Abstract
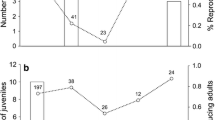
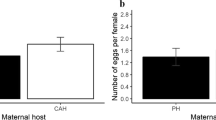
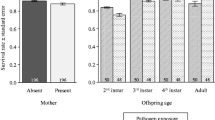
Parents may alter offspring phenotype depending on the type of environment they encounter. Parasitism is a common stressor; therefore, maternal reproductive investment could change in response to parasitic infection. However, few experiments have investigated the relationship between parasitism and maternal investment, whereas earlier field studies provided contradictory evidence. We investigated number, sex ratio, and growth of offspring in two rodent species, solitary altricial Meriones crassus and social precocial Acomys cahirinus, exposed to parasitism by fleas Xenopsylla ramesis and Parapulex chephrenis. No effect of treatment on litter size or sex ratio of a litter was found in either rodent species. Flea parasitism was found to affect pre-weaning body mass gain in M. crassus, but not in A. cahirinus pups. Furthermore, it appeared that female M. crassus invested resources into their offspring differently in dependence of litter size. In small litters (1–3 offspring), pups from infested females gained more body mass before weaning than pups from uninfested mothers. However, this trend was reversed in females with large litters indicating that parasitized females have a finite amount of resources with which to provision their young. Thus, M. crassus mothers parasitized by fleas seemed to receive some sort of external cues (e.g., stress caused by infestation) that prompted them to alter offspring provisioning, depending on species-specific possibilities and constraints. Therefore, parasites could be a mediator of environmentally induced maternal effects and offspring provisioning may have adaptive value against parasitism.



Similar content being viewed by others
References
Agnew P, Koella JC, Michalakis Y (2000) Host life history responses to parasitism. Microbes Infect 2:891–896
Barton K (2016) MuMIn: multi-model inference. R package version. 1.15.6. http://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bernardo J (1996) Maternal effects in animal ecology. Am Zool 36:83–105
Bize P, Roulin A, Bersier LF, Pfluger D, Richner H (2003) Parasitism and developmental plasticity in Alpine swift nestlings. J Anim Ecol 72:633–639
Bize P, Roulin A, Tella JL, Bersier LF, Richner H (2004) Additive effects of ectoparasites over reproductive attempts in the long-lived Alpine swift. J Anim Ecol 73:1080–1088
Bonduriansky R, Day T (2013) Nongenetic inheritance and the evolution of costly female preference. J Evol Biol 26:76–87
Burdge GC, Hoile SP, Uller T, Thomas NA, Gluckman PD, Hanson MA, Lillycrop KA (2011) Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition. PLoS One 6:10
Burgess SC, Marshall DJ (2014) Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123:769–776
Cameron EZ (2004) Facultative adjustment of mammalian sex ratios in support of the Trivers-Willard hypothesis: evidence for a mechanism. Proc R Soc B 271:1723–1728
Chiaverano LM, Graham WM, Costello JH (2015) Parasites alter behavior, reproductive output, and growth patterns of Aurelia medusae in a marine lake. Mar Ecol Prog Ser 540:87–98
Clancey E, Byers JA (2016) A comprehensive test of the Trivers-Willard hypothesis in pronghorn (Antilocapra americana). J Mammal 97:179–186
Cook RC, Cook JG, Vales DJ, Johnson BK, Mccorquodale SM, Shipley LA, Riggs RA, Irwin LL, Murphie SL, Murphie BL (2013) Regional and seasonal patterns of nutritional condition and reproduction in elk. Wildl Monogr 184:1–45
Dama MS, Novakova LM, Flegr J (2016) Do differences in Toxoplasma prevalence influence global variation in secondary sex ratio? Preliminary ecological regression study. Parasitology 143:1193–1203
Dlugosz EM, Downs CJ, Khokhlova IS, Degen AA, Krasnov BR (2014) Host reproductive status and reproductive performance of a parasite: offspring quality and trade-offs in a flea parasitic on a rodent. Parasitology 141:914–924
Fisher DO (1999) Offspring sex ratio variation in the bridled nailtail wallaby, Onychogalea fraenata. Behav Ecol Sociobiol 45:411–419
Forbes MRL (1993) Parasitism and host reproductive effort. Oikos 67:444–450
Hannam K (2006) Ectoparasitic blow flies (Protocalliphora sp.) and nestling eastern bluebirds (Sialia sialis): direct effects and compensatory strategies. Can J Zool 84:921–930
Hasselquist D, Nilsson JA (2009) Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philos Trans R Soc B 364:51–60
Heins DC, Baker JA (2003) Reduction of egg size in natural populations of threespine stickleback infected with a cestode macroparasite. J Parasitol 89:1–6
Hothorn H, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Huck UW, Pratt NC, Labov JB, Lisk RD (1988) Effects of age and parity on litter size and offspring sex-ratio in golden hamsters (Mesocricetus auratus). J Reprod Fertil 83:209–214
Kankova S, Kodym P, Frynta D, Vavrinova R, Kubena A, Flegr J (2007a) Influence of latent toxoplasmosis on the secondary sex ratio in mice. Parasitology 134:1709–1717
Kankova S, Sulc J, Nouzova K, Fajfrlik K, Frynta D, Flegr J (2007b) Women infected with parasite toxoplasma have more sons. Naturwissenschaften 94:122–127
Khokhlova IS, Krasnov BR, Kam M, Burdelova NI, Degen AA (2002) Energy cost of ectoparasitism: the flea Xenopsylla ramesis on the desert gerbil Gerbillus dasyurus. J Zool 258:349–354
Khokhlova IS, Serobyan V, Krasnov BR, Degen AA (2009a) Effect of host gender on blood digestion in fleas: mediating role of environment. Parasitol Res 105:1667–1673
