Abstract
Cryptosporidium is a major cause of diarrheal disease worldwide. In developing countries, this infection is endemic and in children, associated with growth faltering and cognitive function deficits, with the most severe impact on those aged <2 years. Little has been reported about symptoms and risk factors for children in industrialized countries, although the disease incidence is increasing in such regions. In November 2010, a large waterborne outbreak of C. hominis occurred in the city of Östersund in Sweden. Approximately 27,000 of the 60,000 inhabitants were symptomatic. We aimed to describe duration of symptoms and the risk factors for infection with C. hominis in children aged <15 years in a Western setting. Within 2 months after a boil water advisory, a questionnaire was sent to randomly selected inhabitants of all ages, including 753 children aged <15 years. Those with ≥3 loose stools/day were defined as cases of diarrhoea. The response rate was 70.3%, and 211 children (39.9%) fulfilled the case definition. Mean duration of diarrhoea was 7.5 days (median 6, range 1–80 days). Recurrence, defined as a new episode of diarrhoea after ≥2 days of normal stools, occurred in 52.5% of the cases. Significant risk factors for infection, besides living within the distribution area of the contaminated water plant, included a high level of water consumption, male sex, and a previous history of loose stools. The outbreak was characterized by high attack and recurrence rates, emphasizing the necessity of water surveillance to prevent future outbreaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, Cryptosporidium accounts for a majority of waterborne outbreaks of protozoan parasitic diseases (Baldursson and Karanis 2011; Checkley et al. 2015; Efstratiou et al. 2017; Karanis et al. 2007). Cryptosporidiosis is self-limiting in individuals who are immunocompetent but can be very severe in those who are immunocompromised, and this illness most often affects children, especially those <2 years of age (Caccio and Chalmers 2016). To date, 31 species of Cryptosporidium have been identified, and C. hominis and C. parvum account for >90% of human infections (Chalmers et al. 2011; Zahedi et al. 2016). The route of transmission is faecal–oral via ingestion of oocysts present primarily in contaminated water or food, although direct transmission can also occur through direct physical contact with an infected person or animal or inhalation of oocysts (Bouzid et al. 2013; Karanis et al. 2007; Mor et al. 2010).
Once ingested, an oocyst releases sporozoites that penetrate and infect the host’s intestinal epithelial cells, leading to diarrhoea, abdominal pain, nausea, and vomiting. The severity of cryptosporidiosis depends chiefly on factors associated with the host, particularly the individual’s immune status. The incubation period is normally 2–10 days (average 7 days), and the symptoms of acute gastroenteritis last approximately 2 days to 3 weeks in adults (Cama et al. 2008; Frisby et al. 1997; Insulander et al. 2005; Tangermann et al. 1991; Widerstrom et al. 2014; Yamamoto et al. 2000). The acute symptoms are equivalent in patients infected with C. hominis and those with C. parvum, although more variable duration of the symptoms has been described in the former group (Chalmers and Davies 2010). Indeed, some individuals infected with C. hominis experience more prolonged complaints, including sequelae of extraintestinal symptoms such as joint pain, eye pain, and fatigue, although these occur less often in children (Hunter et al. 2004; Rehn et al. 2015). Shedding of oocysts usually ends within a week after cessation of diarrhoea but can continue for several weeks (Chalmers and Davies 2010).
Most studies of children with cryptosporidiosis have been performed in developing countries, where diarrhoea due to this illness is a major concern (Kotloff et al. 2013), and the infection is known to be associated with cognitive function deficits and growth faltering and stunting (Ajjampur et al. 2010). Re-infections frequently occur; that is, children suffer multiple infections with the same or different Cryptosporidium spp. or with some other genotype (Ajjampur et al. 2010; Cama et al. 2008). Recurrence of diarrhoea within a week after the end of a previous episode has also been described (MacKenzie et al. 1995; Newman et al. 1999). Several studies in developing countries have confirmed that young age is a risk factor for acquiring cryptosporidiosis (Kotloff et al. 2013; MacKenzie et al. 1995), although the severity of symptoms and the incidence of infections decrease with age, possibly due to the development of immunity (Ajjampur et al. 2010; Bouzid et al. 2013). Other risk factors described in such countries are low birth weight and male sex, whereas breast-feeding has been reported to offer protection (Creek et al. 2010; Molbak et al. 1994; Newman et al. 1999).
Considering Western countries, the literature offers little information regarding risk factors and symptoms in children. One investigation of a large outbreak in Milwaukee, Wisconsin, in the USA identified immune suppression, HIV, and age ≥1 year as risk factors (Cicirello et al. 1997). Also, a study in London, England, showed a higher rate of chronic diarrhoea following Cryptosporidium infection in children ≤2 years of age (Phillips et al. 1992).
