Abstract
The treatment of acanthamoebiasis is a great problem. Most cerebral invasions end with death, and the treatment of ocular invasions is usually long-lasting and not very effective. Numerous plant extracts and substances isolated from plants, which are effective against trophozoites or cysts, have been studied in the treatment of acanthamoebiasis. However, no agents that are simultaneously effective against both developing forms of amoebae have been discovered yet. It seems that such a plant which fulfils both tasks is Artemisia annua L. Our studies showed that water, alcohol and chloroform extracts from the herb A. annua L. can be applied in general and local treatment or in combined therapy with antibiotics in the treatment of acanthamoebiasis. Extracts from this plant show not only in vitro but also in vivo effects. Studies carried out on experimental animals infected with amoebae show that the application of these extracts significantly prolongs the survival of the animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free-living amoebae belonging to the genus Acanthamoeba are organisms commonly occurring in the environment surrounding humans. They feed on bacteria, mushrooms and other protozoa and are perfectly adjusted to the environment (Khan 2009). These organisms have been found in samples of soil, air, and fresh and salt water, as well as in air conditioning systems, in water supplied by waterworks, showers, sanitary appliances, swimming pools, dialysis machines and contact lens fluid. Trophozoites and cysts of amoebae have also been discovered in oceanic deposits, bottled mineral water and nasal and throat mucosal smears (De Jonckheere 1991; Mergeryan 1991; Szenasi et al. 1998; Visvesvara and Stehr-Green 1990). The first suggestions that amoebae may cause diseases in humans come from 1958 from the USA (Culbertson et al. 1959; Fowler and Carter 1965). At present, human cases of granulomatous amoebic encephalitis (GAE), aspiration pneumonia (AP) and skin inflammations, and in particular, Acanthamoeba keratitis (AK) are noted worldwide (Yoder et al. 2012; Marciano-Cabral et al. 2000; Marciano-Cabral and Cabral 2003; Wanachiwanawin et al. 2012; Kao et al. 2012).
Chemotherapy in the case of Acanthamoeba sp. infection is a great problem. Most cerebral infections end with the patient’s death, while the treatment of ocular acanthamoebiasis is usually long-lasting and not very effective. Only a few cases of effective chemotherapy in the very early stage of infection and by using highly toxic drugs have been reported (Seal 2003; Kitagawa et al. 2003; Polat and Vural 2012). In the late stage of infection, most medications are not effective (Dougherty et al. 1994; Ficker et al. 1990; Horne et al. 1994; Murdoch et al. 1998; Berra et al. 2013).
The broad applicability of chemotherapeutic agents in Acanthamoeba sp. infection is not doubted, but most drugs are highly toxic for humans, causing adverse reactions. Hence, alternative and natural medicinal substances which could prove suitable for use in cases of amoeba infection are sought.
Due to its potential antiparasitic properties, we decided to study the water, alcohol and chloroform extracts of Artemesia annua.
We investigated the possibility to use them externally as well as internally to treat infections caused by free-living amoebae, in particular in the treatment of Acanthamoeba keratitis (AK) and granulomatous amoebic encephalitis (GAE) or Acanthamoeba pneumonitis (PA).
Material and methods
Dried aerial parts of A. annua L. from China were obtained from the company Magiczny Ogród (Poland). One hundred millilitre of hot distilled water was poured on the pulverized plant material in the quantity of 2–3 g and a hot water infusion (tea) was obtained (De Donno et al. 2012; Suberu et al. 2013). The next portions of the dried material in the quantity of 5–10 g were extracted with ethanol or chloroform (Sharma et al. 2014) in the Soxhlet apparatus (about 30 cycles). The obtained methanol or chloroform extracts were filtered and then vaporized to dryness under a vacuum. The dry remnant was dissolved in hot distilled water. Also, a ready-dried extract from A. annua 10:1 made in China (Magiczny Ogród, Poland), which was also dissolved in hot distilled water, and pure artemisinin (from the company Sigma Chemical Company) were used in the study.
