Abstract
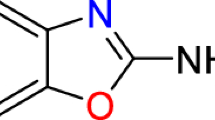
Neospora caninum is an apicomplexan parasite considered one of the main causes of abortion in cattle worldwide; thus, there is an urgent need to develop novel therapeutic agents to control the neosporosis. Enoyl acyl carrier protein reductase (ENR) is a key enzyme of the type II fatty acid synthesis pathway (FAS II), which is essential for apicomplexan parasite survival. The antimicrobial agent triclosan has been shown to be a very potent inhibitor of ENR. In this study, we identified an E. coli ENR-like protein in N. caninum. Multiple sequence alignment showed all the requisite features of ENR existed in this protein, so we named this protein NcENR. Swiss-Model analysis showed NcENR interacts with triclosan. We observed that ENR is localized in the apicoplast, a plastid-like organelle. Similar to the potent inhibition of triclosan on other apicomplexa parasites, this compound markedly inhibits the growth of N. caninum at low concentrations. Further research showed that triclosan attenuated the invasion ability and proliferation ability of N. caninum at low concentrations. The results from in vivo studies in the mouse showed that triclosan attenuated the virulence of N. caninum in mice mildly and reduced the parasite burden in the brain significantly. Taken together, triclosan inhibits the growth of N. caninum both in vitro and in vivo at low concentrations.





Similar content being viewed by others
References
Baldock C, Rafferty JB, Sedelnikova SE, Baker PJ, Stuitje AR, Slabas AR, Hawkes TR, Rice DW (1996) A mechanism of drug action revealed by structural studies of enoyl reductase. Science 274:2107–2110
Chakraborty A (2016) Understanding the biology of the Plasmodium falciparum apicoplast; an excellent target for antimalarial drug development. Life Sci 158:104–110
Dubey JP, Schares G (2011) Neosporosis in animals—the last five years. Vet Parasitol180:90–108
Dubey JP, Schares G, Ortega-Mora LM (2007) Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 20:323
El-Zawawy LA, El-Said D, Mossallam SF, Ramadan HS, Younis SS (2015) Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp Parasitol 149:54–64
Goodman CD, Mcfadden GI (2007) Fatty acid biosynthesis as a drug target in apicomplexan parasites. Curr Drug Targets 8:15–30
Heaslip AT, Florence D, Barry S, Ke H (2010) TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii. PLoS Pathog 6:e1000754
Heath RJ, Rock CO (1995) Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem 270:26538–26542
Hui W, Tao L, Jing L, Muzi L, Huizhu N, Qun L (2014) A nuclear factor of high mobility group box protein in Toxoplasma gondii. PLoS One 9:e111993
Jolly M, Emma HW, Kate M, Christopher AH, Boris S (2006) Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. P NATL ACAD SCI USA 103:13192–13197
Long S, Brown KM, Drewry LL, Anthony B, Iqh P, Sibley LD (2017) Calmodulin-like proteins localized to the conoid regulate motility and cell invasion by Toxoplasma gondii. PLoS Pathog 13:e1006379
Lu JZ, Muench SP, Allary M, Campbell S, Roberts CW, Mui E, McLeod RL, Rice DW, Prigge ST (2007) Type I and type II fatty acid biosynthesis in Eimeria tenella: enoyl reductase activity and structure. Parasitology 134:1949–1962
Ma L, Liu G, Liu J, Li M, Zhang H, Tang D, Liu Q (2017a) Neospora caninum ROP16 play an important role in the pathogenicity by phosphorylating host cell STAT3. Vet Parasitol 243:135–147
Ma L, Liu J, Li M, Fu Y, Zhang X, Liu Q (2017b) Rhoptry protein 5 (ROP5) is a key virulence factor in Neospora caninum. Front Microbiol 8
McLeod R, Muench SP, Rafferty JB, Kyle DE, Mui EJ, Kirisits MJ, Mack DG, Roberts CW, Samuel BU, Lyons RE, Dorris M, Milhous WK, Rice DW (2001) Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int J Parasitol 31:109–113
Mili K, Jayashree G, Namita S, Avadhesha S (2004) Mutational analysis of the triclosan-binding region of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum. Biochem J 381:735
Muench SP, Prigge ST, Zhu L, Kirisits MJ, Roberts CW, Wernimont S, McLeod R, Rice DW (2006) Expression, purification and preliminary crystallographic analysis of the Toxoplasma gondii enoyl reductase. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:604–606
Muench SP, Stec J, Zhou Y, Afanador GA, McPhillie MJ, Hickman MR, Lee PJ, Leed SE, Auschwitz JM, Prigge ST, Rice DW, McLeod R (2013) Development of a triclosan scaffold which allows for adaptations on both the A- and B-ring for transport peptides. Bioorg Med Chem Lett 23:3551–3555