Cryptosporidium oocysts frequently occur in surface water. In Sweden in 2011, Cryptosporidium oocysts were found in 11.5% of analysed samples of raw water, which is equivalent to levels that have been observed in other countries (Chalmers et al. 2010; Robertson and Gjerde 2001). Clearly, to prevent waterborne outbreaks, it is essential to monitor the quality of both raw water and drinking water and to evaluate the efficiency of current barriers in water treatment plants (DeSilva et al. 2016). Also of importance in this context is that a rising number of outbreaks associated with recreational water facilities have been reported (Yoder and Beach 2010), and it is expected that climate changes will further increase the number of outbreaks in the future (Young et al. 2015).
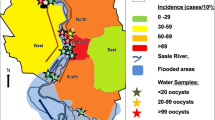
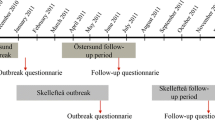
In November 2010, an extensive waterborne outbreak of Cryptosporidium occurred in the city of Östersund, which is located in central Sweden and has a population of 60,000. An estimated 27,000 inhabitants were symptomatic, thus representing the largest outbreak in Europe to date. Oocysts were detected in raw water from Lake Storsjön, in drinking water from the waterworks that supplied approximately 85% of the inhabitants of the city, and in faecal samples from sick patients. All of the analysed samples contained the C. hominis subtype IbA10G2. The number of individuals with gastrointestinal symptoms increased from mid-November, and a boil water advisory was issued on November 26; thereafter, the number of new cases rapidly declined. On 23 December, a UV water disinfection system was installed as a complement to the previous standard purification using ozonation and chloramination, and the boil water advisory was cancelled on 18 February 2011 (Widerstrom et al. 2014).
The aim of this study was to describe the duration of symptoms of C. hominis infection and the risk factors for such illness in children aged <15 years in a Western setting.
Materials and methods
A retrospective study was conducted within 2 months after the boil water advisory was issued. One thousand five hundred eight randomly selected inhabitants of all ages living in the Östersund municipality were sent a questionnaire concerning the following: onset and occurrence of possible symptoms of cryptosporidiosis and possible risk factors, such as medical history, number of family members infected, and how many glasses of water that were consumed daily. Parents were asked to respond on behalf of children <15 years of age. A reminder was sent to those who had not responded within 2 weeks. The methodology has previously been described in detail (Widerstrom et al. 2014). Concurrently, the same questionnaire was sent to an additional 600 randomly selected children (i.e. subjects that were not included in the cited study) who were aged 0–5 years and lived in Östersund municipality. In total, 753 children aged <15 years received the questionnaire. Information about the distribution of water from the waterworks to the respondents’ homes was ascertained through population registers.
We used the following definition of diarrhoea set by the World Health Organization (WHO): ≥3 loose or liquid stools/day (WHO 2016). Children aged <15 years with onset of diarrhoea after 1 November 2010 and before 31 January 2011 were defined as cases. A relapse was defined as recurrent diarrhoea after ≥2 days of normal stools.
Statistical analyses were performed using SPSS (version 19, SPSS Inc. Chicago, IL, USA). Logistic regression was applied to investigate relationship between risk factors and clinical cryptosporidiosis as the outcome variable. Independent samples t test was used to evaluate differences between age and sex groups. All analyses of age-related differences among children were performed using the following four age groups: <2, 2–5.99, 6–9.99, and 10–14.99 years. Probabilities of <0.05 were considered statistically significant. Six respondents that stated unlikely high water consumption (i.e. >30 glasses of water/day) were excluded from analyses regarding water consumption. Some of the risk factors that were analysed in the adult population included in our previous study (i.e. irritable bowel syndrome, inflammatory bowel disease, diabetes, cortisone medication, rheumatic disease, and peptic ulcer medication) (Widerstrom et al. 2014) were not assessed in children in the present investigation due to too few positive answers.
Results
The overall questionnaire response rate was 70.3%, and male and female children of all ages responded at equivalent rates. The proportion of children aged 0–5 years was 79.7%, and 39.9% (211/529) of all the children (more males than females: 45.1 vs. 34.5%, P = 0.015) met the case definition (Table 1). The attack rate was 43.6% for children who lived in the parts of Östersund served by the contaminated water treatment plant compared to 25.7% for children who lived in areas that were not served by that plant (P = 0.001). Most cases (>50%) were detected within ±2 weeks of the date the boil water advisory was issued. Thereafter, the number of cases rapidly declined, but symptoms appeared in January 2011 in 11.0% of the cases. A median of three individuals living in the same household as a case exhibited gastrointestinal symptoms, and the corresponding number for those living together with non-cases was one. A small percentage (0.4%) reported that they did not follow the boil water advisory, and 7.6% indicated that they occasionally failed to do so.