The studies on the influence of the extracts on amoebae were carried out on the strain 309 Acanthamoeba castellanii—pathogenic for mice and isolated from the environment (Kasprzak and Mazur 1972)—and on the strain Ac32 Acanthamoeba sp—pathogenic for humans and isolated from a case of Acanthamoeba keratitis, genotype T4 (accession number KP184479). The amoebae were grown in axenic liquid cultures containing 2 % Bacto Casiton (Difco) and 10 % normal horse serum according to the procedure described by Červa (1969) and on non-nutrient agar (NN) containing 2 % non-nutrient agar Difco poured on the Petri dish and covered with a suspension of the bacterium Enterobacter aerogenes.
The pathogenic properties of amoebae were tested by infecting 2-week-old white mice of the BALB-c strain using the procedure described by Kasprzak and Mazur (1972) and Mazur (1984).
To the axenic culture of amoebae containing 5 × 104 cells/ml, we added hot water infusion (tea), methanol or chloroform extract in the quantity corresponding to 1–300 mg of dry mass of the plant in 1 ml, ready dry extract 10:1 made in China in the quantity from 1 to 20 mg/ml and pure artemisinin (Sigma) in the quantity of 0.005 to 0.2 mg/ml. The increase in the number of amoebae was studied 24, 48 and 72 h after adding to the culture the extracts or pure substance in the log phase of growth using a Thoma counting chamber. The control was the amoeba culture without the plant extract. The half maximal inhibitory concentration (IC50), i.e. the lowest concentration of the studied substance inhibiting the increase of amoebae by 50 %, was determined.
The second method of investigating plant extracts was based on the amoeba grown on NN agar, on which a filter paper was saturated with the extract solution. Solutions with the concentration of 5 and 50 mg of dry mass/ml were used. The controls were filter papers saturated with sterile water. The influence of the extract on the culture increase and migration of amoebae was observed for 7 days.
Study on the influence of A. annua extracts on the course of infection with amoebae was tested on the mice strain BALB/c (Kasprzak and Mazur 1972; Mazur 1984). The infected mice received extracts per os, from the first day after becoming infected until the seventh day, in the volume of 0.5 ml containing 200 mg of dry mass in 1 ml.
All of the experiments were repeated five to seven times. Tests on the animals were repeated five times, using five to ten animals for each test series.
Results
Table 1 presents the IC50 values for the studied extracts obtained from A. annua. IC50 was determined at 24, 48 and 72 h after infection with amoebae of the genus Acanthamoeba. It was found in the in vitro study that all extracts effectively inhibited the increase of amoebae and caused encystation in cultures. There was no statistically significant difference between the two studied strains of Acanthamoeba sp.
The pure artemisinin preparation affected amoebae from 100 to 300 times more strongly than the studied extracts. The most active anti-amoeba extract was chloroform extract. The Chinese water extract 10:1 was approximately 50 % weaker than the best chloroform extract.
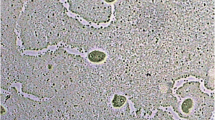
Studies on the effect of extracts on amoebae grown on agar plates showed that filter papers already saturated with the extract with the concentration of 5 mg/ml inhibited the growth and migration of amoebae, and moreover caused the increase in the volume of amoebae and their strong vacuolation (Fig. 1). Vacuolated amoebae did not transform into cysts and after several days became decayed. In Fig. 1, strongly vacuolated amoebae within the region of extract action can be observed.
Tests concerning the therapeutic action of plant extracts on the experimental infection with Acanthamoeba show that, following the application of the extracts, the animals survived considerably longer (three to four times) and infection passed into a chronic condition. Table 2 presents the survival time for mice infected with Acanthamoeba sp. following the application of the plant extracts.
Discussion
Clinical symptoms of granulomatous amoebic encephalitis in humans are primarily strong headache, neurological disorders such as hallucinations, disorientation and visual disturbances, high fever and coma (Martinez and Visvesvara 1997). Keratitis meanwhile is characterized by severe eye pain, sensitivity to light and petechial hemorrhage (Kosik-Bogacka et al. 2010; Wanachiwanawin et al. 2012; Hadaś and Derda 2013). In the lungs, amoebae develop numerous inflammatory foci with a serous exudate containing trophozoites and cysts (Vernon et al. 2005). Skin lesions are characterized by numerous ulcers of varying extent. All forms of infection are usually chronic (Paltiel et al. 2004; Galarza et al. 2009). The chronic character of amoebic invasions in human is caused by the ability of trophozoites to transform into cysts. Cysts, in turn, are resistant to most chemotherapeutic agents.
