Abstract
Parasitic roundworms cause significant sickness and mortality in animals and humans. In livestock, these nematodes have severe economic impact and result in losses in food production on a global scale. None of the currently available drugs ideally suit all treatment circumstances, and the development of drug-resistant nematode strains has become a challenge to control the infection. There is an urgent need to develop novel anthelmintic compounds. According to our previous report, N-methylbenzo[d]oxazol-2-amine (1) showed anthelmintic activity and lowest cytotoxicity. In this study, in vivo anthelmintic properties were evaluated using Trichinella spiralis infected mice. Toxicity was evaluated using the rats and mode of action using molecular docking and metabolomics approaches. The in vivo results demonstrate that a dose of 250 mg/kg reduced the T. spiralis abundance in the digestive tract by 49%. The 250 mg/kg Albendazole was served as control. The relatively low acute toxicity was categorized into chemical category 5, with an LD50 greater than 2000 mg/kg body. Molecular docking analysis showed the T. spiralis tubulin beta chain and glutamate-gated channels might not be the main targets of compound 1. Metabolomics analysis was used to explain the effects of compound 1 on the T. spiralis adult worm. The results demonstrated that compound 1 significantly up-regulated the metabolism of purine, pyrimidine and down-regulated sphingolipid metabolism. In conclusion, compound 1 could be a potential molecule for anthelmintic development. The bioavailability, pharmacokinetics, and absorption of this compound should be studied further to provide information for its future efficacy improvement.
Similar content being viewed by others
Introduction
Almost 300 nematode species cause zoonotic diseases in humans1. Some parasitic nematodes enter the human body through open wounds or penetrate the skin, while others infect people when they are consumed via food products contaminated with embryonated eggs or parasitic nematode larvae. Around 24% of the world's population is infected with parasitic nematodes. Ascaris lumbricoides, Ancylostoma duodenale, Gnathostoma spinigerum, Halicephalobus gingivalis, and Trichinella spiralis are considered harmful parasites. Not only human, nearly every organ in an animal's body also can harbor nematode parasites. The digestive, circulatory, and respiratory systems are the most frequently infected organs. Eelworm, lungworm, pinworm, threadworm, and hookworm are common found in animals2. In order to effectively control the parasitic nematodes, "One Health" approach should be concerned. Since there are no vaccines for parasitic nematodes, the most promising long-term control strategy currently relies on chemotherapeutic medicines to kill parasitic nematodes and limit the spread of infections.
Nematicides are separated into several classes based on similarities in their chemical structures and modes of action. Piperazine, initially used as an anthelmintic in the 1950s, has a GABA-mimetic effect and paralyzes the nematode body muscle3. The first benzimidazole class compound discovered was thiabendazole in 1961 and, subsequently, different benzimidazole structures have been introduced as broad spectrum anthelmintics. It is clear that the anthelmintic efficacy of benzimidazoles is through their selective interaction with β-tubulin4. Levamisole, pyrantel, and morantel are nicotinic receptor agonists that cause the excitation of nicotinic acetylcholine receptors on muscles, resulting in spastic muscular paralysis5. Paraherquamide A and marcfortine A are members of the oxindole alkaloid family originally isolated from Penicillium paraherquei and Penicillium roqueforti, respectively. They both induce paralysis in parasitic nematodes and act as typical competitive antagonists of acetylcholine-stimulated muscle contractions6. Avermectins are a macrocyclic lactone endectocides that are produced naturally or semi-synthetically7. They are made from the fermentation products of Streptomyces avermitilis, a soil-dwelling actinomycete that is able to efficiently control a wide range of endo- and ectoparasites8. This drug and its analogs demonstrate inhibitory activity on glutamate-gated chloride channels (GluCl), leading to the inhibition of pharyngeal pumping, motility, and egg or microfilaria release and a loss of host immunosuppression9. Milbemycins are products of Streptomyces species' fermentation. They action by a similar mechanism, but their half-life is longer than that of avermectins. Invertebrate neurons and myocytes' glutamate-sensitive chloride channels are opened, which causes these cells to become hyperpolarized and impairs signal transmission10. Derivatives of aminoacetonitrile are effective helminthicides. They function as nematode-specific nematode ACh agonists, resulting in a spastic paralysis and quick ejection from the host11. The cyclodepsipeptide molecule emodepside is a semi-synthetic derivative of PF1022A, a fermentation product obtained from a fungus, Mycelia Sterilia. The emodepside causes paralysis in nematodes by stimulating excessive neurotransmitter release at neuromuscular sites12. Nitazoxanide, a pyruvate ferredoxin oxidoreductase inhibitor, acts against a broad spectrum of protozoa and helminths that occur in the intestinal tract, and anaerobic electron transport enzymes are its potential targets13.