Surolia N, Surolia A (2001) Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat Med 7:167–173
Pan H, Alaraj IQM, Aldulayymi JR, Baird MS, Jing L, Liu Q (2016) Sterculic acid and its analogues are potent inhibitors of Toxoplasma gondii. Korean J Parasitol 54:139–145
Paul KS, Bacchi CJ, Englund PT (2004) Multiple triclosan targets in Trypanosoma brucei. Eukaryot Cell 3:855–861
Reichel MP, Ayanegui-Alcérreca M, Alejandra GLFP, Ellis JT (2013) What is the global economic impact of Neospora caninum in cattle - the billion dollar question. Int J Parasitol 43:133–142
Sabine B, Naoaki Y, Tomohide M, Claveria FG, Kozo F, Ikuo I (2003) Growth inhibitory effect of triclosan on equine and bovine Babesia parasites. Am J Trop Med Hyg 68:334–340
Shears MJ, Botté CY, Mcfadden GI (2015) Fatty acid metabolism in the Plasmodium apicoplast: drugs, doubts and knockouts. Mol Biochem Parasit 199:34–50
Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren GG, Hiltunen JK, Kastaniotis AJ, McConville MJ, Striepen B (2012) Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J Biol Chem 287:4957–4971
Turnowsky F, Fuchs K, Jeschek C, Högenauer G (1989) envM genes of Salmonella typhimurium and Escherichia coli. J Bacteriol 171:6555–6565
Vaishnava S, Striepen B (2010) The cell biology of secondary endosymbiosis--how parasites build, divide and segregate the apicoplast. Mol Microbiol 61:1380–1387
Vandhana S, Deepa PR, Aparna G, Jayanthi U, Krishnakumar S (2010) Evaluation of suitable solvents for testing the anti-proliferative activity of triclosan - a hydrophobic drug in cell culture. Indian J Biochem Bio 47:166–171
Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, Aly ASI, Cowman AF, Kappe SHI (2010) Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11:506–520
Ward WH, Holdgate GA, Rowsell S, McLean EG, Pauptit RA, Clayton E, Nichols WW, Colls JG, Minshull CA, Jude DA, Mistry A, Timms D, Camble R, Hales NJ, Britton CJ, Taylor IW (1999) Kinetic and structural characteristics of the inhibition of enoyl (acyl carrier protein) reductase by triclosan. Biochemistry 38:12514–12525
White SW, Zheng J, Zhang YM (2005) The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem 74:791–831
Yang C, Liu J, Ma L, Zhang X, Zhang X, Zhou B, Zhu X, Liu Q (2018) NcGRA17 is an important regulator of parasitophorous vacuole morphology and pathogenicity of Neospora caninum. Vet Parasitol 264:26–34
Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, Valderramos JC, Vilcheze C, Siedner M, Tsai JH, Falkard B, Sidhu AB, Purcell LA, Gratraud P, Kremer L, Waters AP, Schiehser G, Jacobus DP, Janse CJ, Ager A, Jacobs WR Jr, Sacchettini JC, Heussler V, Sinnis P, Fidock DA (2008) The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4:567–578
Acknowledgments
We thank Professor Xuenan Xuan (Obihiro University of Agriculture and Veterinary Medicine, Japan) for kindly providing the Nc-1 strain. We thank Jin Zhu (Therapeutic Goods Administration, Australia) for the helpful comments on the manuscript.
Availability of data and material
Data supporting the conclusions of this article are included within the article.
Funding
This study was supported by the National Key Basic Research Program (973 program) of China (No.2015CB150300) and the National Key Research and Development Program of China (2017YFD0501200).
Author information
Authors and Affiliations
Contributions
HZ and JL designed the study and analyzed the data. HZ, YF, and CSY carried out the experiments. JHX provided help for instrument operation. QL and JL contributed reagents and materials and offered advice during the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedures were performed strictly according to the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China and approved by the Institutional Animal Care and Use Committee of China Agricultural University (under the certificate of Beijing Laboratory Animal employee ID: CAU20161210-2).
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Xing-Quan Zhu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, J., Yang, C. et al. Triclosan inhibits the growth of Neospora caninum in vitro and in vivo. Parasitol Res 118, 3001–3010 (2019). https://doi.org/10.1007/s00436-019-06449-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06449-w