So far, in the therapy of acanthamoebiasis, the possibility to use plant extracts and substances isolated from such plants as Buddleia cordata (Rodríguez-Zaragoza et al., 1999), Pterocaulon polystachyum (Rodio et al., 2008; Sauter et al., 2011), Arachis hypogaea, Curcuma longa, Pancratinum maritimum (El-Sayed et al., 2012), Eryngium planum, Eryngium maritimum, Solidago virgaurea, Solidago graminifolia, Pueraria lobata, Rubus chamaemorus, Tanacetum vulgare (Derda et al., 2009, 2012, 2013), Peucedanum caucasicum, Peucedanum palimbioides, Peucedanum chryseum, Peucedanum longibracteolatum, Satureja cuneifolia, Melissa officinalis (Malatyali et al., 2012a, b), Pouzolzia indica (Roongruangchai et al., 2010), Salvia sclarea (Kuźma et al., 2015), Teucrium polium, Teucrium chamaedrys (Tepe et al., 2012), Croton pallidulus, Croton ericoides, Croton isabelli (Vunda et al., 2012) and others have been studied.
Some of these plants are commonly used in natural medicine. They show antiseptic or amoebicidal properties. Some inhibit the development of amoebae, and others cause the encystation of trophozoites. Some extracts are lethal only for trophozoites but are not effective against cysts. A desirable property of the plant extracts is a capacity for amoebostatic and amoebicidal effects against trophozoites as well as cysts. Until now, no plants that are effective in both cases have been found. It seems that such a plant which fulfils both these requirements is A. annua L.
A. annua is an annual plant which was described by Linnaeus. It grows wild in Asia (mainly Siberia, Japan, Korea and China) and in southern Europe (Cullen 1975; Wąsowicz 2004). It was introduced to Poland, Denmark, Holland, France, Italy, Lichtenstein and Austria, where it became domesticated and bred (Tutin 1976; Wąsowicz 2004). As an introduced plant, it also occurs in North America (Żukowski and Piaszyk 1971; Cullen 1975).
A. annua is considered to be a medicinal plant, but in the herbal literature, it is rarely mentioned (Jędrzejko et al. 1997; Wąsowicz 2004). Its medicinal properties in malaria are considered most important (De Donno et al. 2012; Ho et al. 2013). This plant is a species of particular significance in tropical countries where the danger of malaria is the greatest (Woerdenbag et al. 1994). In China, A. annua is officially recognized as a medicinal plant and was listed in the Pharmacopoeia (Woerdenbag et al. 1994). It is cultivated on a mass scale in India, China and Vietnam.
The main medicinal substances of A. annua are artemisinins, which are sesquiterpene lactones containing an unusual peroxide bridge. The action of artemisinins involves, among other things, the creation of free radicals which facilitate the fight against parasites with the result of splitting endoperoxide bonds in their structure. This peroxide is believed to be responsible for the drug’s mechanism of action.
Moreover, artemisinin derivatives are effective against viruses (Efferth et al. 2008), protozoans (e.g. Toxoplasma gondii (de Oliveira et al., 2009); Trypanosoma cruzi (Sülsen et al., 2008); and Plasmodium falciparum (Sülsen et al., 2011), flatworms (e.g. Schistosoma japonicum, Schistosoma mansoni, Fasciola hepatica, Clonorchis sinensis (Fathy, 2011), bacteria and mushrooms (Lopes-Lutz et al. 2008). Artemisinin and its derivatives show cytotoxic effects against cancer cells by disrupting the cell cycle, promoting apoptosis and preventing angiogenesis. The action of artemisinins also involves inhibition of Toll-like receptors (Ho et al. 2013).