Because of the extraordinary effectiveness of modern anthelmintics, with more than 95% parasite reduction, as well as their generally good safety margin, broad-spectrum nature, and reasonable pricing, the chemical control of parasites in animals has been very successful over the past 50 years. Unfortunately, the prolonged use of these therapies and inappropriate drug doses have resulted in the growth of drug resistance among parasitic nematodes, primarily the gastrointestinal nematodes of cattle, sheep, goats, and horses14. Currently, instances of nematode resistance to all available anthelmintic drugs, including piperazine, benzimidazoles, levamisole, paraherquamide, ivermectin (IVM), emodepside, nitazoxanide, milbemycins and aminoacetonitrile derivatives have been documented15,16. In a study in Argentina, resistance was determined in vivo by a fecal egg count reduction test. The resistances for ivermectin, albendazole and levamisole were 60%, 32% and 28%, respectively. A high level of anthelmintic resistance was observed in this study17. In New Zealand, a cross-sectional prevalence study was performed using a standardized fecal nematode egg count reduction test. Resistance to ivermectin, ivermectin-levamisole, ivermectin-albendazole and ivermectin-levamisole-albendazole were found on 36, 10, 13 and 8% farms, respectively. The prevalence of resistance to ivermectin was showed high prevalent on these farms18. Moreover, climate change has already affected parasite levels and species composition, and large-scale outbreaks have become more frequent, endangering both animal welfare, food security, and public health19. Therefore, there is an urgent need for further anthelmintic drug development. According to our previous study, N-methylbenzo[d]oxazol-2-amine (1) has good anthelmintic properties. The chemical structure of this compound is different from that of known anthelmintic drugs (Fig. 1). An in vitro study indicated they have comparable potency to albendazole (ABZ) in a free-living nematode Caenorhabditis elegans strain N2 and parasitic nematodes (T. spiralis muscle stage and third-stage Gnathostoma spinigerum larvae)20. The 50% effective concentration (EC50) of compound 1 on C. elegans and T. spiralis were 5.77 and 3.8 µM, respectively. In addition, compound 1 demonstrated low cytotoxicity at 50% cytotoxic concentration of 1282 mM to human embryonic kidney 293 cell (HEK293)20. In fact, compound 1 showed about 10 times lower cytotoxicity towards human embryonic kidney cells than ABZ.
Since the complete life cycle of T. spiralis could be simply maintained in the laboratory, it does not require additional intermediate host and alternative non-laboratory animals. In addition, it could provide two important parasite stages including muscle and intestinal stages which could be used for researching. Number of worms in each batch are also sufficient for drug screening experiment. Therefore, T. spiralis was selected as a parasitic nematode model in this study.
The in vivo anthelmintic efficiency, toxicity in animal models, and potential mechanism of action of compound 1 were evaluated. The in vivo infection of the adult parasitic worm T. spiralis in an animal model was used to test the effectiveness and toxicity of anthelmintics. Our findings provide the necessary information to evaluate the potential of compound 1 as a treatment for helminth infections.
Material and methods
Compound
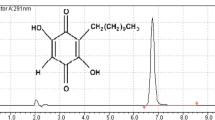
All N-Methylbenzo[d]oxazol-2-amine in this study was evaluated its purity by an Agilent High Performance Liquid Chromatography (HPLC). HPLC Column Hichrom, C18, 150 Å, 5 µm, 4.6 × 250 mm was used for separation with 0.5ml/min flowrate. Mobile phase A and B were water and isopropanol, respectively. The gradient was started from 30%B to 95%B in 77 min. A diode array was the detector at 254nm absorbance. The HPLC trace of compound 1 was > 95% pure (Fig. S1).
Anthelmintic activity on T. spiralis adult worm
The experimental procedures were approved by the Faculty of Tropical Medicine Animal Care and Use Committee, Mahidol University (Approval number: FTM-ACUC 033/2020). All animal experiments were conducted in accordance with ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations. The laboratory strain of T. spiralis used in this study was maintained in the Animal Care Unit, Faculty of Tropical Medicine, Mahidol University. For the in vitro anthelmintic assay, 8-week-old female ICR mice (three mice) were fed 250 larvae by oral gavage. After 7 days of infection, the mice were euthanized by compressed carbon dioxide gas. Adult worms were collected from the digestive tracts by flushing with normal saline. The worms were cultured in RPMI 1640 media, with L-glutamine (SH30027.01) purchased from Hyclone (Logan, UT, USA) for anthelmintic testing. Compound 1 concentrations in RPMI were generated in triplicate, from 10 ng/mL to 100 μg/mL, and used to calculate the half maximum effective concentration (EC50) (final concentration in wells with 0.5% DMSO). Positive was ABZ (CAS No. 54965-21-8, purity ≥ 98.0%) purchased from Sigma-Aldrich (MO, USA) with the same concentration range with compound 1. While, 0.5% DMSO (Sigma-Aldrich, MO, USA) was negative control in this experiment. Each well received 20 worms, which were then cultured at 37 °C for 24 h. Worm motility was assessed under an inverted microscope. Data were shown as the mean ± S.E.M. two-way ANOVA was used (with significance level set at P < 0.05). All statistics were carried out using GraphPad Prism software. GraphPad Prism 9 software was used to calculate the EC50. The molecular weights of compounds were used to calculate EC50 to µM unit.
For the in vivo anthelmintic assay, 8-week-old female ICR mice (six mice/group) weighing 30 ± 3 g from Nomura Siam International Co., Ltd., (Thailand, Bangkok) were fed 250 larvae by oral gavage after 7 days acclimatization. After 24 h of infection, single oral doses of 250, 500, and 1000 mg/kg compound 1 were fed to the infected mice by oral gavaging of the suspension in the tween 80 (15% v/v) (Sigma-Aldrich, MO, USA). Water and 250 mg/kg ABZ were administered to the negative and positive controls, respectively. After 7 days of infection, adult worms were recovered from mouse digestive tracts as described above. The number of worms was evaluated under an inverted microscope.
Acute oral toxicity
The experimental procedures were approved by the Faculty of Tropical Medicine Animal Care and Use Committee, Mahidol University (Approval number: FTM-ACUC 006/2020). All animal experiments were conducted in accordance with ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations. Sprague–Dawley (SD) rats, 5 weeks old, weighing 109 ± 5.62 g, from Nomura Siam International Co., Ltd. (Thailand, Bangkok) were used for toxicity testing according to Organization for Economic Co-operation and Development (OECD) Guidelines for the Testing of Chemicals 425. After 7 days acclimatized at the room temperature of 22 ± 2 °C, relative humidity of 55 ± 5%, light and dark cycle of 12:12 h, each rat was fasted for approximately 16 h before compounds 1 gavage. The compounds were dissolved in a 15% v/v solution of Tween 80 and orally administered to rats at doses of 0, 175, 550, and 2000 mg/kg body weight, in accordance with the OECD 425 guidelines, with administration conducted at 10:00 a.m. During testing, unlimited feeding a standard diet and water are provided until the end of the 14-day period. Then, each rat was fasted for approximately 16 h and euthanized with carbon dioxide. Serum samples were then collected for glucose, lipid and general health indicators using an automated blood sample testing instrument (Cobas analyzer, Roche Diagnostics, Switzerland). Organs including the spleen, kidney and liver were collected and weighed. longitudinally Longitudinal sections were immersed overnight in 10% buffer formalin for biopsy and stained with hematoxylin and eosin for microscopic study. Weight and blood biochemical test data are presented as means ± standard deviations (SD). Data were analyzed using one-way analysis of variance (ANOVA) following with Tukey’s range tests (SPSS Statistics software Version 18, NY, USA). Statistical significance was accepted when P < 0.05.
