomic analyses on individual adult flukes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fasciolosis, caused by the trematode liver flukes Fasciola hepatica and F. gigantica, is a foodborne zoonotic affecting grazing animals and humans worldwide (Andrews 1999). Liver fluke causes economic losses of over US$3 billion worldwide per annum to livestock via a decrease in production of milk, meat and wool, susceptibility to other infections, condemnation of livers and mortality (Boray 1997). There are no commercial vaccines as yet available, with triclabendazole (TCBZ) currently the most commonly used fasciolicide due to its activity against both adults and juvenile stage fluke (Brennan et al. 2007). TCBZ is absorbed in the rumen and passes through the blood to the liver where it is rapidly oxidised to the likely main active metabolites: triclabendazole sulphoxide (TCBZ-SO) (Alvarez et al. 2005) and triclabendazole sulphone (TCBZ-SO2) (Alvarez et al. 2009; Alvarez et al. 2005). Unfortunately, TCBZ-resistant liver flukes are wide spread, with resistance first encountered in Australia; but it is now evident in Western Europe (Brennan et al. 2007) including the UK (Thomas et al. 2000).
At present, our understanding of the mode of action and detoxification of TCBZ is fragmented and mechanisms underpinning resistance may need to be resolved in order to measure early TCBZ resistance in populations and thus preserve efficacy (Brennan et al. 2007). To this end, the glutathione transferase (GST) superfamily have been identified as the major phase II detoxification system present in all parasitic helminths. GSTs have been implicated in both drug metabolism and resistance in other groups of organisms, e.g. insects and human tumours (Hayes and Pulford 1995). Eight cytosolic GST classes have been identified and are often species-independent, including Alpha, Mu, Pi, Omega, Sigma, Theta, Phi and Zeta (Mannervik et al. 2005). In F. hepatica, GSTs belonging to four classes have been revealed by biochemistry and bioinformatics: Omega (ω), Mu (μ), Sigma (σ) and Zeta (ζ) (Chemale et al. 2006; Morphew et al. 2012). Chemale et al. (2010) further reported that Mu class GST levels vary, with Mu class GST-1 reduced in abundance while Mu class GST-26 increased in TCBZ-resistant and susceptible F. hepatica under TCBZ sulphoxide (TCBZ-SO) exposure. In addition, Scarcella et al. (2012) identified that fluke resistant to TCBZ expressed significantly higher levels of GST activity compared to susceptible flukes. Furthermore, an amino acid mutation in Mu class GST-26 has been linked to a TCBZ-resistant liver fluke strain (Fernandez et al. 2015). However, to date, there has not been a robust sub-proteomic study that compared the expression of GST isotypes in individual liver fluke under TCBZ-SO stress. Thus, we purified GSTs from the cytosol of single adult flukes using a combination of glutathione (GSH) and S-hexyl-GSH agarose, resolved GST isotypes by 2-DE and identified individual GSTs by MS/MS with the support of genomic and transcriptomic databases. As a consequence, we have identified a novel Mu, Sigma and Omega class GST-designated FhGST-Mu5, FhGST-S2 and FhGST-O2 respectively. FhGST-Mu5 has been cloned and expressed in a recombinant and active form and characterised.
Materials and methods
In vitro TCBZ culture
Individual liver flukes from natural infections were collected and exposed to TCBZ-SO as described previously (Morphew et al. 2014). In brief, live adult F. hepatica were collected from a local abattoir (Randall Parker Foods, Llanidloes, Wales, UK) and washed in PBS at 37 °C. Flukes were washed for 1 h with PBS replacement every 15 min. Post-washes, replicates of 10 adult, sized matched, worms were placed into fluke Dulbecco’s Modified Eagle Medium (DMEM) culture media containing 15 mM HEPES, 61 mM glucose, 2.2 mM calcium acetate, 2.7 mM magnesium sulphate, 1 μM serotonin and gentamycin (5 μg/ml) as previously described (Morphew et al. 2011). Flukes were maintained in culture at 37°C for 2 h (including transport to the laboratory) to establish a baseline protein expression profile. Upon completion of the initial 2-h incubation, culture media was replaced and supplemented with TCBZ-SO (LGC Standards, UK) at 50 μg/ml (lethal dose) or 15 μg/ml (sub-lethal dose) in DMSO (final conc. 0.1% v/v). For control samples, only DMSO was added to a final volume of 0.1% v/v. Fluke cultures were then allowed to incubate at 37°C for a 6-h time period after which the media was refreshed, with DMSO and TCBZ-SO as required. Fluke cultures were incubated at 37°C for a further 6 h. A final refreshment of culture media was conducted and fluke cultures incubated for an additional 12 h at 37 °C. Upon completion of the culture, flukes were removed from the media and snap frozen individually in liquid N2. All samples were stored at − 80°C until required.
GST assay and purification
Individual adult F. hepatica were homogenised in an all-glass homogeniser on ice using 2 ml of lysis buffer containing 20 mM potassium phosphate pH 7.4, 0.1% v/v Triton-X 100 and EDTA-free protease inhibitors (Roche, Complete-Mini, EDTA-free). Samples were centrifuged at 100,000×g for 45 min at 4 °C to obtain the supernatant, the cytosolic fraction. Protein levels were quantified by the method of Bradford (1976). GST enzymatic specific activity was determined according to the conditions outlined by Habig et al. (1974) described previously (LaCourse et al. 2012) and stored at − 80 °C until needed. Briefly, GST activity was measured using 1 mM of the model substrate 1-chloro-2,4-dinitrobenzene (CDNB) and 1 mM reduced GSH in 20 mM potassium phosphate pH 6.5 and 25 °C. Activity was monitored at 340 nm over a period of 3 min. Specific activity data was log10 transformed prior to statistical comparison carried out by a two-way ANOVA.
Cytosolic proteins were applied to a GSH-agarose (Sigma-Aldrich) or an S-hexyl-GSH-agarose (Sigma-Aldrich) affinity matrix and purified at 4°C according to the manufacturer’s instructions and as described previously (Morphew et al. 2012). Eluted proteins were concentrated using 10-kDa molecular weight cutoff filters (Amicon Ultra, Millipore) and washed with ddH2O. All samples were quantified again by the method of Bradford (Sigma-Aldrich).
