Abstract
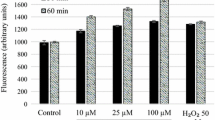
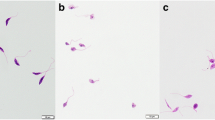
Despite the available drug options, leishmaniasis treatment remains unsatisfactory. The repurposing of calpain inhibitors originally developed for human diseases became an interesting alternative, since Leishmania cells express calpain-related proteins. The susceptibility of six Leishmania species (L. amazonensis, L. braziliensis, L. major, L. mexicana, L. chagasi, and L. donovani) to the calpain inhibitor MDL28170 was determined. Promastigote and intracellular amastigote viability in the presence of MDL28170 was evaluated. MDL28170 was able to reduce promastigote proliferation in a dose-dependent manner for all the parasites. A significant reduction on the general parasite metabolism was detected, as judged by resazurin assay, as well as induced important morphological alterations, including rounding promastigotes and loss of the flagellum. MDL28170 was also able to reduce the number of intracellular amastigotes in RAW macrophages. The susceptibility of both parasite stages (promastigotes and amastigotes) to MDL28170 was similar for all Leishmania species tested. MDL28170 showed a much higher toxicity to Leishmania amastigotes when compared with mammalian macrophages, displaying selectivity index values varying from 13.1 to 39.8. These results suggest that the development of calpain inhibitors may represent an interesting alternative in the treatment of leishmaniasis.





Similar content being viewed by others
References
Aulner N, Danckaert A, Rouault-Hardoin E, Desrivot J, Helynck O, Commere PH, Munier-Lehmann H, Späth GF, Shorte SL, Milon G, Prina E (2013) High content analysis of primary macrophages hosting proliferating Leishmania amastigotes: application to anti-leishmanial drug discovery. PLoS Negl Trop Dis 7:e2154
Branquinha MH, Marinho FA, Sangenito LS, Oliveira SSC, Gonçalves KC, Ennes-Vidal V, D’Avila-Levy CM, Santos ALS (2013) Calpains: potential targets to alternative chemotherapeutic intervention against human pathogenic trypanosomatids. Curr Med Chem 20:3174–3185
Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW (2009) Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. J Biomed Mater Res 88:858–871
Croft SL, Seifert K, Yardley V (2006) Current scenario of drug development for leishmaniasis. Indian J Med Res 123:399–410
D’Avila-Levy CM, Marinho FA, Santos LO, Martins JL, Santos AL, Branquinha MH (2006) Antileishmanial activity of MDL28170, a potent calpain inhibitor. Int J Antimicrob Agents 28:138–142
Donkor IO (2015) An updated patent review of calpain inhibitors (2012-2014). Expert Opin Ther Pat 25:17–31
Ennes-Vidal V, Menna-Barreto RF, Santos ALS, Branquinha MH, D’Avila-Levy CM (2010) Effects of the calpain inhibitor MDL28170 on the clinically relevant forms of Trypanosoma cruzi in vitro. J Antimicrob Chemother 66:1395–1398
Ennes-Vidal V, Menna-Barreto RF, Santos ALS, Branquinha MH, D’Avila-Levy CM (2011) MDL28170, a calpain inhibitor, affects Trypanosoma cruzi metacyclogenesis, ultrastructure and attachment to Rhodnius prolixus midgut. PLoS One 6:e18371
Ennes-Vidal V, Menna-Barreto RF, Santos ALS, Branquinha MH, D’Ávila-Levy CM (2017) Why calpain inhibitors are interesting leading compounds to search for new therapeutic options to treat leishmaniasis? Parasitology 144:117–123
Ersfeld K, Barraclough H, Gull K (2005) Evolutionary relationships and protein domain architecture in an expanded calpain superfamily in kinetoplastid parasites. J Mol Evol 61:742–757
Kervic I, Cappel MA, Keeling JH (2015) New world and old world Leishmania infections—a practical review. Dermatol Clin 33:579–593
Kim SI, Kim HJ, Lee HJ, Lee K, Hong D, Lim H, Cho K, Jung N, Yi Y (2016) Application of a non-hazardous vital dye for cell counting with automated cell counters. Anal Biochem 492:8–12
Kulshrestha A, Bhandari V, Mukhopadhyay R, Ramesh V, Sundar S, Maes L, Dujardin JC, Roy S, Salotra P (2013) Validation of a simple resazurin-based promastigote assay for the routine monitoring of miltefosine susceptibility in clinical isolates of Leishmania donovani. Parasitol Res 112:825–828