Docking compound 1 with ABZ and IVM target proteins
Three-dimensional (3D) models of tubulin beta chain and glutamate-gated chloride channel (GluCl) from Trichinella spiralis were constructed by SWISS-MODEL21. The tubulin beta of decorated ciliary doublet microtubule (PDB ID: 6U42) and the C. elegans GluCl (PDB ID: 4TNW) were used as templates for tubulin beta chain and GluCl, respectively. T. spiralis tubulin beta chain and GluCl shared 87.10% and 60.06% sequence identity with the templates, respectively. Subsequently, the obtained 3D models were validated using SAVESv6.0 (https://servicesn.mbi.ucla.edu/SAVES/). Albendazole and N-methylbenzo[d]oxazol-2-amine and ivermectin and N-methylbenzo[d]oxazol-2-amine were docked to the T. spiralis tubulin beta chain and GluCl, respectively, by Autodock Vina22 using CB-Dock server23.
Metabolomics analysis
For metabolite extraction, 0.5% DMSO and EC50 compound 1 treated T. spiralis adult worms were homogenized in 500 μL methanol. The tubes were liquid nitrogen-snap frozen, thawed, then centrifuged at 800g for one minute at 4 °C. The pellet was extracted once more using the same procedure. The supernatant was collected and put in a fresh tube. The second extraction's supernatant was added into the first extraction's supernatant. The pellet was redissolved in 250 μL of deionized water, the pellet was frozen in liquid nitrogen and thawed. Centrifugation at 15,000g for 1 min at 4 °C was used to collect the supernatant, which was then combined into the previous extraction. To get rid of the last bits of debris, the pooled supernatants were centrifuged at 15,000g for one minute at 4 °C. The clear supernatant was collected and dried in a speed vacuum (Tomy Digital Biology, Tokyo, Japan). The metabolite pellet was reconstituted in 200 μL of mobile phase A:B at a ratio of 50:50 (vol/vol) and subjected to the ultra-high performance liquid chromatography (UHPLC; Agilent 1260 Quaternary pump, Agilent 1260 High Performance Autosampler, and Agilent 1290 Thermostatted Column Compartment SL, Agilent Technologies) coupled to a quadrupole time-of-flight mass spectrometer (Q-TOF-MS) (TripleTOF 5600+, SCIEX, US). The mobile phase A and B was water containing 0.1% formic acid and acetonitrile containing 0.1% formic acid, respectively. A C18 reversed phase column (ACQUITY UPLC HSST3, 2.1 × 100 mm, 1.8 μM, Waters) protected by a pre-column (ACQUITY UPLC HSST3, 2.1 × 5 mm, 1.8 μM, Waters) was used for separation with a flow rate of 0.3 mL/min at 40 °C. The gradient was started at 5% mobile phase B for 2.0 min (0.0–2.0 min), 5–60% B for 0.5 min (2.0–2.5 min), 60–80% B for 1.5 min (2.5–4.0 min), 80–100% B for 8.0 min (4.0–12.0 min), 100% B for 5 min (12.0–17.0 min), 100–5% B for 0.1 min (17.0–17.1 min), and 5% B for 2.9 min (17.1–20.0 min). The UHPLC-Q-TOF-MS system, mass ion chromatogram, and mass spectra were acquired by Analyst Software version 1.7 (SCIEX). The Q-TOF-MS was operated in positive (+ESI) and negative (−ESI) electrospray ionization modes. Equal aliquots of each metabolite sample were pooled to form the quality control (QC) samples. The QC samples were injected before, during, and after sample analysis to assess the system performance. Raw mass spectra files from the UHPLC-Q-TOF-MS (.wiff and .wiff.scan files) were processed using the XCMS online software version 3.7.1 (The Scripps Research Institute, CA, USA). The comparison between control and treated groups were performed using “Pairwise” mode with “UPLC/Triple TOF pos” protocol. Metabolomic data from XCMS were then analyzed using MetaboAnalyst online software version 5.0 (https://www.metaboanalyst.ca/)24 in both “Statistical Analysis (one factor)” and “Pathway Analysis” modules. For statistical analysis module, metabolites with their concentrations were filtered by “Interquantile range (IQR)” and normalized using quantile normalization, cube root data transformation, and data range scaling. Data visualization was performed using Partial Least Squares-Discriminant Analysis (PLS-DA), and Volcano plot. PLS-DA were demonstrated with 95% confidence regions. Log2 of fold change and -log of p-value were used to generate the Volcano plot. Differential metabolites were identified with the specific cutoff (> 1.5-fold change, p-value < 0.01). Pathway analysis of differential metabolites was performed in MetaboAnalyst and the STITCH database version 5.0 (http://stitch.embl.de/)25 with the p-value less than 0.01 as a statistical significance.
Compliance with ethics guidelines
The animal study were approved and performed in complete compliance with the ethical approval granted by the Faculty of Tropical Medicine Animal Care and Use Committee, Mahidol University (Approval number: FTM-ACUC 006/2020 and FTM-ACUC 033/2020).