Symptoms
The most commonly presented symptoms are listed in Table 2. Watery diarrhoea was reported by 82.0% of cases but also by 6.5% of non-cases. Among cases, two symptoms were more common in female children: headache (51 vs. 28% in males, P = 0.03) and abdominal pain (76 vs. 61.1% in males, P = 0.035). The mean duration of diarrhoea was 7.5 days (median 6, range 1–80 days), and no differences in duration were found between male and female children or between different age groups (Table 3). However, the duration of diarrhoea was longer for some children: 13.2% reported ≥14 days, and 2.0% reported ≥30 days. Also, 33.5% of the participants reported one relapse, 11.2% reported two relapses, and 7.8% indicated ≥3 relapses; thus, no relapses were reported by 47.6% of the respondents. There was no difference in recurrence rate between the sexes or between age groups.
Risk factors and underlying chronic conditions
Table 4 presents risk factors for clinical cryptosporidiosis. Male sex and also high consumption of water increased the risk. Male and female children consumed the same amount of water (4.7 glasses/day). The youngest children aged 0–1 years consumed less water than those aged 2–5 years (mean 3.7 vs. 4.4 glasses/day, P = 0.003).
Discussion
To our knowledge, this is the most extensive study describing risk factors and clinical symptoms among children aged <15 years with cryptosporidiosis in a Western setting. In particular, this investigation focused on the youngest children aged 0–5 years, who constituted the largest group in the evaluation. We found no evidence that young age was a significant risk factor for cryptosporidiosis, as is often found in studies conducted in developing countries (Kotloff et al. 2013; Molbak et al. 1994). However, male children were at significantly higher risk of cryptosporidiosis, which concurs with previous reports (Agnew et al. 1998; Molbak et al. 1994) and with increasing data showing that morbidity from various infectious diseases occurs more frequently in male than that in female children (Muenchhoff and Goulder 2014). The general findings of our investigation with regard to symptoms and the duration of diarrhoea are also consistent with previous studies (Cicirello et al. 1997; Phillips et al. 1992).
No differences between age groups were found with respect to incidence, symptoms, or duration of diarrhoea, which might be partly explained by nutritional factors, considering that cryptosporidiosis has been observed to be more prevalent in malnourished children (Bouzid et al. 2013; Caccio and Chalmers 2016). Most children in Östersund were probably exposed to Cryptosporidium for the first time during the noted outbreak, whereas older children in countries where Cryptosporidium is endemic may develop partial immunity over time (Checkley et al. 2015). In our study, more water was consumed by the older children than those who were youngest, which might have resulted in ingestion of a larger number of oocysts. Oocyst density in the drinking water was relatively low during the outbreak in Östersund, although this was measured after the boil water advisory had been issued (Widerstrom et al. 2014). Notably, previous reports have described a protective impact of breast-feeding (Creek et al. 2010; Molbak et al. 1994), an aspect that might also have contributed to a lower incidence of infection among the youngest children in our study.
Although >90% of the questionnaire respondents followed the boil water advisory, new cases of cryptosporidiosis occurred in January. Symptoms were reported by more individuals in households with cases than in households with non-cases, possibly indicating the presence of secondary transmission or potentially the habit of consuming large amounts of drinking water within such household. Secondary transmission among children was suggested to be an important transmission route in several outbreaks (Heijbel et al. 1987; Johansen et al. 2015), but it is also possible that some of the affected children in those studies drank tap water without the parents’ knowledge. In addition to this, asymptomatic carriage is prevalent (Ajjampur et al. 2010; Collinet-Adler and Ward 2010; Cordell et al. 1997; Davies and Chalmers 2009); for example, only 78% of the children found to be positive for Cryptosporidium in Milwaukee presented with diarrhoea (Cicirello et al. 1997).
A high rate of recurrence of diarrhoea in individuals affected by cryptosporidiosis as reported by Hunter et al. (2004) is consistent with the recurrence rates observed in adults in both Östersund (Widerstrom et al. 2014) and Milwaukee (MacKenzie et al. 1995). Whether recurrence in diarrhoea in the present subjects was due to autoinfection or new secondary transmission is not possible to determine based on our data. It is well documented that oocyst shedding continues after cessation of symptoms (Chalmers and Davies 2010), potentially representing a risk that individuals will re-infect themselves or others. In Östersund, it is unlikely that recurring diarrhoea could have been due to other pathogens, because no laboratory-confirmed cases during the outbreak in that city were positive for any gastrointestinal pathogens other than Cryptosporidium (Widerstrom et al. 2014).