Our study showed that water, alcohol and chloroform extracts of the herb A. annum L. can be used in acanthamoebiasis for general and local treatment or in combined therapy with antibiotics. Medicinal substances contained in this plant show not only in vitro but also in vivo effects. In the case of animals experimentally infected with amoebae of the genus Acanthamoeba, it significantly prolongs their survival. Plant extracts administered to experimentally infected animals have considerably lengthened their survival time in comparison with control animals that were not given any treatment. The control animals that were infected usually died after 7 days. The animals which received a plant extract monotherapy survived for a period three to four times longer or more. In the case of animals that were not infected, the therapeutic doses of drugs given did not display any toxic activity.
References
Berra M, Galperin G, Boscaro G, Zarate J, Tau J, Chiaradia P, Berra A (2013) Treatment of Acanthamoeba keratitis by corneal cross-linking. Cornea 32(2):174–178
Červa L (1969) Amoebic meningoencephalitis: axenic culture of Naegleria. Science 163:576
Culbertson CG, Smith JW, Minner JR (1959) Experimental infection of mice and monkeys by Acanthamoeba. Am J Pathol 35(1):185–197
Cullen J (1975) Artemisia L. – w: Davis P.H. (red.). Flora of Turkey and the East Aegean Islands. Edinburgh, University Press, 311–324
De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Caccioppola A, Villanova L, Merendino A, Bagordo F, Fanizzi FP (2012) First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg 106(11):696–700
De Jonckheere JF (1991) Ecology of Acanthamoeba. Rev Infect Dis 13(Suppl 5):S385–S387
De Oliveira TC, Silva DA, Rostkowska C, Béla SR, Ferro EA, Magalhães PM, Mineo JR (2009) Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol 122(3):233–241
Derda M, Hadaś E, Thiem B (2009) Plant extracts as natural amoebicidal agents. Parasitol Res 104(3):705–708
Derda M, Hadaś E, Thiem B, Wojt WJ, Wojtkowiak-Giera A, Cholewiński M, Skrzypczak Ł (2012) Tanacetum vulgare L. as a plant with potential medicinal properties for Acanthamoeba keratitis. Now Lek 81(6):620–625
Derda M, Thiem B, Budzianowski J, Hadaś E, Wojt WJ, Wojtkowiak-Giera A (2013) The evaluation of the amebicidal activity of Eryngium planum extracts. Acta Pol Pharm 70:1027–1034
Dougherty PJ, Binder PS, Mondino BJ, Glasgow BJ (1994) Acanthamoeba sclerokeratitis. Am J Ophthalmol 117(4):475–479
Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M (2008) The antiviral activities of artemisinin and artesunate. Clin Infect Dis 47(6):804–811
El-Sayed NM, Ismail KA, Ahmed SA, Hetta MH (2012) In vitro amoebicidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and Pancratium maritimum L. on Acanthamoeba castellanii cysts. Parasitol Res 110(5):1985–1992
Fathy FM (2011) Anthelmintic effect of artesunate in experimental heterophyid infection. J Egypt Soc Parasitol 41(2):469–483
Ficker L, Seal D, Warhurst D, Wright P (1990) Acanthamoeba keratitis—resistance to medical therapy. Eye 4(6):835–838
Fowler M, Carter RF (1965) Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br Med J 2(5464):740–742
Galarza C, Ramos W, Gutierrez EL, Ronceros G, Teran M, Uribe M, Navincopa M, Ortega-Loayza AG (2009) Cutaneous acanthamebiasis infection in immunocompetent and immunocompromised patients. Int J Dermatol 48(12):1324–1329
Hadaś E, Derda M (2013) Acanthamoeba keratitis—the new epidemiological threat. Prob Hig Epidemiol 94(4):730–733
Ho WE, Peh HY, Chan TK, Wong WS (2013) Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 142(1):126–139