Protein preparation and 2-DE
IPG strips (7 cm, linear pH 3–10) were rehydrated with 100 μl of buffer (containing 8 M Urea, 2% w/v CHAPS, 33 mM DTT, 0.5% ampholytes pH range 3–10) plus 25 μl of sample protein and ddH2O to load 20 μg of GSH or Hexyl-GSH affinity bound proteins. Samples were in-gel rehydrated for 16 h and isoelectrically focused on 7 cm pH 3–10 IPG strips to 10,000 Vh on a Protean® IEF Cell (BioRad). After focusing, strips were then equilibrated for 15 min in reducing equilibration buffer (30% v/v glycerol, 6 M urea, 1% DTT) followed by 15 min in alkylating equilibration buffer (30% v/v glycerol, 6 M urea, 4% iodoacetamide). IPG strips were run upon SDS PAGE (12.5% acrylamide) using the Protean® II 2-D Cell (BioRad). Gels were then Coomassie blue stained (Phastgel Blue R, Amersham, Biosciences), and imaged on a GS 800 calibrated densitometer (BioRad). Quantitative differences between 2-DE protein spots were analysed using Progenesis PG220, software version 200 (Nonlinear Dynamics Ltd.), using 5 biological replicates. Spots were automatically detected on gels and manually edited. Normalisation of spots was calculated using total spot volume multiplied by the total volume (Moxon et al. 2010). All gel images were warped using manual matching before average gels (5 gels were used to make the average gels) for each treatment group were produced. Unmatched protein spots were also detected on appropriate gel comparisons. Two-fold differences between protein spots with a p < 0.05 were considered significant when average gels were compared.
Western blotting
Following 2-DE, resolved proteins were transferred to nitrocellulose membranes. The nitrocellulose membrane was soaked in ddH2O for 1 min. The gel, membrane, filter paper and porous pads were equilibrated in 1× Western Blot Transfer Buffer (NuPAGE Transfer Buffer, Life Technologies) for 20 min.
Proteins were transferred at 40 V for 2 h in 1× Western blot transfer buffer (50 ml NuPage transfer Buffer, 850 ml ddH2O, 100 ml methanol). To ensure proteins were transferred, the membrane was removed and stained with Amido black staining solution (0.1% w/v Amido black, 10% v/v Acetic Acid, 25% v/v isopropanol) for 1 min to detect the success of the transfer. The membrane was then washed with ddH2O. The membrane was then placed in Amido black de-stain (25% v/v isopropanol and 10% v/v acetic acid) for 30 min. The membrane was imaged using the GS-800 calibrated densitometer (BioRad). Amido black stain was removed with several washes of Tris-buffered saline, 1% v/v Tween 20 (TTBS).
The nitrocellulose membrane was blocked in blocking buffer (TTBS + 5% milk powder) overnight. The membrane was then washed with TTBS and then incubated with the primary antibody for 1–2 h. A 1:5,000 dilution and a 1:30,000 antibody dilution in blocking buffer was used for anti-Mu and anti-Sigma GST respectively. After incubation with the primary antibody, the membrane was washed in TBS for 10 min. The membrane was washed twice more before incubating with the secondary antibody (anti-goat IgG raised in rabbits for the Mu and anti-rabbit IgG raised in goats for the Sigma) for 1–2 h at a 1:30000 dilution in blocking buffer. The membrane was then washed 3 times in Tris-buffered saline (TBS). Interacting spots or bands were detected using the 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in conjugation with nitro blue tetrazolium (NBT), according to manufacturer’s instructions. To develop, a 1:2 solution of BCIP:NBT in substrate buffer consisting of 0.1 M tris, 100 mM NaCl and 5 mM magnesium chloride, adjusted to pH 9.5. To cease the over development, membranes were rinsed in ddH2O. Blots were then scanned with a GS-800 calibrated densitometer (BioRad) and were imaged using Quantity One Version 4.6 software (BioRad, UK).
Protein identification
Protein spots were manually excised from the gels and in-gel digested with trypsin according to the method of Chemale et al. (2006). Tandem mass spectrometry (MSMS) was performed according to the method described by Moxon et al. (2010) Briefly, selected peptides from peptide digests were loaded onto a gold coated nanovial (Waters, UK), and sprayed at 800–900 V at atmospheric pressure and fragmented by collision-induced dissociation using argon as the collision gas. Mass Lynx v 3.5 (Waters, UK) ProteinLynx was used to process the fragmentation spectra. Each fragmented spectrum was individually processed as follows: each spectrum was combined and smoothed twice using the SavitzkyGolay method ± 3 channels, background subtraction (polynominal order 15 and 10% below the curve). Each spectrum was exported and spectra common to each 2-DE spot were merged into a single MASCOT generic format (.mgf) file using the online peak list conversion utility available at www.proteomecommons.org (Falkner et al. 2007).
Mass spectrometry database analysis
Merged files were submitted to MASCOT MSMS ion search set to search the published F. hepatica genomes (Cwiklinski et al. 2015; McNulty et al. 2017). The following parameters were selected for each peptide search: enzyme set at trypsin with one missed cleavage allowed, fixed modifications set for carbamidomethylation with variable modifications considered for oxidation of methionine, monoisotopic masses with unrestricted protein masses were considered, peptide and fragment mass tolerance were set at ± 1.2 Da and 0.6 Da respectively for an ESI QUAD-TDF instrument (Moxon et al. 2010).
In silico investigation of Fasciola transcripts and F. hepatica genome
Sequences representing known GST classes were obtained from NCBI (http://www.ncbi.nlm.nih.gov/). A mammalian and a helminth GST sequence were selected for each GST class where available. GST sequences were used to tBLASTn the F. hepatica transcriptome (Young et al. 2010) and the F. gigantica transcriptome (Young et al. 2011) both available to search at http://bioinfosecond.vet.unimelb.edu.au/wblast2.html. A second F. hepatica transcriptome database (EBI-ENA archive ERP000012: an initial characterisation of the F. hepatica transcriptome using 454-FLX sequencing) was also used to search against. In silico investigation of the known GST sequences and positive transcript hit were blasted against genome sequencing project of F. hepatica (Cwiklinski et al. 2015) and F. gigantica (Choi et al. 2020). The genome of Choi et al. (2020) was chosen over that of Pandey et al. (2020) as the data is freely accessible through NCBI GenBank and Wormbase Parasite. Transcript expression levels for individual F. hepatica GST isoforms were analysed from Cwiklinski et al. (2018) Each specific GST isoform was used to BLASTp the transcriptome to identify the respective expression level.