Results and discussion
Anthelmintic activity on T. spiralis adults
In this study, T. spiralis adult worms served as a model intestinal parasitic nematode. T. spiralis is a parasitic worm with a complex life cycle and sophisticated gene regulation during development, and employing T. spiralis as a model organism may help with the development of drugs26. The EC50 of compound 1 was examined by in vitro assay 1, 2, 3, and 24 h after exposure, and ABZ was applied as positive-control drug. The in vitro anthelmintic activity results are presented in Table 1. According to the results, no T. spiralis adult worms were dead 1 and 2 h after compound 1 or ABZ exposure. Worm motility was evaluated under an inverted microscope. Living nematodes maintain a sinusoidal shape and show locomotion, whereas alive paralysis is characterized by a rod-shaped body posture with integrity and structure of internal organs. The dead nematodes appear as straight, rigid rods and complete disintegration of internal organs. Data were shown as the mean ± S.E.M. two-way ANOVA was used (with significance level set at P < 0.05). Interestingly, the EC50 of compound 1 was 208.6 µM, which was statistically lower than that of ABZ (275.05 µM), after 3 h of exposure. In addition, the EC50 of compound 1 was 1.55 µM, which was lower than that of ABZ (3.09 µM), after 24 h of exposure. These in vitro assay findings demonstrated that compound 1 has an approximately twofold greater activity than ABZ at 24 h. In this study, the latest time exposure was 24 h followed the protocol of Ruo Yu Peng et al.27. The longer exposure time might provide better results. In comparison to previous study, at 24 h after treatment, the EC50 of compound 1 was 3.8 µM, which was approximately threefold less activity than ABZ (1.3 µM). This compound showed better anthelmintic activity in adult than larvae stages. One of limitation of this study was the random selection of adult worm genders. The different worm gender may affect the anthelminthic activity. Therefore, the gender specific experiment should be further performed to evaluate the compound 1 activity.
As compound 1 demonstrated promising activity in vitro, the effectiveness of compound 1 as an anthelmintic was evaluated in animal models. Six infected mice per group were used in this study. The infected mice were given single oral doses of 250, 500, or 1000 mg/kg of compound 1. The in vivo efficacy of compound 1 is shown in Table 2. Since an appropriate dose of ABZ to use against T. spiralis in muscle is 250 mg/kg28,29 mice treated with a single oral dose of 250 mg/kg ABZ were included as positive controls. The oral doses of compound 1 was started from the same concentration of ABZ and increased two-fold more concentration to improve efficiency. Untreated infected mice were used as the negative controls.
The results showed that compound 1 reduced worm abundance in the digestive tracts of the infected mice by 49.17%, 64.54%, and 76.50% when we administrated compound 1 at 250, 500, and 1000 mg/kg, respectively. The anthelmintic efficacy improved when we increased the dose of compound 1 and was statistically different from control (infected mice). However, ABZ cleared all adult worms from the digestive tract of mice at 250 mg/kg. The in vivo anthelmintic efficacy of compound 1 was, therefore, not comparable to that of ABZ. One limitation of this experiment was that compound 1 or ABZ was fed to the mice after 24 h of infection and recorded the adult worm viability after 7 days of infection. The worms might not totally change to adult stage on the day that we fed compounds. Therefore, the result demonstrated the compound 1 anthelmintic activity of mix stages between pre-adult and adult of T. spiralis at 24 h after infection.
Toxicity in animal models
In our study, the toxicity assay in the rat model followed the OECD guidelines for the testing of chemicals30. For acute oral toxicity, the up-and-down procedure (Test no. 425 from the OECD guidelines) was applied. The method allows for the estimation of a 50% lethal dose (LD50) with a confidence interval. A substance can be categorized for acute toxicity in accordance with the Globally Harmonized System of classification and labeling of chemicals. According to the starting dose recommended in the guidelines, 175 mg/kg body weight (BW) of compound 1 was the starting dose used for the rats (n = 5). This dose did not cause changes in the rats’ food and water consumption or excretion over a 14-day experimental period. The dose was progressively increased in accordance with the guidelines. In the first 6 h after applying 550 mg/kg BW, all rats experienced sleepiness and were responsive to their surroundings. In the first 12 h after the concentration was increased to 2000 mg/kg BW, all rats experienced drowsiness, did not consume water or food, and were less responsive to their surroundings. After the first 6 and 12 h and throughout the experimental period, all rats administered doses of 550 and 2000 mg/kg BW, respectively, exhibited normal responses to their surroundings and consumption of food and water. The Korea Institute of Drug Safety & Risk Management monitors drug-safety-related events and collects nationwide reports, and they found that sleepiness, vomiting, dizziness, and nausea were induced by ABZ in the Korean population31. In 828 studies, praziquantel was also found to lead to common adverse effects, for example drowsiness, abdominal pain, headache, fatigue, nausea, dizziness, and weakness32. Thus the adverse effects of compound 1 are similar to those observed for ABZ and praziquantel.
The BW and organs of rats fed compound 1 concentrations of 175, 550, and 2000 mg/kg BW were not significantly different compared with those given 0 mg/kg BW compound 1. The rat liver, spleen, and kidney weights were also not significantly different between the groups (Table 3). No deaths were found in any rats in the 14 days after the administration of compound 1 at 0–2000 mg/kg BW. Therefore, we concluded that the acute oral toxicity of compound 1 is represented by an LD50 (oral, female rat) greater than 2000 mg/kg body weight. ABZ has been reported to have an LD50 of 1320–2400 mg/kg33. Therefore, compound 1 showed comparable acute oral toxicity to ABZ.
Blood chemistry tests provide information on the function of organs, such as the heart, kidneys, and liver34. Serum sugar, total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein were not significantly different between the groups. However, rats that received compound 1 concentrations of 175, 550, and 2000 mg/kg body weight tended to have high blood triglyceride levels compared with the control group (rats not administered compound 1) (Table 4). In experimental type 2 diabetic rats, triglyceride values were found to increase after high-dose ABZ compared with the control group35. This information relates to the rise in triglycerides after compound 1 treatment.
