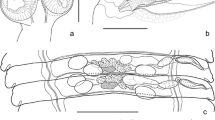
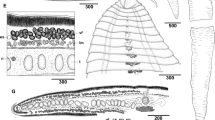
Abstract
Species of the genus Arostrilepis were discovered and definitively identified for the first time in rodents from geographically disparate localities along the Appalachian Mountain range of eastern North America (West Virginia, Virginia, and Maine). These are the first confirmed records for species of Arostrilepis occurring east of the Rocky Mountains and the Mississippi River in North America. Arostrilepis gardneri n. sp. is described on the basis of specimens obtained from two phylogenetically divergent rodent hosts: Southern Red-Backed Vole Myodes gapperi (Cricetidae: Arvicolinae) (from West Virginia) and the Woodland Jumping Mouse Napaeozapus insignis (Dipodidae: Zapodinae) (West Virginia, Virginia, and Maine). Additionally, in a mixed infection, specimens of Arostrilepis insperata n. sp. were also found in a Southern Red-Backed Vole from West Virginia. These previously unknown species are primarily distinguished from congeners based on shape, dimensions, and spination (pattern, shape, and size of spines) of the cirrus. Specimens of A. gardneri n. sp. are further characterized by the relative position and length of the cirrus-sac, arrangement of the testes, and relative size of the external seminal vesicle and seminal receptacle. Specimens of A. insperata n. sp. are structurally most similar to A. macrocirrosa from the western Nearctic and Palearctic but with consistently greater dimensions for the cirrus-sac, testes, and seminal receptacle. Phylogenetic analysis of Arostrilepis spp. using partial sequences of the mitochondrial cytochrome b gene and the nuclear second ribosomal internal transcribed spacer strongly supported the status of A. gardneri n. sp. and A. insperata n. sp. within an unresolved clade of congeners in Red-Backed Voles (Myodini and species of Myodes). Our observations extend the known geographic distribution for species of Arostrilepis to the Appalachian Mountains in either a disjunct or possibly continuous but patchy range across North America. Prior observations, summarizing field and museum collections, had suggested that geographic ranges for a diverse assemblage of Arostrilepis in North America were largely restricted to the north-western region of the continent, with historical connections to Beringia and Eurasia. Recognition of a more extensive distribution is consistent with a history of episodic biotic expansion and isolation under a dynamic of taxon pulses for arvicoline rodents and an associated parasite fauna in the Nearctic during the Quaternary. Occurrence in a dipodid rodent represents an event of host colonization from an arvicoline source.






Similar content being viewed by others
References
Agosta SJ, Janz N, Brooks DR (2010) How specialists can be generalists: resolving the ‘parasite paradox’ and implications for emerging infectious disease. Zoologia 27:151–162. https://doi.org/10.1590/51984-46702010000200001
Araujo SBL, Braga MP, Brooks DR, Agosta S, Hoberg EP, von Hathental F, Boeger WA (2015) Understanding host-switching by ecological fitting. PLoS One 10(10):e0139225. https://doi.org/10.1371/journal.pone.0139225
Brooks DR, Hoberg EP, Boeger W (2019) The Stockholm paradigm: climate change and emerging disease. University of Chicago Press, p 423. https://doi.org/10.7208/chicago/9780226632582.001.0001
Cook JA, Hoberg EP, Koehler A, Henttonen H, Wickström L, Haukisalmi V, Galbreath K, Chernyavski F, Dokuchaev N, Lahzuhtkin A, MacDonald SO, Hope A, Waltari E, Runck A, Veitch A, Popko R, Jenkins E, Kutz S, Eckerlin R (2005) Beringia: intercontinental exchange and diversification of high latitude mammals and their parasites during the Pliocene and quaternary. Mammal Stud 30:S33–S44. https://doi.org/10.3106/1348-6160(2005)30[33:BIEADO]2.0.CO;2
Cook JA, Galbreath KE, Bell KC, Campbell ML, Carrière S, Colella JP, Dawson NG, Dunnam JL, Eckerlin RP, Greiman SE, Federov V, Hass GMS, Haukisalmi V, Henttonen H, Hope AG, Jackson D, Jung T, Koehler AVA, Kinsella M, Krejsa D, Kutz SJ, Liphardt S, MacDonald SO, Malaney JL, Makarikov A, Martin J, Mclean B, Mulders R, Nyamsuren B, Talbot SL, Tkach V, Tsvetkova A, Toman HM, Waltari E, Whitman J, Hoberg EP (2017) The Beringian coevolution project: holistic collections of mammals and associated parasites reveal novel perspectives on changing environments in the north. Arct Sci 3:585–617. https://doi.org/10.1139/as-2016-0042