Horne DD, Frizell ME, Ingam L, Janas RG, Gubash SM, Anand CM, Athar MA (1994) Acanthamoeba keratitis an emerging clinical problem. CMAJ 150(6):923–925
Jędrzejko K, Klama H, Żarnowiec J (1997) Zarys wiedzy o roślinach leczniczych. – Śląska Akademia Medyczna w Katowicach
Kao PM, Hsu BM, Chen NH, Huang KH, Huang SW, King KL, Chiu YC (2012) Isolation and identification of Acanthamoeba species from thermal spring environments in southern Taiwan. Exp Parasitol 130(4):354–358
Kasprzak W, Mazur T (1972) Free living amoebae isolated from waters frequented by people in the vicinity of Poznań, Poland. Experimental studies in mice on the pathogenicity of the isolates. Z Tropenmed Parasitol 23(4):391–398
Khan NA (2009) Acanthamoeba: biology and pathogenesis. Caister Academic Press, Norfolk UK
Kitagawa K, Nakamura T, Takahashi N, Oikawa Y, Ikeda T (2003) A novel combination treatment of chlorohexidine gluconate, natamycin (pimaricin) and debridement for a Acanthamoeba keratitis. Jpn J Ophthalmol 47(6):616–617
Kosik-Bogacka D, Czepita D, Łanocha N (2010) Pełzaki z rodzaju Acanthamoeba jako czynnik etiologiczny zapalenia rogówki oka. Klin Oczna 112(4–6):161–164
Kuźma Ł, Derda M, Hadaś E, Wysokińska H (2015) Abietane diterpenoids from Salvia sclarea transformed roots as growth inhibitors of pathogenic Acanthamoeba spp. Parasitol Res 14(1):323–327
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69(8):1732–1738
Malatyali E, Tepe B, Degerli S, Berk S, Akpulat HA (2012a) In vitro amoebicidal activity of four Peucedanum species on Acanthamoeba castellanii cysts and trophozoites. Parasitol Res 110(1):167–174
Malatyali E, Tepe B, Degerli S, Berk S (2012b) In vitro amoebicidal activities of Satureja cuneifolia and Melissa officinalis on Acanthamoeba castellanii cysts and trophozoites. Parasitol Res 110(6):2175–2180
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microb Rev 16(2):273–307
Marciano-Cabral F, Puffenbarger R, Cabral G (2000) The increasing importance of Acanthamoeba infections. J Eucaryot Microbiol 47(1):29–36
Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunistic amebas. Brain Pathol 7(1):583–598
Mazur T (1984) Występowanie Naegleria fowleri w środowisku wolnym i właściwości biologiczne izolowanych szczepów. Wiad Parazytol 30(1):3–35
Mergeryan H (1991) The prevalence of Acanthamoeba in the human environment. Rev Infect Dis 13(Suppl 5):S390–S391
Murdoch D, Gray TB, Cursons R, Parr D (1998) Acanthamoeba keratitis in New Zealand, including two cases with in vitro resistance to polyhexamethylene biguanide. Aust N Z J Ophthalmol 26(3):231–236
Paltiel M, Powell E, Lynch J, Baranowski B, Martins C (2004) Disseminated cutaneous acanthamebiasis: a case report and review of the literature. Cutis 73(4):241–248
Polat ZA, Vural A (2012) Effect of combined chlorhexidine gluconate and neosporin on experimental keratitis with two pathogenic strains of Acanthamoeba. Parasitol Res 110(5):1945–1950
Rodio C, da Rocha VD, Kowalski KP, Panatieri LF, von Poser G, Rott MB (2008) In vitro evaluation of the amebicida activity of Pterocaulon polystachyum (Asteraceae) against trophozoites of Acanthamoeba castellanii. Parasitol Res 104:191–194
Rodríguez-Zaragoza S, Ordaz C, Avila G, Muñoz JL, Arciniegas A, Romo de Vivar A (1999) In vitro evaluation of the amebicidal activity of Buddleia cordata (Loganiaceae, H.B.K.) on several strains of Acanthamoeba. J Ethnopharmacol 66(3):327–334
Roongruangchai K, Kummalue T, Sookkua T, Roongruangchai J (2010) Comparison of Pouzolzia indica methanolic extract and Virkon against cyst of Acanthamoeba spp. Southeast Asian J Trop Med Public Health 41(4):776–784