Liver aspartate transaminase, alanine transaminase, alkaline phosphatase, blood urea nitrogen, and creatinine values were not significantly different among all groups of rats. Hematoxylin and eosin staining of the spleen and kidney of rats that received compound 1 showed similar histopathological shapes and structures to those of the control group. However, there was evidence of hepatocyte dispersion in the liver sections of rats that received 550 and 2000 mg/kg body weight of compound 1. Some intercellular vacuolization was also observed in liver sections of rats dosed with 2000 mg/kg body weight compound 1 (Fig. 2).
Toxicological screening is very important when exploring the new therapeutic uses for existing molecules36. From the acute oral toxicity results, we concluded that compound 1 is a relatively low-acute-toxicity chemical of category 5 with an LD50 greater than 2000 mg/kg body weight. This is the highest dose for an acute toxicity test according to the OECD 425 guidelines for the testing of the acute oral toxicity of chemicals, acute toxic class method37. Compound 1 showed no effects on the kidney, spleen, or body weight. Compound 1 also caused no mortality in the female rats, which are more sensitive than males, making the results clear. However, the side effects of compound 1 at 550 mg/kg body weight in rats were drowsiness and elevated triglycerides. Previous systematic reviews reported the side effects of anthelmintic drugs on acute liver function38. These should be a concern when applying the continued use of the compound in sensitive groups.
Docking compound 1 with ABZ and IVM targets
To investigate the mechanistic target involved in the anthelmintic activity of compound 1, molecular modeling and docking methods were used. ABZ’s mode of action is thought to involve β-tubulin, as it prevents the production of microtubules39. Whereas IVM targets nematode glutamate-gated chloride channels40. In our study, the binding energy of compound 1 to both T. spiralis β-tubulin and glutamate-gated chloride channels was estimated and compared with those of ABZ and IVM, respectively. Docking ABZ and compound 1 to T. spiralis tubulin beta chain resulted in affinity binding energies of − 6.0 kcal/mol and − 5.4 kcal/mol, respectively. Nonetheless, when compound 1 was docked to the same binding cavity as ABZ, the affinity score was − 4.9 kcal/mol. A more negative energy indicates a stronger binding. Thus, the docking results suggested that the binding of compound 1 to tubulin beta chain was weaker than that of ABZ. Both compounds were predicted to fit into distinct binding sites (Fig. 3). However, the EC50 of compound 1 was 1.55 µM, which is lower than that of ABZ (3.09 µM), after exposure. This in vitro assay finding demonstrated that compound 1 has an approximately twofold greater activity than ABZ. Therefore, the T. spiralis tubulin beta chain might not be the predominant target of compound 1.
Docking of ABZ and N-methylbenzo[d]oxazol-2-amine (compound 1) to the T. spiralis tubulin beta chain. The 3D model of T. spiralis tubulin beta chain was generated with SWISS-MODEL using PDB template 6U42. Discovery Studio Visualizer was used to visualize the model. The T. spiralis tubulin beta chain is shown as a solid blue ribbon. The top right panel depicts ABZ binding, while the bottom right panel depicts compound 1 binding. Hydrogen bond donors are shown in pink, and hydrogen bond acceptors are shown in green.
Trichinella spiralis GluCl docking of IVM and compound 1 resulted in affinity binding energies of − 8.0 kcal/mol and − 4.9 kcal/mol, respectively, suggesting that IVM has a stronger binding affinity than compound 1 (Fig. 4). The greater binding energy of IVM implies that T. spiralis GluCl is not the target of compound 1.
Docking of IVM and N-methylbenzo[d]oxazol-2-amine (compound 1) to T. spiralis glutamate-gated channel (GluCl). The 3D model of T. spiralis GluCl was generated with SWISS-MODEL using PDB template 4TNW. Discovery Studio Visualizer was used to visualize the model. The T. spiralis GluCl is shown as a solid dark blue ribbon. The left panel depicts IVM binding, while the right panel depicts compound 1 binding. Hydrogen bond donors are shown in pink, and hydrogen bond acceptors are shown in green.
Metabolomics analysis
Compound 1 exhibited higher binding energy to the known target proteins of anthelmintic drugs than ABZ and IVM. Metabolomics analysis was therefore used to explain the effects of compound 1 on T. spiralis adult worms. The metabolite profiles of 0.5% DMSO (control) and compound 1 EC50-treated T. spiralis adult worms were studied. Partial least squares discriminant analysis (PLS-DA) was employed for data analysis (Fig. 5).
The results revealed that the metabolite profile of T. spiralis adult worms after compound 1 exposure was clearly different those of the control. A total of 13,469 features were observed by mass spectrometric analysis. There were 94 differential features of T. spiralis adult worms after compound 1 exposure. Among them, 9 metabolites were identified by METLIN database. The volcano plot in Fig. 6. was used to show statistical significance (p-value) versus magnitude of change (fold change). Using the criteria of p-value < 0.01 and fold change ≥ 1.5, we identified 94 differential features after compound 1 exposure. Among them, 61 and 33 were up- and down-regulated, respectively. Metabolite identification using the METLIN database revealed 3 and 6 up- and down-regulated metabolites, respectively (Tables 5, 6, and S1).