Khokhlova IS, Serobyan V, Krasnov BR, Degen AA (2009b) Is the feeding and reproductive performance of the flea, Xenopsylla ramesis, affected by the gender of its rodent host, Meriones crassus? J Exp Biol 212:1429–1435
Koffler BR (1972) Meriones crassus. Mamm Species 9:1–4
Krasnov BR (2008) Functional and evolutionary ecology of fleas: a model for ecological parasitology. Cambridge University Press, Cambridge
Krasnov BR, Shenbrot GI, Khokhlova IS, Degen AA, Rogovin KA (1996) On the biology of Sundevall's jird (Meriones crassus Sundevall, 1842) (Rodentia: Gerbillidae) in the Negev highlands, Israel. Mammalia 60:375–391
Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, Khokhlova IS (1997) Host-habitat relations as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev desert. Parasitology 114:159–173
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001) Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 38:629–637
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NI (2002) Time of survival under starvation in two flea species (Siphonaptera: Pulicidae) at different air temperatures and relative humidities. J Vector Ecol 27:70–81
Lalonde RG (1991) Optimal offspring provisioning when resources are not predictable. Am Nat 138:680–686
Lehmann T (1993) Ectoparasites: direct impact on host fitness. Parasitol Today 9:8–13
Liberman V, Khokhlova IS, Degen AA, Krasnov BR (2011) The effect of host age on feeding performance of fleas. Parasitology 138:1154–1163
Marzal A, de Lope F, Navarro C, Moller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
Merila J, Wiggins DA (1995) Offspring number and quality in the blue tit: a quantitative genetic approach. J Zool 237:615–623
Ming QL, Shen JF, Cheng C, Liu CM, Feng ZJ (2015) Wolbachia infection dynamics in Tribolium confusum (Coleoptera: Tenebrionidae) and their effects on host mating behavior and reproduction. J Econ Entomol 108:1408–1415
Mousseau TA, Dingle H (1991) Maternal effects in insect life histories. Annu Rev Entomol 36:511–534
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Novakova M, Vasakova B, Kutalova H, Galestokova K, Prusova K, Smilauer P, Sumbera R, Frynta D (2010) Secondary sex ratios do not support maternal manipulation: extensive data from laboratory colonies of spiny mice (Muridae: Acomys). Behav Ecol Sociobiol 64:371–379
Podmokla E, Dubiec A, Drobniak SM, Arct A, Gustafsson L, Cichon M (2014) Avian malaria is associated with increased reproductive investment in the blue tit. J Avian Biol 45:219–224
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org
Raveh S, Neuhaus P, Dobson FS (2015) Ectoparasites and fitness of female Columbian ground squirrels. Philos Trans R Soc B 370:9
Salinas S, Munch SB (2012) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15:159–163
Schultz ET, Topper M, Heins DC (2006) Decreased reproductive investment of female threespine stickleback Gasterosteus aculeatus infected with the cestode Schistocephalus solidus: parasite adaptation, host adaptation, or side effect? Oikos 114:303–310
Schwagmeyer PL, Mock DW (2008) Parental provisioning and offspring fitness: size matters. Anim Behav 75:291–298
Schwanz LE (2008) Chronic parasitic infection alters reproductive output in deer mice. Behav Ecol Sociobiol 62:1351–1358
Schwanz LE (2016) Parental thermal environment alters offspring sex ratio and fitness in an oviparous lizard. J Exp Biol 219:2349–2357
Skibiel AL, Speakman JR, Hood WR (2013) Testing the predictions of energy allocation decisions in the evolution of life-history trade-offs. Funct Ecol 27:1382–1391
St Juliana JR, Khokhlova IS, Wielebnowski N, Kotler BP, Krasnov BR (2014) Ectoparasitism and stress hormones: strategy of host exploitation, common host-parasite history and energetics matter. J Anim Ecol 83:1113–1123
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex-ratio of offspring. Science 179:90–92
Warner DA, Uller T, Shine R (2013) Transgenerational sex determination: the embryonic environment experienced by a male affects offspring sex ratio. Sci Rep 3:4
Wilson K, Hardy IC (2002) Statistical analysis of sex ratios: an introduction. In: Hardy IC (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 48–92
Wolf JB, Brodie ED, Cheverud JM, Moore AJ, Wade MJ (1998) Evolutionary consequences of indirect genetic effects. Trends Ecol Evol 13:64–69
Yarmolinsky L, Kam M, Khokhlova IS, Degen AA (2009) Effect of weaning time on the growth rate and food intake of the spiny mouse pup. J Zool 27(9):203–209
Acknowledgements
The authors thank the anonymous reviewer for helpful comments on the manuscript. This study was supported by the Israel Science Foundation (grant number 26/12 to B.R.K. and I.S.K.). E.M.W. received financial support from the US–Israel Education Foundation (USIEF Fulbright Post-Doctoral Fellowship) and the Swiss Institute for Dryland Environmental and Energy Research. E.M.D. received financial support from the Blaustein Center for Scientific Cooperation, the Kreitman School of Advanced Graduate Studies and the Swiss Institute for Dryland Environmental and Energy Research. L.V.D.M. received financial support from the Blaustein Center for Scientific Cooperation and the French Associates Institute for Agriculture and Biotechnology of Drylands. This is publication number 920 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warburton, E.M., Khokhlova, I.S., Dlugosz, E.M. et al. Effects of parasitism on host reproductive investment in a rodent–flea system: host litter size matters. Parasitol Res 116, 703–710 (2017). https://doi.org/10.1007/s00436-016-5336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5336-3