Marinho FA, Gonçalves KC, Oliveira SS, Gonçalves DS, Matteoli FP, Seabra SH, Oliveira AC, Bellio M, Oliveira SS, Souto-Padrón T, D’Avila-Levy CM, Santos ALS, Branquinha MH (2014) The calpain inhibitor MDL28170 induces the expression of apoptotic markers in Leishmania amazonensis promastigotes. PLoS One 9:e87659
Marinho FA, Sangenito LS, Oliveira SSC, De Arruda LB, D’Avila-Levy CM, Santos ALS, Branquinha MH (2017) The potent cell permeable calpain inhibitor MDL28170 affects the interaction of Leishmania amazonensis with macrophages and shows anti-amastigotes activity. Parasitol Int 66:579–583
Menezes JPB, Guedes CES, Petersen ALOA, Fraga DBM, Veras PST (2015) Advances in development of new treatment for Leishmaniasis. Biomed Res Int 2015: 815023, 1, 11
Mikus J, Steverding D (2000) A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int 48:265–269
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
Nassif WP, Melo TFP, Navasconi TR, Mota CA, Demarchi IG, Aristides SMA, Lonardoni MVC, Teixeira JJV, Silveira TGV (2017) Safety and efficacy of current alternatives in the topical treatment of cutaneous leishmaniasis: a systematic review. Parasitology 144:995–1004
No JH (2016) Visceral leishmaniasis: revisiting current treatments and approaches for future discoveries. Acta Trop 155:113–123
Nogueira PM, Assis RR, Torrecilhas AC, Saraiva EM, Pessoa NL, Campos MA, Marialva EF, Rios-Velasquez CM, Pessoa FA, Secundino NF, Rugani JN, Nieves E, Turco SJ, Melo MN, Soares RP (2016) Lipophosphoglycans from Leishmania amazonensis strains display immunomodulatory properties via TLR4 and do not affect sand fly infection. PLoS Negl Trop Dis 10:e0004848
Ono Y, Sorimachi H (2012) Calpains—an elaborate proteolytic system. Biochim Biophys Acta 1824:224–236
Ono Y, Saido TC, Sorimachi H (2016) Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov 6:854–876
Polonio T, Efferth T (2008) Leishmaniasis: drug resistance and natural products (review). Int J Mol Med 22:277–286
Sangenito LS, Ennes-Vidal V, Marinho FA, Da Mota FF, Santos ALS, Avila-Levy CM D’, Branquinha MH (2009) Arrested growth of Trypanosoma cruzi by the calpain homologues in epimastigotes forms. Parasitology 136:433–441
Santos ALS (2011) Protease expression by microorganisms and its relevance to crucial physiological/pathological events. World J Biol Chem 26:48–58
Santos LO, Marinho FA, Altoé EF, Vitório BS, Alves CR, Brito C, Motta MC, Branquinha MH, Santos ALS, d'Avila-Levy CM (2009) HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonensis. PLoS One 4:e4918
Savoia D (2015) Recent updates and perspectives on leishmaniasis. J Infect Dev Ctries 9:588–596
Toté K, Vandenberghe D, Levecque S, Bénéré E, Maes L, Cos P (2009) Evaluation of hydrogen peroxide-based disinfectants in a new resazurin microplate method for rapid efficacy testing of biocides. J Appl Microbiol 107:606–615
World Health Organization (2013) Leishmaniasis: background information. http://www.who.int/leishmaniasis/en/
Zulfiqar B, Shelper TB, Avery VM (2017) Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov Today 22:1516–1531
Acknowledgments
The authors would like to thank Denise Rocha de Souza, who is supported by a FAPERJ scholarship, for her technical assistance.
Funding
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação Oswaldo Cruz (FIOCRUZ). ALSS, MHB, and CMd’A-L were supported by CNPq and FAPERJ fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Sarah Hendrickx
André Luis Souza dos Santos and Marta Helena Branquinha share senior authorship.
Rights and permissions
About this article
Cite this article
de Sousa Araújo, P.S., de Oliveira, S.S.C., d’Avila-Levy, C.M. et al. Susceptibility of promastigotes and intracellular amastigotes from distinct Leishmania species to the calpain inhibitor MDL28170. Parasitol Res 117, 2085–2094 (2018). https://doi.org/10.1007/s00436-018-5894-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5894-7