Cloning of newly identified genes
PCR amplification was carried out on an Applied Biosystems 96 Well Thermal Cycler. PCR of cDNA was performed using MyFi Taq (Bioline) following the manufacturer’s instructions. Standard thermocycler conditions involved an initial denaturation at 95 °C for 2 min, followed by 25–35 cycles of denaturation (95 °C, 30 s), annealing (gradient temperature specific for each gene of interest, 30 s) and extension (72 °C, 30–90 s), before a final extension at 72 °C for 5 min and holding period at 4 °C until products removed. Primers were based on the scaffolds from the F. hepatica genome (FhGST-S2 For: GGGCGATACTATCTATCAACGT Rev: GTGCGACTGACTTTGAATC; FhGST-O2 For: CACACAGCTGGAATTGA TTA Rev: TAATATTGACGGATCCAAACA). PCR products were ligated into pGEM-T-Easy, according to the manufacturer’s protocol and sequenced in house. Sequences were translated using Expasy Translate (https://web.expasy.org/translate/) and molecular weight and pI calculated using Expasy Compute pI/Mw (https://web.expasy.org/compute_pi/). GST domains were predicted using PFam (El-Gebali et al. 2019).
Protein sequence alignment and phylogenetic tree construction
All sequences were aligned using ClustalW through BioEdit Version 7.0.5.3 (10/28/05) (Hall 1999). To construct a phylogenetic tree, an alignment of all GST sequences was exported into Molecular Evolutionary Genetics Analysis (MEGA) software version X (Tamura et al. 2007). Analysis was performed using a neighbour-joining method, 1000-replicate, bootstrapped tree. The amino acid data were corrected for a gamma distribution (level set at 1.0) and with a Poisson correction.
Recombinant Fasciola hepatica glutathione transferase Mu class (rFhGST-Mu5) production
FhGST-Mu5 was amplified via PCR using the following primer pair: rFhGST-Mu5 forward primer, 5′ CATATGGCTCCAGTCTTA 3′; rFhGSTMu5 reverse primer, 5′ GCGGCCGCTTAACTGGGTGGTGCA 3′; and a second reverse primer containing the stop codon 5′ GCGGCCGCACTTTAACTGGGTGGTGCA 3′. Restriction enzyme sites (in bold type and underlined) for NdeI (forward primer) and NotI (reverse primer) were included so that the entire ORF could be directly cloned into the pET23a (Novagen) vector. Recombinant proteins were produced in Escherichia coli BL21 (DE3) cells (Bioline) as described previously (LaCourse et al. 2012; Morphew et al. 2012).
E.coli preparations containing rFhGST-Mu5 were suspended in lysis buffer (containing 5 mM MgCl, 400 mM NaCl and 20 mM sodium phosphate pH 7.4) and were lysed through a freeze/thaw method, freezing in liquid nitrogen followed by thawing at 42 °C three times. This was followed by 3 cycles of ultrasonication; the samples were sonicated for 30 s with 30-s intervals in ice. The samples were centrifuged at 13,200×g for 20 min at 4°C, and purified by GSH affinity chromatography as described previously.
Results
Limited induction of soluble F. hepatica GST by TCBZ-SO
Prior to affinity chromatography, GST enzymatic specific activity was assessed to examine if overall cytosolic GST activity was induced by TCBZ-SO exposure (Table 1). In general, there was a trend to increased GST-specific activity following exposure to TCBZ-SO for treatment groups compared to controls (Online Resource 1). However, following ANOVA, no significant difference was noted between any of the treatment groups or the controls (2df, F = 1.25, P = 0.320).
GST proteomic profiling of individual fluke
Two affinity matrices were used to isolate GST isoforms from individual adult F. hepatica. Eighteen individuals were homogenised independently and all 18 independently processed through GSH or S-Hexyl GSH agarose columns to separate F. hepatica GST proteins from other soluble proteins. Following purification, it was possible to compare the GST-ome from each individual F. hepatica exposed to TCBZ-SO, either a sub-lethal concentration (15 μg/ml) or a lethal concentration (50 μg/ml), versus those not exposed using 2-DE proteomics.
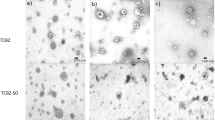
Proteomic arrays of the GSTs purified from S-hexyl GSH-agarose consistently yielded 13 protein spots (Fig. 1a), whereas those purified from GSH-agarose column yielded 11 prominent protein spots (Fig. 1b). All protein spots from both purification systems were confirmed as containing Fasciola GSTs using tandem mass spectrometry (Table 2; full proteomic analysis Online Resource 2). Comparison of 2-DE protein arrays was then performed to establish if there was a change in abundance of the identified GSTs relating to the different treatment of TCBZ-SO (Fig. 1c–f).
Representative 2-DE arrays of GSTs purified from F. hepatica using S-hexyl GSH and GSH agarose columns following TCBZ-SO exposure. (a) S-hexyl GSH agarose-purified GSTs from control samples (TCBZ-SO 0 μg/ml). (b) GSH agarose-purified GSTs from control samples (TCBZ-SO 0 μg/ml). (c) S-hexyl GSH agarose-purified GSTs from sub-lethal samples (TCBZ-SO 15 μg/ml). (d) GSH agarose-purified GSTs from sub-lethal samples (TCBZ-SO 15 μg/ml). (e) S-hexyl GSH agarose-purified GSTs from lethal samples (TCBZ-SO 50 μg/ml). (f) GSH agarose-purified GSTs from lethal samples (TCBZ-SO 50 μg/ml). Spot numbers relate to GST putative identifications seen in Table 2. Spot 14 shown in (a) and (c) was only present on 1 replicate of the lethal arrays and is therefore not shown in the representative array shown in (e)
When comparing the S-Hexyl GSH control array with both the S-Hexyl GSH TCBZ-SO exposed arrays (Fig. 1a, c and e), it was noted that spot 14 was present on all control arrays, thus present on the average control array. However, the presence of this protein spot varied on the TCBZ-SO treatment arrays (sub-lethal and lethal). This particular protein spot was only present on 2 sub-lethal arrays (numbered in Fig. 1c) and 1 lethal array (not visible on the representative array shown in Fig. 1e). MSMS analysis identified this spot as Mu class GST29 (THD21358).
The comparison of the arrays produced via GSH agarose affinity columns identified two protein spots of interest when the average control and average sub-lethal arrays were examined with both spots increased in abundance in sub-lethal samples: spot 5, Mu class GST 26 (1df, F = 3.89, P = 0.089) and spot 7, Mu class GST 29 (1df, F = 4.83, P = 0.064) both approaching statistical significance (Fig. 1b and d; Online Resource 3). No additional changes in protein abundance were observed for GSH purifications.