Doran DJ (1954) A catalogue of the protozoa and helminths of north American rodents II. Cestodes. Am Midl Nat 52:469–480
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797
Erickson AB (1938) Parasites of some Minnesota Cricetidae and Zapodidae, and a host catalogue of helminth parasites of native American mice. Am Midl Nat 20:575–589
Fedorov KP (1986) Patterns of spatial distribution of parasitic worms. Nauka, Novosibirsk, p 256 (In Russian)
Galbreath KE, Hoberg EP (2015) Host responses to historical climate change shape parasite communities in North America’s intermountain west. Folia Zool 64:218–232. https://doi.org/10.25225/fozo.v64.i3.a4.2015
Galbreath KE, Ragaliauskaitė K, Kontrimavicius LV, Makarikov AA, Hoberg EP (2013) A widespread distribution for Arostrilepis tenuicirrosa (Eucestoda: Hymenolepididae) in Myodes voles (Cricetidae: Arvicolinae) from the Palearctic based on molecular and morphological criteria. Historical and biogeographic implications. Acta Parasitol 58:441–452. https://doi.org/10.2478/s11686-013-0170-6
Galbreath KE, Hoberg EP, Cook JA, Bell KC, Campbell ML, Dunnum JL, Eckerlin RP, Gardner SL, Greiman SE, Henttonen H, Agustín Jiménez F, Koehler VA, Tkach VV, Hope AG (2019) Building an integrated infrastructure for exploring biodiversity: field collections and archives of mammals and parasites. J Mammal 100:382–393. https://doi.org/10.1093/jmammal/gyz048
Gulyaev VD, Chechulin AI (1997) Arostrilepis microtis n. sp. (Cyclophyllidea: Hymenolepididae), a new cestode species from Siberian rodents. Res Rev Parasitol 57:103–107
Haukisalmi V, Hardman LM, Foronda P, Feliu C, Laakonen J, Niemimaa J, Lehtonen JT, Henttonen H (2010) Systematic relationships of hymenolepidid cestodes of rodents and shrews inferred from sequences of 28S ribosomal RNA. Zool Scr 39:631–641. https://doi.org/10.1111/j.1463-6409.2010.00444.x
Haukisalmi V, Hardman LM, Hoberg EP, Henttonen H (2014) Phylogenetic relationships and taxonomic revision of Paranoplocephala Lühe, 1910 sensu lato (Cestoda, Cyclophyllidea, Anoplocephalidae). Zootaxa 3873:371–415. https://doi.org/10.11646/zootaxa.3873.4.3
Hoberg EP, Brooks DR (2008) A macroevolutionary mosaic: episodic host-switching, geographic colonization, and diversification in complex host-parasite systems. J Biogeogr 35:1533–1550. https://doi.org/10.1111/j.1365-2699.2008.01951.x
Hoberg EP, Brooks DR (2015) Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos Trans R Soc B 370:20130553. https://doi.org/10.1098/rstb.2013.0553
Hoberg EP, Kutz SJ, Galbreath KE, Cook J (2003) Arctic biodiversity: from discovery to faunal baselines- revealing the history of a dynamic ecosystem. J Parasitol 89(supplement):S-84–S-95
Hoberg EP, Galbreath KE, Cook JA, Kutz SJ, Polley L (2012) Northern host-parasite assemblages: history and biogeography on the borderlands of episodic climate and environmental transition. In: Rollinson D, Hays SI (eds) Adv Parasitol. 79. Elsevier, pp 1–97. https://doi.org/10.1016/B978-0-12-398457-9.00001-9
Hoberg EP, Makarikov AA, Tkach VV, Meagher SA, Nims TN, Eckerlin RE, Galbreath KE (2016) Insights on the host associations and geographic distribution of Hymenolepis folkertsi (Cestoda: Hymenolepididae) among rodents across temperate latitudes of North America. Parasitol Res 115:4627–4638. https://doi.org/10.1007/s00436-016-5255-3
Hoberg EP, Cook JA, Agosta SJ, Boeger W, Galbreath KE, Laaksonen S, Kutz SJ, Brooks DR (2017) Arctic systems in the quaternary: ecological collision, faunal mosaics and the consequences of wobbling climate. J Helminthol 91:409–421. https://doi.org/10.1017/S0022149X17000347
Holden ME, Musser GG (2005) Family Dipodidae. In: Wilson DE, Reeder DM (eds) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore, Maryland, pp 871–893
Kohli BA, Speer KA, Kilpatrick CW, Batsaikhan N, Damdinbaza D, Cook JA (2014) Multilocus systematics and non-punctuated evolution of Holarctic Myodini (Rodentia: Arvicolinae). Mol Phylogenet Evol 76:18–29. https://doi.org/10.1016/j.ympev.2014.02.019