Sauter IP, dos Santos JC, Apel MA, Cibulski SP, Roehe PM, von Poser GL, Rott MB (2011) Amoebicidal activity and chemical composition of Pterocaulon polystachyum (Asteraceae) essential oil. Parasitol Res 109(5):1367–1371
Seal DV (2003) Acanthamoeba keratitis update—incidence, molecular epidemiology and new drugs for treatment. Eye 17(8):893–905
Sharma G, Kapoor H, Chopra M, Kumar K, Agrawal V (2014) Strong larvacidal potential of Artemisia annua leaf extract against malaria (Anopheles stephensi Liston) and dengue (Aedes aegypti L.) vectors and bioassay-driven isolation of the marker compounds. Parasitol Res 113(1):197–209
Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA (2013) Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—possible synergistic and resistance mechanisms. PLoS ONE 8(11), e80790. doi:10.1371/journal.pone.0080790
Sülsen V, Frank FM, Cazorla SI, Anesini CA, Malchiodi EL, Fleixa B, Vila R, Muschietti LV, Martino VS (2008) Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob Agents Chemother 52(7):2415–2419
Sülsen V, Gutierrez Yappu D, Laurella L, Anesini C, Gimenez Turba A, Martino V, Muschietti L (2011) In vitro antiplasmodial activity of sesquiterpene lactones from Ambrosia tenuifolia. Evid Based Complement Alternat Med 2011:352938
Szenasi Z, Endo T, Yagita K, Nagy E (1998) Isolation, identification and increasing importance of “free-living” amoebae causing human disease. J Med Microb 47(1):5–16
Tepe B, Malatyali E, Degerli S, Berk S (2012) In vitro amoebicidal activities of Teucrium polium and T. chamaedrys on Acanthamoeba castellanii trophozoites and cysts. Parasitol Res 110(5):1773–1778
Tutin TG (1976) Flora Europaea. T. IV. University Press, Cambridge
Vernon SE, Acar BC, Pham SM, Fertel D (2005) Acanthamoeba infection in lung transplantation: report of a case and review of the literature. Transplant Infect Dis 7(3–4):154–157
Visvesvara GS, Stehr-Green J (1990) Epidemiology of free-living ameba infections. J Protozool 37(4):25S–33S
Vunda SLL, Sauter IP, Cibulski SP, Roche PM, Bordignon SAL, Rott MB, Apel MA, von Poser GL (2012) Chemical composition and amoebicidal activity of Croton pallidulus, Croton ericoides, and Croton isabelli (Euphorbiaceae) essential oils. Parasitol Res 111:961–966
Wanachiwanawin D, Booranapong W, Kosrirukvongs P (2012) Clinical features of Acanthamoeba keratitis in contact lens wearers and non-wearers. Southeast Asian J Trop Med Public Health 43(3):549–556
Wąsowicz A (2004) Occurrence of Artemisia annua L. in Wrocław city area (Lower Silesia, Poland). Acta Botan Silesiaca 1:141–146
Woerdenbag HJ, Pras N, Chan NG, Bang BT, Bos R, van Uden W, Van YP, Van Boi N, Batterman S, Lugt CB (1994) Artemisinin, related sesquiterpens and essential oil in Artemisia annua during a vegetation period in Vietnam. Planta Med 60(3):272–275
Yoder JS, Verani J, Heidman N, Hoppe-Bauer J, Alfonso EC, Miller D, Jones DB, Bruckner D, Langston R, Jeng BH, Joslin CE, Tu E, Colby K, Vetter E, Ritterband D, Mathers W, Kowalski RP, Acharya NR, Limaye AP, Leiter C, Roy S, Lorick S, Roberts J, Beach MJ (2012) Acanthamoeba keratitis: the persistence of cases following a multistate outbreak. Ophthal Epidemiol 19(4):221–225
Żukowski W, Piaszyk M (1971) Rozmieszczenie niektórych gatunków synantropijnych z rodzaju Artemisia L. w Polsce. Badania Fizjograficzne nad Polską Zachachodnią. Biologia 24:107–129
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Derda, M., Hadaś, E., Cholewiński, M. et al. Artemisia annua L. as a plant with potential use in the treatment of acanthamoebiasis. Parasitol Res 115, 1635–1639 (2016). https://doi.org/10.1007/s00436-016-4902-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4902-z