According to the fold-change values, 4alpha-hydroxymethyl-5alpha-cholesta-8-en-3beta-ol was the most up-regulated metabolite after compound 1 exposure. It has been found as an ergosterol intermediate in sterol biosynthetic pathway of Leishmania spp. Amphotericin B is the compound of choice for leishmaniasis treatment. One of its mechanisms is rapidly alteration of lipid metabolism of the Leishmania parasite41. In addition, several antiparasitic drugs interfere the sterol biosynthetic pathway such as zaragozic acids, quinuclidines and bisphosphonates for treating human African trypanosomiasis, Chagas disease, and leishmaniasis42,43. The up-regulation of 4alpha-hydroxymethyl-5alpha-cholesta-8-en-3beta-ol after compound 1 exposure might interfere sterol biosynthetic pathway and demise of T. spiralis. MG(18:0/0:0/0:0) was also up-regulated after compound 1 exposure. This compound is monoacylglyceride, an ester of the trihydric alcohol glycerol and a long-chain fatty acid. Monoacylglycerol lipase is an enzyme producing monoacylglycerides. This enzyme is a target protein of an antiplasmodial compound, salinipostin A. The alteration of monoacylglyceride level could affect vitality of P. falciparum44. In addition, a monoacylglyceride from Nordic seaweeds demonstrated as a major anti-parasitic compound against Ascaris suum45. Therefore, altered monoacylglyceride level may have an effect on T. spiralis survivability similar to P. falciparum.
The 2-amino-14,16-dimethyloctadecan-3-ol and phytosphingosine were down-regulated metabolites after compound 1 exposure involving in sphingolipid biosynthesis. The 2-amino-14,16-dimethyloctadecan-3-ol is natural analogs of sphinganine and 1-deoxysphinganine which could control sphingolipid production46. While phytosphingosine is a sphingoid base, a fundamental structure for generating sphingolipids. Phytosphingosine is abundant in plants and fungi and present in animals47. No information relating to 2-amino-14,16-dimethyloctadecan-3-ol and phytosphingosine functions in the parasite is available. However, the alteration of sphingolipid level could inhibit P. falciparum development48. The development of T. spiralis may be inhibited by interfering sphingolipid biosynthesis after exposure to compound 1. Palmitamide was also down-regulated after compound 1 exposure, and this compound has inflammatory properties49. In general, helminths are successful at modulating their host’s immune response50. Schistosoma mansoni secretes an anti-inflammatory molecule to modulate and circumvent the host's innate and adaptive immune responses51. The reduction in palmitamide may affect the parasite’s ability to modulate its host’s immunity, potentially restoring the host’s defense mechanism52,53,54. The differential metabolites were subjected to pathway analysis using the STITCH bioinformatics tool (Fig. 7). The significantly up-regulated metabolic pathway was purine and pyrimidine metabolisms, while the most down-regulated was sphingolipid metabolism. The purine and pyrimidine metabolisms are important pathways generating purine and pyrimidine molecules for DNA replication, RNA synthesis, and cellular bioenergetics. The imbalance in purine and pyrimidine metabolisms are linked with inability to maintaining cell homeostasis55. Accordingly, the elevation of these mechanisms promotes uncontrolled growth of tumors and is one of characteristic of cancer56. Since the crucial role of the purine and pyrimidine metabolic, they have been proposed as antiparasitic drug targets in Plasmodium falciparum57 and Schistosoma spp58. The highly regulated purine and pyrimidine metabolisms in T. spiralis after compound 1 exposure may bring imbalance of nucleotide molecule, failure to maintain parasite cell homeostasis and cause death of the parasite. For down-regulation of sphingolipid metabolism, the alteration of sphingolipid level could affect parasite survival as described above.
Pathway analysis of significantly altered metabolites after compound 1 exposure. Circles indicate the first shell of interactors related to the predicted pathway. Purine and pyrimidine metabolisms, and sphingolipid metabolism were predicted to be the related pathway with the false discovery rate of 2.27e−21 and 1.26e−19, respectively.
Further pathway analysis was performed on all altered metabolites using MetaboAnalyst (Fig. 8), and the most significantly altered pathway for all metabolites was glycerophospholipid metabolism. Both bioinformatics tools confirmed that glycerophospholipid metabolism plays an important role in compound 1 activity mechanisms. Glycerophospholipids, which are fatty acid diglycerides with a phosphatidyl ester molecule linked to the terminal carbon, are components of cellular or vesicle membranes59. The up-regulated glycerophospholipid metabolic pathway was shown to play an important role in the low-oxygen supply stress response in Saccharomyces cerevisiae60. In addition, glycerophospholipid metabolism was reported to be a potential pathway for antiparasitic drug development for Trichinella papuae61, P. falciparum62, Leishmania, Trypanosoma brucei, and Trypanosoma cruzi63. Therefore, T. spiralis glycerophospholipid metabolism might be affected by the stress generated by compound 1. The down-regulation of sphingolipid metabolism was also observed following compound 1 treatment. Sphingolipids are a class of lipids containing a sphingoid base backbone and the organic aliphatic amino alcohol sphingosine. Sphingolipids are abundant membrane components of pathogenic protozoans such as Leishmania major64, Trypanosoma brucei65, P. falciparum66, and Giardia lamblia67. Sphingolipid metabolism potentially contributes to parasite survival and host defense68. Moreover, sphingolipid production is necessary for cell cycle progression and cell survival in T. brucei, but it is not necessary for the regular movement of proteins associated with the flagellar membrane or the creation of lipid rafts69. Therefore, T. spiralis parasite survival may be impacted by the down-regulation of the sphingolipid pathway.
Conclusion
We investigated the anthelmintic activity and toxicity of compound 1 in this study using rodents and T. spiralis. Through this study, we found that compound 1 has good in vitro anthelmintic activity and acceptable toxicity in an animal model. However, it demonstrated lower in vivo anthelmintic efficacy compared with ABZ. The metabolomics results showed that purine and pyrimidine metabolisms and sphingolipid metabolism were significantly affected by compound 1. Further studies into its absorption, bioavailability, pharmacokinetics, and efficacy would help to understand this lack of in vitro and in vivo correlation. The information could also be advantages for further development of compound 1 as an anthelmintic.
Data availability
The datasets generated and/or analysed during the current study are available in the Science Data Bank repository (https://www.scidb.cn/anonymous/Qk5CYnFh).