The WHO definition of persistent diarrhoea (i.e. lasting ≥14 days) (WHO 2016) was fulfilled by 13.2% of cases in our investigation, and a high rate of persistent diarrhoea among children with cryptosporidiosis was described in a previous study conducted in London, England (Phillips et al. 1992). In Östersund, only 2.0% met the WHO definition of chronic diarrhoea (i.e. lasting ≥30 days), although this may be an underestimation, because the follow-up of diarrhoea ended in February 2011.
Strengths of our investigation include a large study population and a high response rate only a short time after a C. hominis outbreak. A major weakness is that cases may have been misclassified. In short, it is possible that some cases of diarrhoea were not caused by C. hominis, considering that many were not verified by analysis of faecal samples. On the other hand, a number of non-cases also presented with gastrointestinal symptoms, indicating that some of them might also have been infected with Cryptosporidium.
In conclusion, the outbreak in Östersund was characterized by a high attack rate and a high rate of recurrent diarrhoea that led to many days with symptoms in a large number of the investigated children. In addition to living within the distribution area of the contaminated water plant, other significant risk factors included consumption of large amounts of water, male sex, and a previous history of loose stools. This study underlines that employing insufficient drinking water control measures can have a pronounced impact on public health.
References
Agnew DG, Lima AA, Newman RD, Wuhib T, Moore RD, Guerrant RL, Sears CL (1998) Cryptosporidiosis in northeastern Brazilian children: association with increased diarrhea morbidity. J Infect Dis 177:754–760
Ajjampur SS et al (2010) Symptomatic and asymptomatic Cryptosporidium infections in children in a semi-urban slum community in southern India. Am J Trop Med Hyg 83:1110–1115. https://doi.org/10.4269/ajtmh.2010.09-0644
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2004–2010. Water Res 45:6603–6614. https://doi.org/10.1016/j.watres.2011.10.013
Bouzid M, Hunter PR, Chalmers RM, Tyler KM (2013) Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev 26:115–134. https://doi.org/10.1128/CMR.00076-12
Caccio SM, Chalmers RM (2016) Human cryptosporidiosis in Europe. Clin Microbiol Infect 22:471–480. https://doi.org/10.1016/j.cmi.2016.04.021
Cama VA et al (2008) Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis 14:1567–1574. https://doi.org/10.3201/eid1410.071273
Chalmers RM, Davies AP (2010) Minireview: clinical cryptosporidiosis. Exp Parasitol 124:138–146. https://doi.org/10.1016/j.exppara.2009.02.003
Chalmers RM et al (2010) Detection of Cryptosporidium species and sources of contamination with Cryptosporidium hominis during a waterborne outbreak in north west Wales. J Water Health 8:311–325
Chalmers RM, Smith R, Elwin K, Clifton-Hadley FA, Giles M (2011) Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004–2006. Epidemiol Infect 139:700–712. https://doi.org/10.1017/S0950268810001688
Checkley W et al (2015) A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. https://doi.org/10.1016/S1473-3099(14)70772-8
Cicirello HG, Kehl KS, Addiss DG, Chusid MJ, Glass RI, Davis JP, Havens PL (1997) Cryptosporidiosis in children during a massive waterborne outbreak in Milwaukee, Wisconsin: clinical, laboratory and epidemiologic findings. Epidemiol Infect 119:53–60
Collinet-Adler S, Ward HD (2010) Cryptosporidiosis: environmental, therapeutic, and preventive challenges. Eur J Clin Microbiol Infect Dis 29:927–935
Cordell RL et al (1997) Impact of a massive waterborne cryptosporidiosis outbreak on child care facilities in metropolitan Milwaukee, Wisconsin. Pediatr Infect Dis J 16:639–644
Creek TL et al (2010) Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr 53:14–19. https://doi.org/10.1097/QAI.0b013e3181bdf676
Davies AP, Chalmers RM (2009) Cryptosporidiosis. BMJ 339:b4168. https://doi.org/10.1136/bmj.b4168
Desilva MB et al (2016) Communitywide cryptosporidiosis outbreak associated with a surface water-supplied municipal water system—Baker City, Oregon, 2013. Epidemiol Infect 144:274–284. https://doi.org/10.1017/S0950268815001831
Efstratiou A, Ongerth JE, Karanis P (2017) Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2011–2016. Water Res 114:14–22. https://doi.org/10.1016/j.watres.2017.01.036