Kohli BA, Federov VB, Waltari E, Cook JA (2015) Phylogeography of a Holarctic rodent (Myodes rutilus): testing high-latitude biogeographical hypotheses and the dynamics of range shifts. J Biogeogr 42:377–389. https://doi.org/10.1111/jbi.12433
Kontrimavichus VL, Smirnova LV (1991) Hymenolepis beringiensis sp. n. from the Siberian lemming (Lemmus sibiricus Kerr) and the problem of the sibling species in helminthology. In: Krasnoschekov GP, Roitman VA, Sonin MD, Chesnova LV (eds) Evoljucia parazitov. Materialy I Vsesojuznogo Simpoziuma, Akademiya Nauk SSSR, Tol’yatti, pp 90–104 (In Russian)
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Linstow O (1901) Taenia horrida, Tetrabothrium macrocephalum, und Heterakis distans. Archiv Naturgesch Berlin 67:1–10
Linzey DW (2001) Mammals of the Great Smoky Mountains National Park. University of Tennessee Press, Knoxville
Makarikov AA, Hoberg EP (2016) Broadening diversity in the Arostrilepis horrida complex: Arostrilepis kontrimavichusi n. sp. (Cyclophyllidea: Hymenolepididae) in the western red-backed vole Myodes californicus (Merriam) (Cricetidae: Arvicolinae) from temperate latitudes of the Pacific northwest, North America. Syst Parasitol 93:467–477. https://doi.org/10.1007/s11230-016-9640-1
Makarikov AA, Kontrimavichus VL (2011) A redescription of Arostrilepis beringiensis (Kontrimavichus et Smirnova, 1991) and descriptions of two new species from Palearctic microtine rodents, Arostrilepis intermedia sp. n. and A. janickii sp. n. (Cyclophyllidea: Hymenolepididae). Folia Parasitol 58:289–301. https://doi.org/10.14411/fp.2011.029
Makarikov AA, Gulyaev VD, Kontrimavichus VL (2011) A redescription of Arostrilepis horrida (Linstow, 1901) and descriptions of two new species from Palearctic microtine rodents, Arostrilepis macrocirrosa sp. n. and Arostrilepis tenuicirrosa sp. n. (Cestoda: Hymenolepididae). Folia Parasitol 58:108–120. https://doi.org/10.14411/fp.2011.011
Makarikov AA, Gardner SL, Hoberg EP (2012) New species of Arostrilepis (Eucestoda: Hymenolepididae) in members of Cricetidae and Geomyidae (Rodentia) from the Western Nearctic. J Parasitol 98:617–626. https://doi.org/10.1645/GE-2943.1
Makarikov AA, Galbreath KE, Hoberg EP (2013) Parasite diversity at the Holarctic nexus: species of Arostrilepis (Eucestoda: Hymenolepididae) in voles and lemmings (Cricetidae: Arvicolinae) from greater Beringia. Zootaxa 3608:401–439. https://doi.org/10.11646/zootaxa.3608.6.1
Makarikov AA, Nims TN, Galbreath KE, Hoberg EP (2015) Hymenolepis folkertsi n. sp. (Eucestoda: Hymenolepididae) in the oldfield mouse Peromyscus polionotus (Wagner) (Rodentia: Cricetidae: Neotominae) from the southeastern Nearctic with comments on tapeworm faunal diversity among deer mice. Parasitol Res 114:2107–2117. https://doi.org/10.1007/s00436-015-4399-x
Makarikov AA, Dokuchaev NE, Konyaev SV (2016) Cestodes of rodents in northern Priokhotye. Bulletin of the North-East Scientific Center, Russian Academy of Sciences Far East Branch 4:52–61 (in Russian). http://vestnik.north-east.ru/2016/n4/r_Makarikov.htm
Mas-Coma S, Tenora F (1997) Proposal of Arostrilepis n. gen. (Cestoda: Hymenolepididae). Res Rev Parasitol 57:93–101
Musser GG, Carelton MD (2005) Superfamily Muroidea. In: Wilson DE, Reeder DM (eds) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore, Maryland, pp 894–1522
Nylin S, Agosta S, Bensch S, Boeger WA, Braga MP, Brooks DR, Forister ML, Hambäck PA, Hoberg EP, Nyman T, Schäpers A, Stigall AL, Wheat CW, Österling M, Janz N (2018) Embracing colonizations: a new paradigm for species association dynamics. Trends Ecol Evol 33:4–14. https://doi.org/10.1016/j.tree.2017.10.005
Okamoto M, Agatsuma T, Kurosawa T, Ito A (1997) Phylogenetic relationships of three hymenolepidid species inferred from nuclear ribosomal and mitochondrial DNA sequences. Parasitology 115:661–666
Patton JL (2005) Family Geomyidae. In: Wilson DE, Reeder DM (eds) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore, Maryland, pp 859–870
Rausch RL (1951) Biotic interrelationships of helminth parasitism. Public Health Rep:66928–66934