References
World Health Organization. Fact Sheet: Soil-Transmitted Helminth Infections (World Health Organ, 2020).
Borkow, G. et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J. Clin. Investig. 106, 1053–1060 (2000).
Martin, R. J. Gamma-Aminobutyric acid-and piperazine-activated single-channel currents from Ascaris suum body muscle. Br. J. Pharmacol. 84, 445–461 (1985).
Borgers, M. & De Nollin, S. Ultrastructural changes in Ascaris suum intestine after mebendazole treatment in vivo. J. Parasitol. 61, 110–122 (1975).
Aceves, J., Erlij, D. & Martinez-Maranon, R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br. J. Pharmacol. 38, 602 (1970).
Lee, B. H. et al. Marcfortine and paraherquamide class of anthelmintics: Discovery of PNU-141962. Curr. Top. Med. Chem. 2, 779–793 (2002).
Premanathan, M., Karthikeyan, K., Jeyasubramanian, K. & Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine. 7, 184–192 (2011).
Venkatasubbu, G. D., Baskar, R., Anusuya, T., Seshan, C. A. & Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B. 148, 600–606 (2016).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Willson, J., Amliwala, K., Harder, A., Holden-Dye, L. & Walker, R. J. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 126, 79–86 (2003).
Gilles, H. M. & Hoffman, P. S. Treatment of intestinal parasitic infections: A review of nitazoxanide. Trends Parasitol. 18, 95–97 (2002).
Lee, H. H. & Terada, M. In vitro effects of milbemycin oxime: Mechanism of action against Angiostrongylus cantonensis and Dirofilaria immitis. Parasitol. Res. 78, 349–353 (1992).
Kaminsky, R. et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 452, 176–180 (2008).
Potârniche, A. V. et al. First report of anthelmintic resistance in gastrointestinal nematodes in goats in Romania. Animals. 11, 2761 (2021).
Ploeger, H. W. & Everts, R. R. Alarming levels of anthelmintic resistance against gastrointestinal nematodes in sheep in the Netherlands. Vet. Parasitol. 262, 11–15 (2018).
Idris, O. A., Wintola, O. A. & Afolayan, A. J. Helminthiases; prevalence, transmission, host-parasite interactions, resistance to common synthetic drugs and treatment. Heliyon. 5, e01161 (2019).
Suarez, V. H. & Cristel, S. L. Anthelmintic resistance in cattle nematode in the western Pampeana Region of Argentina. Vet. Parasitol. 144, 111–117 (2007).
Waghorn, T. S. et al. Prevalence of anthelmintic resistance on sheep farms in New Zealand. N. Z. Vet. J. 54, 271–277 (2006).
Fox, N. J., Marion, G., Davidson, R. S., White, P. C. L. & Hutchings, M. R. Climate-driven tipping-points could lead to sudden, high-intensity parasite outbreaks. R. Soc. Open Sci. 2, 140296 (2015).
Laohapaisan, P. et al. Discovery of N-methylbenzo [d] oxazol-2-amine as new anthelmintic agent through scalable protocol for the synthesis of N-alkylbenzo[d]oxazol-2-amine and N-alkylbenzo[d]thiazol-2-amine derivatives. Bioorg. Chem. 131, 106287 (2023).
Waterhouse, A. et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, 296–303 (2018).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Liu, Y. et al. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 41, 138–144 (2020).
Pang, Z. et al. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49, 388–396 (2021).
Szklarczyk, D. et al. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, 380–384 (2016).
Gao, F., Wang, R. & Liu, M. Trichinella spiralis, potential model nematode for epigenetics and its implication in metazoan parasitism. Front. Physiol. 4, 410 (2014).
Peng, R. Y. et al. Comparative proteomics analysis of Trichinella spiralis muscle larvae exposed to albendazole sulfoxide stress. Acta Trop. 185, 183–192 (2018).
Li, R. H. et al. Efficacy of albendazole orally administered at different dosages against Trichinella spiralis encapsulated larvae in mice. Chin. J. Parasitol. Parasit. Dis. 30, 184–188 (2012).
Codina, A. V. et al. Efficacy of albendazole: β-cyclodextrin citrate in the parenteral stage of Trichinella spiralis infection. Int. J. Biol. Macromol. 77, 203–206 (2015).
OECD Test No. 425. Acute Oral Toxicity: Up-and-Down Procedure; OECD Guidelines for the Testing of Chemicals, Section 4 (OECD Publishing, 2008).
Hong, S. T. Albendazole and praziquantel: Review and safety monitoring in Korea. J. Infect. Chemother. 50, 1–10 (2018).
Zwang, J. & Olliaro, P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: A meta-analysis. Parasites Vectors. 10, 1–16 (2017).
Dayan, A. D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 86, 141–159 (2003).
Bokhoven, M. A. et al. Why do patients want to have their blood tested? A qualitative study of patient expectations in general practice. BMC Fam. Pract. 7, 75 (2006).
Dik, B., Coşkun, D., Bahçivan, E. & Üney, K. Potential antidiabetic activity of benzimidazole derivative albendazole and lansoprazoledrugs in different doses in experimental type 2 diabetic rats. Turk. J. Med. Sci. 51, 1579–1586 (2021).
Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2, 74–79 (2011).
OECD Test No. 425. Acute Oral Toxicity: Up-and-Down Procedure; OECD Guidelines for the Testing of Chemicals, Section 4 (OECD Publishing, 2002).
Onakpoya, I. J. Antihelminthic drugs. In Side Effects of Drugs Annual Vol. 41 (ed. Sidhartha, D. R.) 339–349 (Elsevier, 2019).
Chambers, E. et al. Liver fluke β-tubulin isotype 2 binds albendazole and is thus a probable target of this drug. Parasitol. Res. 107, 1257–1264 (2010).
Chen, I. S. & Kubo, Y. Ivermectin and its target molecules: Shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J. Physiol. 596, 1833–1845 (2018).