GST expression in the cytosol of individual fluke and affinity binding
Given the recorded potential of Mu class GSTs responding to TCBZ-SO exposure, Western blotting was used to estimate the number of Mu class GSTs present in liver fluke cytosol prior to affinity chromatography, given previous indications that some GST isoforms may fail to bind to affinity matrices (Brophy et al. 1990). Assays were undertaken on five individual adult flukes using anti-S. mansoni Mu class polyclonal antibodies, previously shown to recognise F. hepatica Mu class GSTs (Chemale et al. 2006). The anti-flatworm GST Mu class antibody recognised 8 GST subunits within the cytosolic profile (Fig. 2a). Post purification, the blot patterns display the same distinctive GST protein profiles following both GSH and S-Hexyl GSH affinity 2-DE gels (Fig. 2b, c). A distinctive and reproducible 2-DE GST profile provides evidence that 8 GST subunits are recognised by the Mu antibody post purification.
Assessment of Mu class GST binding affinity through Western blotting with anti-S. mansoni Mu of affinity-purified GSTs in comparison to cytosolic fractions. (a) Visualisation of 100 μg TCA precipitated cytosolic proteins of F. hepatica adult worms using two-dimensional gel electrophoresis (2-DE) and Western blot analysis probing for Mu class GSTs. (b, c) Visualisation of 5 μg of GSH or S-Hexyl GSH agarose-purified GST subunits of F. hepatica adult worms using two-dimensional gel electrophoresis (2-DE) and Western blot analysis probing for Mu class GSTs
Bioinformatic characterisation of GSTs identified in F. hepatica
Following analysis of available transcript and genome sequences, the known 4 Mu class GSTs were identified alongside a fifth Mu class GST designated FhGST-Mu5. Following cloning and sequencing of FhGST-Mu5, multiple alignment of all Mu class GSTs of F. hepatica revealed the extent of identity and similarity across this class of GSTs (Fig. 3a). Amino acid sequence similarity when comparing the newly identified FhGST-Mu5 (Genbank MT613329) with the previously known F. hepatica Mu class GSTs identified the closest sequence similarity was with FhGST-7 at approximately 54%. It is also worth noting that GenBank entry THD26413 matches to FhGST-Mu5 with 91.9% sequence identity but is an incomplete sequence lacking the N-terminus. When transcript expression was analysed for FhGST-Mu5 based on Cwiklinski et al. (2018), the levels of transcript within the adult are significantly lower than those in alternative life cycle stages such as metacercariae and newly excysted juveniles from 1 to 24 h (Fig. 3b). Of note was the identification of a homologue of FhGST-Mu5 within F. gigantica. Two accession numbers (TPP60771 and TPP66459) were retrieved from genome searches albeit both representing incomplete sequences.
Bioinformatics, expression and purification of recombinant rFhGST-Mu5. a Multiple sequence alignment of the 4 established F. hepatica Mu class GSTs and the newly identified FhGST-Mu5. No other Mu class GSTs were identified within the genome of F. hepatica. A putative SNAIL/TRAIL motif and their synonymous sequences in parasites are in the solid-line grey box. FhGST-Mu5 demonstrates 3 out of the 5 residues match with this motif with a fourth a highly conserved switch of the leucine residue to an isoleucine. The residues forming the μ-loop are in dotted-line grey box. Arrowed are predicted GSH-binding sites. Amino acid sequence identity of FhGST-Mu5 with the four previously known Mu class GSTs is provided at the end of the alignment. Accession numbers for each Mu class GST used: FhGST-Mu29/1 (P56598), FhGST-Mu28/7 (P31671), FhGST-Mu27/47 (P31670) and FhGST-Mu26/51 (P30112). b Transcript expression levels for FhGST-Mu5 were analysed from Cwiklinski et al. (2018). c SDS-PAGE gel of the expression and purification of rFhGST-Mu5. L: E. coli total cytosolic protein lysate, 10 μg. W1 and W2: column washes removing non-binding proteins, 10 μl. FT: flow through proteins collected after passing through a GSH-agarose column, 10 μg. E: eluted GSH affinity-purified recombinant rFhGST-Mu5 protein, 2 μg. Arrowed is the band representing rFhGST-Mu5
Further transcript and genome investigation allowed the examination of the complete GST-ome of F. hepatica and a more complete understanding of F. gigantica. In addition to FhGST-Mu5, in silico investigation revealed the identification of a second Sigma class and a second Omega class GST within F. hepatica. Bioinformatic characterisation of the new FhGST-S2 and FhGST-O2 was undertaken to identify the structural features and characteristics of these genes/proteins. Only a single homologue for each was identified in the original F. hepatica genome. For FhGST-S2, gene BN1106_s1104B000225 (Scaffold 1104) was identified yet this is now fragmented and incomplete in the most recent version of the genome (PRJEB25283) despite transcript support (Online Resource 4). In addition, a F. gigantica homologue of FhGST-S2 was also identified in the recent genome (TPP56382). However, unlike F. hepactica, the F. gigantica genome revealed a third potential Sigma class GST (FgGST-S3; TPP56383). For FhGST-O2, gene BN1106_s50B000678 (Scaffold 50) was revealed and is now designated as maker-scaffold10x_938_pilon-snap-gene-0.52/D915_03058. This was mirrored within F. gigantica (TPP65079). Each gene encoded for a predicted single protein isoform.
Both the newly predicted FhGST-S2 and FhGST-O2 were cloned (Online Resource 5a) and sequenced. Confirmation of the correct class assignment was performed with multiple alignment (Online Resource 5b and 5c) and comparison of gene intron exon structure (Online Resource 6). Of note was a significant N-terminal extension of 20 amino acids in FhGST-S2 when compared to FhGST-S1. FhGST-O2 in comparison to FhGST-O1 revealed the addition of 1 amino acid to each of exons 1 and 5. Further confirmation of class assignment was supported with both FhGST-S2 and FhGST-O2 subjected to a PFam domain analysis revealing key-predicted GST features: FhGST-S2 with a predicted C-terminal domain (PFam GST_C_3) and FhGST-O2 with a predicted N- and C-terminal domain (PFam GST_N_3 and GST_C_2).
Following a full phylogenetic analysis of the completed F. hepatica GST-ome, all of the newly identified FhGST-Mu5, FhGST-S2 and FhGST-O2, in addition to the F. gigantica homologues, were assigned to their respective clades (Fig. 4). Of note is the close association of FhGST-Mu5 to the Schistosome Mu class GSTs rather than to the previously established four Fasciola Mu class isoforms.