Rausch RL (1952) Studies on the helminth fauna of Alaska. XI Helminth parasites of microtine rodents – taxonomic considerations. J Parasitol 38:415–444. https://doi.org/10.2307/3273922
Rausch RL (1957) Distribution and specificity of helminths in microtine rodents: evolutionary implications. Evolution 11:361–368
Rausch RL, Tiner JD (1949) Studies on the parasitic helminths of the north central states. II. Helminths of voles (Microtus spp.) preliminary report. Am Midl Nat 41:665–694
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Runck AM, Cook JA (2005) Postglacial expansion of the southern red-backed vole (Clethrionomys gapperi) in North America. Mol Ecol 14:1445–1456. https://doi.org/10.1111/j.1365-294X.2005.02501.x
Schiller EL (1952) Studies on the helminth fauna of Alaska. X. Morphological variation in Hymenolepis horrida (von Linstow, 1901) (Cestoda: Hymenolepididae). J Parasitol 38:554–568. https://doi.org/10.2307/3273983
Sikes RS, The Animal Care and Use Committee of the American Society of Mammalogists (2016) Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Spassky AA (1947) The phenomenon of confluence of proglottides and uteri in cestodes. Doklady Akademii Nauk SSSR 58:723–724 (In Russian)
Spassky AA (1963) Hymenolepidid celstodes—tapeworms of wild and domestic birds, vol 2. Izdatel’stvo Akademii Nauk SSSR, Moscow 418 pp. (In Russian)
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4:vey016. https://doi.org/10.1093/ve/vey016
Swofford DL (2000) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland
Voge M (1952) Variation in some unarmed Hymenolepididae (Cestoda) from rodents. In: Kirby H, Eakin RM, Miller A, Stern C (eds) Univ Calif Pubs Zool 57, pp 1–52
Whitaker JO Jr, Hamilton WJ (1998) Mammals of the eastern United States. Cornell University Press, Ithaca, p 583
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Unpublished Ph.D. dissertation, University of Texas at Austin, p 1–115
Acknowledgments
Dr. Alfred L. Gardner contributed to field collections in Virginia and Maine and prepared the symbiotype host specimens for deposition in the mammal collections of National Museum of Natural History. We thank the Virginia Division of Game and Inland Fisheries, the West Virginia Division of Natural Resources, and the Maine Division of Natural Resources for granting Scientific Collecting Permits. Funding which contributed to field collections was also provided from the Washington Biologists Field Club (to RPE).
Funding
AAM was supported in part by the Federal Fundamental Scientific Research Program for 2013–2020, grant no. VI.51.1.5 (АААА-А16-116121410121-7) and the Russian Foundation for Basic Research (Project No. 17-04-00227-a). RPE received partial funding for field collections from the Washington Biologists Field Club. Our study represents a continuing contribution of the Beringian Coevolution Project and the Integrated Inventories of Biomes of the Arctic through grants from the National Science Foundation (USA) to Joseph A. Cook, EPH, and KEG (DEB- 0196095, 0415668, 1250810, and 1256943).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Mammal specimens were collected under state scientific collector permits (Maine, Virginia, and West Virginia) and guidelines of the National Museum of Natural History Institutional Animal Care and Use Committee and American Society of Mammalogists (Sikes and The Animal Care and Use Committee of the American Society of Mammalogists 2016).
Ethical approval
The authors carefully reviewed the ethical standards of the journal and hereby certify that the procedures used with the investigated species comply fully with those standards. All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Section Editor: Georg von Samson-Himmelstjerna
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makarikov, A.A., Galbreath, K.E., Eckerlin, R.P. et al. Discovery of Arostrilepis tapeworms (Cyclophyllidea: Hymenolepididae) and new insights for parasite species diversity from Eastern North America. Parasitol Res 119, 567–585 (2020). https://doi.org/10.1007/s00436-019-06584-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06584-4