Alpizar-Sosa, E. A. et al. Amphotericin B resistance in Leishmania mexicana: Alterations to sterol metabolism and oxidative stress response, PLoS Negl. Trop. Dis. 16(9), e0010779 (2022).
Leaver, D. J. Synthesis and Biological Activity of Sterol 14α-Demethylase and Sterol C24-Methyltransferase Inhibitors. Molecules 23(7), 1753 (2018).
de Souza, W. Rodrigues, J. C. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs interdiscip perspect infect Dis. 642502 (2009).
Yoo, E. et al. The antimalarial natural product salinipostin A identifies essential α/β serine hydrolases involved in lipid metabolism in P. falciparum parasites. Cell Chem. Biol. 27, 143–157 (2020).
Bonde, C. S. et al. Bio-guided fractionation and molecular networking reveal fatty acids to be principal anti-parasitic compounds in Nordic Seaweeds. Front. Pharmacol. 12 674520 (2021).
Hu, Z. & Duan, J. 1-Deoxysphingolipids and their analogs in foods: The occurrence and potential Iipact on human health. J. Nutr. Sci. Vitaminol. 68, S146–S148 (2022).
Li, J. et al. Phytosphingosine-induced cell apoptosis via a mitochondrially mediated pathway. Toxicology 482, 153370 (2022).
Sah, R. K. et al. Sphingosine-1-phosphate regulates Plasmodium histone deacetylase activity and exhibits epigenetic control over cell death and differentiation. bioRxiv. (2022).
Kuehl, F. A. Jr, Jacob, T. A., Ganley, O. H., Ormond, R. E. & Meisinger, M. A. P. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 79, 5577–5578 (1957).
R. M. Maizels, H. H. Smits, H. J. McSorley, Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules, Immunity. 49 (2018) 801–818.
Smith, P. et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J. Exp. Med. 202, 1319–1325 (2005).
Kuehl, F. A. Jr., Jacob, T. A., Ganley, O. H., Ormond, R. E. & Meisinger, M. A. P. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 79, 5577–5578 (1957).
Maizels, R. M., Smits, H. H. & McSorley, H. J. Modulation of host immunity by helminths: The expanding repertoire of parasite effector molecules. Immunity. 49, 801–818 (2018).
Smith, P. et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J. Exp. Med. 202, 1319–1325 (2005).
Weber, G. Enzymes of purine metabolism in cancer. Clin. Biochem. 16, 57–63 (1983).
Siddiqui, A. & Ceppi, P. A. A non-proliferative role of pyrimidine metabolism in cancer. Mol. Metab. 35, 100962 (2020).
Cassera, M. B., Zhang, Y., Hazleton, K. Z. & Schramm, V. L. Purine and pyrimidine pathways as targets in Plasmodium falciparum. Curr. Top. Med. Chem. 11, 2103–2115 (2011).
Kouni, M. H. E. Pyrimidine metabolism in schistosomes: A comparison with other parasites and the search for potential chemotherapeutic targets. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 213, 55–80 (2017).
Tao, B. Y. Industrial Applications for Plant Oils and Lipids: Bioprocessing for Value-Added Products from Renewable Resources 611–627 (Elsevier, 2007).
Xia, Z. et al. Multiple-omics techniques reveal the role of glycerophospholipid metabolic pathway in the response of Saccharomyces cerevisiae against hypoxic stress. Front. Microbiol. 10, 1398 (2019).
Mangmee, S. et al. Lipid profile of Trichinella papuae muscle-stage larvae. Sci. Rep. 10, 1–11 (2020).
Vial, H. J. et al. Lipids as drug targets for malaria therapy. In Apicomplexan Parasites: Molecular Approaches Toward Targeted Drug Development (ed. Becker, K.) 137–162 (Wiley-VCH, 2011).
Biagiotti, M., Dominguez, S., Yamout, N. & Zufferey, R. Lipidomics and anti-trypanosomatid chemotherapy. J. Transl. Med. 6, 1–11 (2017).
Zhang, K. et al. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 22, 6016–6026 (2003).
Güther, M. L. S., Lee, S., Tetley, L., Acosta-Serrano, A. & Ferguson, M. A. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol. Biol. Cell. 17, 5265–5274 (2006).
Gerold, P. & Schwarz, R. T. Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 112, 29–37 (2001).
Kaneda, Y. & Goutsu, T. Lipid analysis of Giardia lamblia and its culture medium. Ann. Trop. Med. Parasitol. 82, 83–90 (1988).
Zhang, K., Bangs, J. D. & Beverley, S. M. Sphingolipids in parasitic protozoa. In Sphingolipids as Signaling and Regulatory Molecules (eds Chalfant, C. & Poeta, M. D.) 238–248 (Springer, 2010).
Fridberg, A. et al. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J. Cell. Sci. 121, 522–535 (2008).
Acknowledgements
We sincerely thank Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University for equipment support.
Funding
This research project is supported by Mahidol University (MU’s Strategic Research Fund: 2023) to O.R. and Innovation Project grant [69864] and Thailand Science Research and Innovation (TSRI), Grant No. 36824/4274394 to J.T.
Author information
Authors and Affiliations
Contributions
P.P., J.T., P.A. and O.R. designed experiments, participated in all experiments, wrote and revised manuscript. P.A. maintained T. spilaris life cycle. N.U. analyzed metabolomics data. U.B. performed molecular docking experiment. P.T. and J.T. carried out mass spectrometric analysis. P.L., C.T., S.R. synthesized compound. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prangthip, P., Tummatorn, J., Adisakwattana, P. et al. Anthelmintic efficacy evaluation and mechanism of N-methylbenzo[d]oxazol-2-amine. Sci Rep 13, 22840 (2023). https://doi.org/10.1038/s41598-023-50305-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50305-y
- Springer Nature Limited