Phylogenetic analysis of the soluble cytosolic GST superfamily. All reported accession numbers are from GenBank. Where sequences were identified in silico, only contig numbers are reported. Those from F. gigantica were taken from the study of Choi et al. (2020), Young et al. (2011) and transcripts produced by Aberystwyth University. Those from F. hepatica were taken from the study of Young et al. (2010) and transcripts produced by the University of Liverpool (EBI-ENA archive ERP000012: an initial characterisation of the F. hepatica transcriptome using 454-FLX sequencing)
Expression, purification and characterisation of rFhGST-Mu5
Full sequence length recombinant F. hepatica Mu class GST (rFhGST-Mu5) was expressed and purified from transformed E. coli cytosol following expression in BL21 (DE3) cells. Purity was assessed on SDS-PAGE gels (Fig. 3c). Interestingly, rFhGST-Mu5 was not able to be produced as a pure protein with significant levels of contaminating E. coli proteins remaining in the sample following GSH affinity purification. However, rFhGST-Mu5 was produced as an active protein for further studies displaying enzymatic activity towards the model GST substrate 1-chloro-2,4-dinitrobenzene (CDNB). The specific activity for the rFhGST-Mu5 preparation was confirmed at 243.27 ± 92.45 nmol/min/mg.
Discussion
The 2-DE mapping of GSTs has been shown to be a useful tool to delineate the function of individual members of this soluble protein superfamily (Chemale et al. 2006; Morphew et al. 2012), particularly as these proteins play a role in phase II detoxification (Cvilink et al. 2009). To date, research has only been completed on pooled cytosol samples from wild-type fluke and defined isolates and there has not been a robust sub-proteomic study that compared the expression of GST isotypes in individual fluke populations under TCBZ-SO challenge in culture. This study has adapted the pooled approach and, for the first time, performed analytical scale 2-DE mapping of GSTs from individual F. hepatica adult parasites. Thus, GSTs were purified from the cytosol of single adult flukes using either S-Hexyl-GSH or GSH agarose columns, resolved using analytical 2-DE and identified individual GSTs by MSMS with the support of liver fluke transcriptomic and genomic databases. In doing so, we can identify individual fluke responses within the GST superfamily following exposure to chemotherapeutics. Furthermore, this finding has major implications for future population and resistance monitoring studies specifically on, but not limited to, liver fluke GSTs.
In the current study, both S-Hexyl GSH agarose and GSH agarose columns were used for GST purification at the individual fluke level. Previous studies (Chemale et al. 2006; Morphew et al. 2012) have demonstrated that S-Hexyl GSH agarose columns have the ability to purify a greater range of GSTs in both F. hepatica and F. gigantica population mixes respectively, thus was a useful inclusion in the current work at the individual fluke level. Using biochemical techniques and analytical sub-proteomics identified both Sigma and Mu class GSTs purified from individual adult F. hepatica. It was confirmed that both S-Hexyl GSH and GSH agarose columns have the ability to purify both Mu and Sigma class GSTs, but with GSH columns purifying the Sigma class to a much lesser extent expressing a preference to purify Mu class GSTs as observed for pooled samples (Chemale et al. 2006).
The overall GST-ome profile, via GST activity and 2-DE arrays, demonstrated a general trend of response to TCBZ-SO exposure. Following exposure, GST-specific activity increased with increasing TCBZ-SO concentration. In addition, the only changes noted in both S-Hexyl GSH and GSH agarose purifications were recorded abundance changes associated with Mu class GSTs, specifically FhGST-Mu29 and FhGST-Mu26. Therefore, from activity data and proteomic profiling, it is likely that, of the two GST classes identified, Mu class GSTs are likely highly important for xenobiotic detoxification with Sigma class GSTs acting as secondary xenobiotic sequesters with a primary role as a house-keeping enzyme and as, more importantly, an immunomodulatory (LaCourse et al. 2012). This finding of Mu class GST-TCBZ-SO detoxification supports the work of Chemale et al. (2010) examining the TCBZ-SO response of TCBZ-resistant and TCBZ-susceptible isolates. In this case, both FhGST-Mu29 and FhGST-Mu26 responded to TCBZ-SO exposure, in agreement with the current study. We identified changes in response to TCBZ-SO exposure linked to dimer and monomer formation of FhGST-Mu29 and differential purification of both using the two purification methods. S-hexyl GSH purification was more efficient at purifying FhGST-Mu29 dimers compared to GSH agarose purification. On exposure to TCBZ-SO, a reduction in FhGST-Mu29 dimers was observed with a corresponding increase in FhGST-Mu29 monomers purified through GSH agarose. The novel dimer-monomer GST conformational switch might reflect a new liver fluke mechanism in response to TCBZ-SO challenge. GSTs normally function as dimers but active monomeric GSTs have been previously identified in F. hepatica (Brophy et al. 1990).
In F. hepatica, there are four recognised isoforms of Mu class GSTs, i.e. FhGST-Mu26, 27, 28 and 29 (alternatively called FhGST-Mu51, 47, 7 and 1), with a fifth identified only through bioinformatics previously (Morphew et al. 2012), and now cloned and expressed in the current work. Alongside the identification of FhGST-S1, four of the five Mu class GST isoforms were identified in the samples examined in the current study under TCBZ-SO stress. In previous proteomic studies, the same four classes have also been identified. However, the functional significance of multiple Mu GSTs is as yet unknown. Multiple Mu class isoforms might relate to their role in the protection of the parasite from various classes of xenobiotics derived from the host bile environment (Brophy et al. 2012). Specifically, the current work supports a role for FhGST-Mu29 in TCBZ-SO response via conformational changes as identified by evidence of altered in dimer/monomer ratios. Of interest, based on transcriptome evidence of Cwiklinski et al. (2018), FhGST-Mu29 is naturally the highest expressed Mu class GST in adult fluke. In addition, FhGST-Mu26 ranks third in all Mu class GST expression (FhGST-Mu29 > Mu27 > Mu26 > Mu5 > Mu28). Therefore, it is likely that these primary expressed GSTs are important in binding xenobiotics with structures such as such as TCBZ-SO.
In many cases, peptides belonging to different GSTs were identified in a single protein spot providing the identification of multiple GST isoforms. As reported by Chemale et al. (2006), this may result from spot overlapping in the 2-DE gels, as proteins may have a similar pI, potential modifications and co-migration. Of note is the failure to identify the fifth Mu class GST, FhGST-Mu5, despite overlapping GST isoforms identified in multiple spots. Given the sequence similarity of 54% for FhGST-Mu5 compared to FhGST-7, the failure to identify FhGST-Mu5 is unlikely to be from mis-assigning sequenced peptides to alternative Mu class GSTs and likely represents low expression as evidenced from transcriptomics (Cwiklinski et al. 2018) or, given the poor affinity purification of FhGST-Mu5, non-binding to affinity columns.