Frisby HR, Addiss DG, Reiser WJ, Hancock B, Vergeront JM, Hoxie NJ, Davis JP (1997) Clinical and epidemiologic features of a massive waterborne outbreak of cryptosporidiosis in persons with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 16:367–373
Heijbel H, Slaine K, Seigel B, Wall P, SJ MN, Gibbons W, Istre GR (1987) Outbreak of diarrhea in a day care center with spread to household members: the role of Cryptosporidium. Pediatr Infect Dis J 6:532–535
Hunter PR et al (2004) Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis: Off Publ Infect Dis Soc Am 39:504–510. https://doi.org/10.1086/422649
Insulander M, Lebbad M, Stenstrom TA, Svenungsson B (2005) An outbreak of cryptosporidiosis associated with exposure to swimming pool water. Scand J Infect Dis 37:354–360
Johansen OH, Hanevik K, Thrana F, Carlson A, Stachurska-Hagen T, Skaare D, Robertson LJ (2015) Symptomatic and asymptomatic secondary transmission of Cryptosporidium parvum following two related outbreaks in schoolchildren. Epidemiol Infect 143:1702–1709. https://doi.org/10.1017/S095026881400243X
Karanis P, Kourenti C, Smith H (2007) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Kotloff KL et al (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. https://doi.org/10.1016/S0140-6736(13)60844-2
Mackenzie WR et al (1995) Massive outbreak of waterborne cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis: Off Publ Infect Dis Soc Am 21:57–62
Molbak K, Aaby P, Hojlyng N, da Silva AP (1994) Risk factors for Cryptosporidium diarrhea in early childhood: a case-control study from Guinea-Bissau, West Africa. Am J Epidemiol 139:734–740
Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, Griffiths JK (2010) Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin Infect Dis: Off Publ Infect Dis Soc Am 50:1366–1372. https://doi.org/10.1086/652140
Muenchhoff M, Goulder PJ (2014) Sex differences in pediatric infectious diseases. J Infect Dis 209(Suppl 3):S120–S126. https://doi.org/10.1093/infdis/jiu232
Newman RD et al (1999) Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis 180:167–175. https://doi.org/10.1086/314820
Phillips AD, Thomas AG, Walker-Smith JA (1992) Cryptosporidium, chronic diarrhoea and the proximal small intestinal mucosa. Gut 33:1057–1061
Rehn M et al (2015) Post-infection symptoms following two large waterborne outbreaks of Cryptosporidium hominis in Northern Sweden, 2010–2011. BMC Publ Health 15:529. https://doi.org/10.1186/s12889-015-1871-6
Robertson LJ, Gjerde B (2001) Occurrence of Cryptosporidium oocysts and Giardia cysts in raw waters in Norway. Scand J Public Health 29:200–207
Tangermann RH, Gordon S, Wiesner P, Kreckman L (1991) An outbreak of cryptosporidiosis in a day-care center in Georgia. Am J Epidemiol 133:471–476
WHO (2016). http://www.who.int/topics/diarrhoea/en/ Accessed April 11th 2017
Widerstrom M et al (2014) Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis 20:581–589. https://doi.org/10.3201/eid2004.121415
Yamamoto N et al (2000) Outbreak of cryptosporidiosis after contamination of the public water supply in Saitama Prefecture, Japan, in 1996. Kansenshogaku Zasshi 74:518–526
Yoder JS, Beach MJ (2010) Cryptosporidium surveillance and risk factors in the United States. Exp Parasitol 124:31–39
Young I, Smith BA, Fazil A (2015) A systematic review and meta-analysis of the effects of extreme weather events and other weather-related variables on Cryptosporidium and Giardia in fresh surface waters. J Water Health 13:1–17. https://doi.org/10.2166/wh.2014.079
Zahedi A et al (2016) Zoonotic Cryptosporidium species in animals inhabiting Sydney water catchments. PLoS One 11:e0168169. https://doi.org/10.1371/journal.pone.0168169
Acknowledgments
This work was supported by unrestricted grants from Region Jämtland Härjedalen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all parents/legal guardians of children included in the study. The study was approved by the Research Ethics Committee of the Faculty of Medicine, Umeå University, Umeå, Sweden (Reg. no. 2010-392-31M).
Funding
This work was supported by unrestricted grants from Region Jämtland Härjedalen, Sweden.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adler, S., Widerström, M., Lindh, J. et al. Symptoms and risk factors of Cryptosporidium hominis infection in children: data from a large waterborne outbreak in Sweden. Parasitol Res 116, 2613–2618 (2017). https://doi.org/10.1007/s00436-017-5558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5558-z