In an attempt to assess if FhGST-Mu5 was not identified in affinity-purified samples as a result of non-binding, F. hepatica cytosolic material was probed with anti-S. mansoni Mu polyclonal antibodies and compared with the profiles obtained post affinity purification. Given that the same repertoire of protein spots following Western blotting was visualised on both cytosolic and affinity-purified fractions, in addition to FhGST-Mu5 recognition by anti-S. mansoni Mu (data not shown), it seems unlikely that FhGST-Mu5 was missed in the affinity proteomics study. In addition, it seems unlikely that FhGST-Mu5 was missed due to low expression in adults given the identification of FhGST-Mu28 in the current work and in previous studies (Chemale et al. 2006). Thus, the potential exists that FhGST-Mu5 is a low-affinity isoform. In support, Brophy et al. (1990) proposed that an endogenous ligand interacts with GSTs preventing GST binding to the affinity matrix generating a ‘low-affinity’ fraction. Therefore, general inhibitory binding factors are likely present in the liver fluke cytosol and may be important in flatworm GST function.
Following the successful induction and expression of FhGST-Mu5, it is clear that ‘low-affinity’ GSTs are produced within the GST-ome of F. hepatica yet not all GSTs fail to bind from potential inhibitory factors. GSH affinity purification of rFhGST-Mu5 resulted in low impure yields of recombinant protein and suggests that FhGST-Mu5 is a ‘low-affinity’ isoform. Previous studies have all successfully used GSH affinity chromatography for successful purification of native and recombinant GSTs (Chemale et al. 2006; LaCourse et al. 2012; Morphew et al. 2012) yet failed to purify FhGST-Mu5. Thus, to determine if rFhGST-Mu5 is an isoform with ‘low affinity’ for GSH, the specific activity was determined with the model substrate CDNB (Habig et al. 1974). The specific activity of rFhGST-Mu5 was significantly lower than that recorded for the previously known 4 Mu class GSTs from F. hepatica (Kalita et al. 2017; Salvatore et al. 1995). This lower affinity may be correlated with the lower sequence homology and the more distant grouping of FhGST-Mu5 in phylogenetic modelling aligning closer to schistosome Mu class GSTs rather than the previous four F. hepatica Mu class. Brophy et al. (1990) demonstrated that following chromatofocusing, 95% of ‘low-affinity’ GSTs were relieved of their inhibition and thus, based on current evidence, it is likely that FhGST-Mu5 could indeed be classed as a ‘low-affinity’ Mu class GST as part of the remaining 5% of activity. Low GSH affinity most likely accounts for the previous lack of detection during affinity studies with the initial identification achieved through transcriptomic analysis (Morphew et al. 2012). Given that FhGST-Mu5 clustered with schistosome Mu class GSTs during phylogenetics, it is possible that FhGST-Mu5 and schistosome Mu class GSTs perform similar roles within these fluke species. Given FhGST-Mu5 was not purified to homogeneity using GSH affinity, as evidenced by 1D SDS PAGE, additional purification steps are required in order to biochemically confirm the role of FhGST-Mu5.
The current study represents the first 2-DE profiling of TCBZ-SO exposed F. hepatica GSTs. However, TCBZ-SO stress in F. gigantica, and the resulting GST activity, has been previously investigated. Shehab et al. (2009) examined GST activities from crude homogenates of adult and juvenile F. gigantica exposed to TCBZ-SO concentrations. This research indicated that a significant increase in the level of GST was present, in both adult and juvenile flukes, after exposure to TCBZ-SO (Shehab et al. 2009). Such a significant increase in response to TCBZ-SO prior to affinity purification was not noted in the current research and may reflect important differences between F. hepatica and F. gigantica GST expression. Nevertheless, the work of Shehab and colleagues further supports the role of Mu class GST in TCBZ-SO detoxification.
The release of the genome assemblies of F. hepatica (Cwiklinski et al. 2015; McNulty et al. 2017) has allowed for further in-depth and complete investigation of the GST-ome complement of this parasitic flatworm. This has additionally been supported with the release of a genome for F. gigantica (Choi et al. 2020). Two new soluble superfamily GSTs were identified in F. hepatica; a second Sigma (σ) class and a second Omega (ω) class, on original genes BN1106_s1104B000225 and BN1106_s50B000678 (scaffolds 1104 and 50, respectively). Both GSTs contained Pfam IDs for the respective GSTs and both sequences were successfully amplified through PCR and sequence verified. The predicted molecular weight of the sub-units of the newly identified Sigma and Omega GSTs was shown to be 26 and 27 kDa, respectively, and this is in general agreement with known soluble GSTs that have a subunit mass of between 23 and 28 kDa with an average length of 220 amino acids (Torres-Rivera and Landa 2008). Gene structure analysing introns and exons for both the newly identified Sigma and Omega genes in comparison with the previously identified F. hepatica Sigma and Omega supported the confirmation of GST class assignment.
Previous research has demonstrated that model organisms (humans and mice) both encode for 2 Omega class GST genes which are widely expressed (Board 2011) reflecting expression within F. hepatica and now F. gigantica, albeit human and mice omega GSTs comprise of six exons (Board 2011) rather than 5 in F. hepatica omega class GSTs. Interestingly, omega class GSTs have been linked with drug resistance in human cancers (Townsend and Tew 2003) and Alzheimer’s disease (Allen et al. 2012) and thus may have some role in anthelmintic resistance or detoxification not yet discovered.
Sigma class GSTs in F. hepatica were also initially identified by Chemale et al. (2006). A recombinant form of F. hepatica Sigma class GST, FhGST-S1, has since been produced and demonstrated to have multi-functional roles, including general endogenous detoxification, and is strongly linked with prostaglandin synthesis and the modulation of dendritic cell activity (LaCourse et al. 2012). Across trematode species, the exon-intron structure of Sigma class GSTs is conserved. Recently, reports of 5 newly identified Sigma class GSTs from Clonorchis sinensis consist of 4 exons akin to the two F. hepatica genes (Bae et al. 2016). It was also noted that the final exon, exon 4, of Sigma GST genes in the gene predictions of all the trematode species investigated by Bae et al. (2016) consisted of 225 bp; this conservation of gene structure likely reflects conserved biological function. As yet, proteomic investigations have not identified FhGST-S2 from adult flukes despite their presence in adult transcriptomes. It is therefore likely that FhGST-S2 remains part of the unbound fraction of the GST-ome; a likely ‘low-affinity’ sigma class GST. Interestingly, bioinformatics has revealed a potential expansion of the Sigma class GSTs within F. gigantica with a third potential member identified clustering alongside a C. sinensis Sigma class GST. Further in-depth experimentation will be required to confirm this finding.
With a key role for GSTs in the detoxification of TCBZ demonstrated through proteomic profiling, it is now crucial to understand any involvement of GSTs in TCBZ resistance. This is of particular importance given that Scarcella et al. (2012) identified that fluke resistant to TCBZ expressed significantly higher levels of GST activity compared to susceptible flukes. The authors suggest that under TCBZ-SO exposure, there is an increased requirement for phase I detoxification of TCBZ-SO, to the less-effective TCBZ-SO2, and thus also require increased phase II detoxification, principally from GSTs, to catalyse TCBZ intermediates. Given the recent bioinformatics identification of a potential Cytochrome P450 (Cwiklinski et al. 2015), TCBZ-SO exposure is likely to stimulate this phase I pathway leading to an increased requirement for phase II GSTs. Therefore, profiling the specific GST isoforms will give more insight into resistance mechanisms.
Conclusions
GSTs are a multi-gene family of ubiquitous multifunctional proteins that are predicted to have major roles in detoxifying both endogenous and exogenous toxins as part of the phase II system. We have expanded the knowledge on this important protein family in the parasitic flatworm F. hepatica. In doing so, we have revealed 5 Mu class, 2 Sigma class (3 in F. gigantica), 2 Omega class and 1 Zeta class GSTs including novel ‘low-affinity’ Mu and Sigma class enzymes. In addition, it is clear that GSTs respond to TCBZ-SO exposure and the role of GSTs in TCBZ resistance awaits further investigation. Finally, the ability to incorporate individual fluke for proteomic and sub-proteomic studies has implications for potential early TCBZ resistance monitoring in liver fluke populations.
Data availability
Full proteomics datasets are provided as an online resource. All raw files associated with the proteomics are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Allen M, Zou FG, Chai HS, Younkin CS, Miles R, Nair AA, Crook JE, Pankratz VS, Carrasquillo MM, Rowley CN, Nguyen T, Ma L, Malphrus KG, Bisceglio G, Ortolaza AI, Palusak R, Middha S, Maharjan S, Georgescu C, Schultz D, Rakhshan F, Kolbert CP, Jen J, Sando SB, Aasly JO, Barcikowska M, Uitti RJ, Wszolek ZK, Ross OA, Petersen RC, Graff-Radford NR, Dickson DW, Younkin SG, Ertekin-Taner N (2012) Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: an association study with mechanistic implications. Mol Neurodegener 7:13. https://doi.org/10.1186/1750-1326-7-13
Alvarez LI, Solana HD, Mottier ML, Virkel GL, Fairweather I, Lanusse CE (2005) Altered drug influx/efflux and enhanced metabolic activity in triclabendazole-resistant liver flukes. Parasitology 131:501–510. https://doi.org/10.1017/s0031182005007997
Alvarez L, Moreno G, Moreno L, Ceballos L, Shaw L, Fairweather I, Lanusse C (2009) Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet Parasitol 164:211–216. https://doi.org/10.1016/j.vetpar.2009.05.014
Andrews SJ (1999) The life cycle of Fasciola hepatica. In: Dalton JP (ed) Fasciolosis. C. A. B. International, Oxford, pp 1–30
Bae YA, Kim JG, Kong Y (2016) Phylogenetic characterization of Clonorchis sinensis proteins homologous to the sigma-class glutathione transferase and their differential expression profiles. Mol Biochem Parasitol 206:46–55. https://doi.org/10.1016/j.molbiopara.2016.01.002
Board PG (2011) The omega-class glutathione transferases: structure, function, and genetics. Drug Metab Rev 43:226–235. https://doi.org/10.3109/03602532.2011.561353
Boray JC (1997) Chemotherapy of infections with fasciolidae. In: Boray JC (ed) Immunology, pathobiology and control of Fasciolosis. MSD AGVET, Rahway, pp 83–97
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal Biochem 72:248–254
Brennan GP, Fairweather I, Trudgett A, Hoey E, McCoy MCM, Meaney M, Robinson M, McFerran N, Ryan L, Lanusse C, Mottier L, Alvarez L, Solana H, Virkel G, Brophy PM (2007) Understanding triclabendazole resistance. Exp Mol Pathol 82:104–109. https://doi.org/10.1016/j.yexmp.2007.01.009
Brophy PM, Crowley P, Barrett J (1990) Detoxification reactions of Fasciola hepatica cytosolic glutathione transferases. Mol Biochem Parasitol 39:155–162
Brophy PM, Mackintosh N, Morphew RM (2012) Anthelmintic metabolism in parasitic helminths: proteomic insights. Int J Parasitol 139:1205–1217
Chemale G, Morphew R, Moxon JV, Morassuti AL, LaCourse EJ, Barrett J, Johnston DA, Brophy PM (2006) Proteomic analysis of glutathione transferases from the liver fluke parasite, Fasciola hepatica. Proteomics 6:6263–6273. https://doi.org/10.1002/pmic.200600499
Chemale G, Perally S, LaCourse EJ, Prescott MC, Jones LM, Ward D, Meaney M, Hoey E, Brennan GP, Fairweather I, Trudgett A, Brophy PM (2010) Comparative proteomic analysis of triclabendazole response in the liver fluke Fasciola hepatica. J Proteome Res 9:4940–4951
Choi YJ, Fontenla S, Fischer PU, Le TH, Costabile A, Blair D, Brindley PJ, Tort JF, Cabada MM, Mitreva M (2020) Adaptive radiation of the flukes of the family Fasciolidae inferred from genome-wide comparisons of key species. Mol Biol Evol 37:84–99. https://doi.org/10.1093/molbev/msv204
Cvilink V, Lamka J, Skalova L (2009) Xenobiotic metabolizing enzymes and metabolism of anthelminthics in helminths. Drug Metab Rev 41:8–26
Cwiklinski K, Dalton JP, Dufresne PJ, La Course J, Williams DJL, Hodgkinson J, Paterson S (2015) The Fasciola hepatica genome: gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol 16:71. https://doi.org/10.1186/s13059-015-0632-2
Cwiklinski K, Jewhurst H, McVeigh P, Barbour T, Maule AG, Tort J, O’Neill SM, Robinson MW, Donnelly S, Dalton JP (2018) Infection by the helminth parasite Fasciola hepatica Requires Rapid Regulation of Metabolic, Virulence, and Invasive Factors to Adjust to Its Mammalian Host. Mol Cell Proteomics 17:792–809. https://doi.org/10.1074/mcp.RA117.000445
El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD (2019) The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. https://doi.org/10.1093/nar/gky995
Falkner JA, Falkner JW, Andrews PC (2007) ProteomeCommons.org IO Framework: reading and writing multiple proteomics data formats. Bioinformatics 23:262–263. https://doi.org/10.1093/bioinformatics/btl573
Fernandez V, Estein S, Ortiz P, Luchessi P, Solana V, Solana H (2015) A single amino acid substitution in isozyme GST mu in triclabendazole resistant Fasciola hepatica (Sligo strain) can substantially influence the manifestation of anthelmintic resistance. Exp Parasitol 159:274–279. https://doi.org/10.1016/j.exppara.2015.10.007
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hayes JD, Pulford DJ (1995) The glutathione S-Transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600. https://doi.org/10.3109/10409239509083491
Kalita J, Shukla R, Shukla H, Gadhave K, Giri R, Tripathi T (2017) Comprehensive analysis of the catalytic and structural properties of a mu-class glutathione s-transferase from Fasciola gigantica. Sci Rep 7:17547. https://doi.org/10.1038/s41598-017-17678-3
LaCourse JE, Perally S, Morphew RM, Moxon JV, Prescott MC, Dowling D, O’Neill SM, Kipar A, Hetzel U, Hoey E, Zafra R, Buffoni L, Arevalo JP, Brophy PM (2012) The sigma class glutathione transferase of the liver fluke Fasciola hepatica. Plos Neglect Trop D 6:e1666
Mannervik B, Board PG, Hayes JD, Listowsky I, Pearson WR (2005) Nomenclature for mammalian soluble glutathione transferases. Methods Enzymol 401:1–8
McNulty SN, Tort JF, Rinaldi G, Fischer K, Rosa BA, Smircich P, Fontenla S, Choi YJ, Tyagi R, Hallsworth-Pepin K, Mann VH, Kammili L, Latham PS, Dell’Oca N, Dominguez F, Carmona C, Fischer PU, Brindley PJ, Mitreva M (2017) Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of potomac horse and human sennetsu fevers. PLoS Genet 13(1):e1006537. https://doi.org/10.1371/journal.pgen.1006537
Morphew RM, Wright HA, LaCourse EJ, Porter J, Barrett J, Woods DJ, Brophy PM (2011) Towards delineating functions within the Fasciola secreted cathepsin l protease family by integrating in vivo based sub-proteomics and phylogenetics. PLoS Negl Trop Dis 5:e937. https://doi.org/10.1371/journal.pntd.0000937
Morphew RM, Eccleston N, Wilkinson TJ, McGarry J, Perally S, Prescott M, Ward D, Williams D, Paterson S, Raman M, Ravikumar G, Saifullah MK, Abidi SMA, McVeigh P, Maule AG, Brophy PM, LaCourse EJ (2012) Proteomics and in silico approaches to extend understanding of the glutathione transferase superfamily of the tropical liver fluke Fasciola gigantica. J Proteome Res 11:5876–5889
Morphew RM, MacKintosh N, Hart EH, Prescott M, LaCourse EJ, Brophy PM (2014) In vitro biomarker discovery in the parasitic flatworm Fasciola hepatica for monitoring chemotherapeutic treatment. EuPA Open Proteom 3:85–99
Moxon JV, LaCourse EJ, Wright HA, Perally S, Prescott MC, Gillard JL, Barrett J, Hamilton JV, Brophy PM (2010) Proteomic analysis of embryonic Fasciola hepatica: characterization and antigenic potential of a developmentally regulated heat shock protein. Vet Parasitol 169:62–75. https://doi.org/10.1016/j.vetpar.2009.12.031
Pandey T, Ghosh A, Todur VN, Rajendran V, Kalita P, Kalita J, Shukla R, Chetri PB, Shukla H, Sonkar A, Lyngdoh DL, Singh R, Khan H, Nongkhlaw J, Das KC, Tripathi T (2020) Draft genome of the liver fluke Fasciola gigantica. Acs Omega 5:11084–11091. https://doi.org/10.1021/acsomega.0c00980
Salvatore L, Wijffels G, Sexton JL, Panaccio M, Mailer S, McCauley I, Spithill TW (1995) Biochemical analysis of recombinant glutathione S-transferase of Fasciola hepatica. Mol Biochem Parasitol 69:281–288
Scarcella S, Lamenza P, Virkel G, Solana H (2012) Expression differential of microsomal and cytosolic glutathione-S-transferases in Fasciola hepatica resistant at triclabendazole. Mol Biochem Parasitol 181:37–39. https://doi.org/10.1016/j.molbiopara.2011.09.011
Shehab AY, Ebeid SM, El-Samak MY, Hussein NM (2009) Detoxifying and anti-oxidant enzmes of Fasciola gigantica worms under triclabendazole sulphoxide (TCBZ-SX): an in vitro study. J Egypt Soc Parasitol 39:73–83
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thomas I, Coles GC, Duffus K (2000) Triclabendazole-resistant Fasciola hepatica in southwest Wales. Vet Rec 146:200–200
Torres-Rivera A, Landa A (2008) Glutathione transferases from parasites: a biochemical view. Acta Trop 105:99–112. https://doi.org/10.1016/j.actatropica.2007.08.005
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375. https://doi.org/10.1038/sj.onc.1206940
Young ND, Hall RS, Jex AR, Cantacessi C, Gasser RB (2010) Elucidating the transcriptome of Fasciola hepatica - a key to fundamental and biotechnological discoveries for a neglected parasite. Biotechnol Adv 28:222–231. https://doi.org/10.1016/j.biotechadv.2009.12.003
Young ND, Jex AR, Cantacessi C, Hall RS, Campbell BE, Spithill TW, Tangkawattana S, Tangkawattana P, Laha T, Gasser RB (2011) A portrait of the transcriptome of the neglected trematode, Fasciola gigantica-biological and biotechnological implications. PLoS Negl Trop Dis 5:e1004. https://doi.org/10.1371/journal.pntd.0001004
Acknowledgements
The authors are grateful to Randall Parker Foods (Wales) for providing F. hepatica–infected sheep livers.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council through an IBERS PhD Scholarship award and through Innovate UK (Grant Number: 102108).
Author information
Authors and Affiliations
Contributions
Peter Brophy and Russell Morphew contributed to the study conception and design. Rebekah Stuart, Suzanne Zwaanswijk, Neil MacKintosh, Boontarikaan Witikornkul and Russell Morphew contributed to material preparation, data collection and analysis. The first draft of the manuscript was written by Rebekah Stuart and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Section Editor: Xing-Quan ZHU
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stuart, R.B., Zwaanswijk, S., MacKintosh, N.D. et al. The soluble glutathione transferase superfamily: role of Mu class in triclabendazole sulphoxide challenge in Fasciola hepatica. Parasitol Res 120, 979–991 (2021). https://doi.org/10.1007/s00436-021-07055-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07055-5