Abstract
A total of 853 questing Ixodes ricinus males, females, and nymphs and of 582 questing Dermacentor reticulatus males and females were collected from vegetation on the territory of the Lublin province (eastern Poland). The ticks were examined for the presence of Babesia by PCR detecting part of 18S ribosomal RNA (rRNA) gene and nuclear small subunit rRNA (SS-rDNA) for determining of Babesia spp. and Babesia microti, respectively. The overall incidence of Babesia strains in I. ricinus ticks was 4.6 %. Three species of Babesia were identified. The prevalent species was B. microti which occurred in 2.8 % of ticks, while Babesia venatorum, Babesia divergens, and unidentified Babesia species were found at the frequency of 1.2, 0.2, and 0.3 %, respectively. Altogether, B. microti constituted 61.5 % of the total strains detected in I. ricinus, B. venatorum—25.7 %, B. divergens—5.1 %, and unidentified Babesia species—7.7 %. The prevalence of Babesia species in I. ricinus did not depend significantly on locality (χ 2 = 1.885, P = 0.390) nor on the tick stage (χ 2 = 4.874, P = 0.087). The incidence of Babesia strains in D. reticulatus ticks was 2.7 %. Two species of Babesia were identified. Again, the prevalent species was B. microti which occurred in 2.1 % of ticks, while B. canis was found in 0.7 % of ticks. In one D. reticulatus female, B. canis and B. microti co-infection was found. Altogether, B. microti constituted 75 % of the total strains detected in D. reticulatus while B. canis formed 25 % of the total strains. The frequency of the occurrence of Babesia species in D. reticulatus did not depend significantly on locality (χ 2 = 0.463, P = 0.793). The difference between the prevalence of Babesia in males and females of D. reticulatus was insignificant (P = 0.0954); nymphs were not found. The dominance of B. microti in the species composition of tick-borne Babesia found in this study was typical for eastern Europe. In conclusion, the results revealed that the population inhabiting the forested area of eastern Poland could be exposed to Babesia parasites, especially to those from the species B. microti, by a bite of I. ricinus, a competent vector of human babesiosis, and probably also by a bite of D. reticulatus whose role in the transmission of human babesiosis needs to be clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protozoan genus Babesia Starcovici, 1893 (Apicomplexa: Piroplasmida: Babesiidae) comprises intraerythrocytic parasites of mammals and birds which are transmitted by hard ticks (Ixodidae) from the genera Amblyomma, Boophilus, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus. These hemoprotozoans cause babesiosis, a disease of animals and humans manifested in severe cases by fever and hemolysis leading to anemia, hyperbilirubinuria, hemoglobinuria, and possible organ failure (Peirce 2000; Hunfeld and Brade 2004; Hamel et al. 2012; Altay et al. 2012; Hildebrandt and Hunfeld 2014; Aydin et al. 2015). To date, more than 100 Babesia species have been identified worldwide, of which the most important parasites of domestic animals are Babesia bigemina, Babesia bovis, and Babesia divergens in cattle and Babesia canis (formerly Babesia canis canis) in dogs. Babesiosis in humans is regarded as an emerging disease with the greatest number of cases (above 1000 per annum) caused by Babesia microti in North America (Yabsley and Shock 2012). The disease, transmitted by Ixodes scapularis, can range from asymptomatic and mild infections to severe disease and death. In Europe, about 50 cases of babesiosis have been recorded up to date, caused primarily by B. divergens in splenectomized individuals, less so by Babesia venatorum (formerly Babesia sp. EU1) and B. microti. The disease may also develop in the immunocompetent individuals (Martinot et al. 2011).
Ixodes ricinus is regarded as the most important vector of Babesia in Europe; other potential vectors are Dermacentor reticulatus and Ixodes persulcatus (Hildebrandt et al. 2013; Wójcik-Fatla et al. 2012; Katargina et al. 2011). The aim of the present study was to determine the prevalence and species diversity of Babesia in I. ricinus and D. reticulatus ticks collected in eastern Poland.
Materials and methods
Collection of ticks
A total of 582 questing D. reticulatus ticks (341 females and 241 males) and a total of 853 questing I. ricinus ticks (314 females, 268 males, and 271 nymphs) were collected during spring/summer season in the years 2011–2012 on the areas of six localities situated in the Lublin province (eastern Poland). D. reticulatus ticks were collected on the territory of three localities: Ostrów Lubelski (51° 46′ N, 22° 84′ E), Suchawa (51° 49′ N, 23° 40′ E), and Parczew (51° 64′ N, 22° 90′ E). I. ricinus ticks were collected on the territory of Wilków (51° 25′ N, 21° 88′ E), Suchawa, and Dąbrowa (51° 17′ N 22° 57′ E). Ticks were collected by dragging a woolen flag over the lower vegetation and litter along the paths and edges of deciduous and mixed forests, including suburban localities and recreational areas.
DNA isolation from ticks
Total DNA was isolated from the adult ticks separately and from nymphs in pools of five specimens (Rijpkema et al. 1996) by boiling in 0.7 M ammonium hydroxide and stored at −20 °C for further analysis. Prevalence of infection in nymphs was expressed as the minimum infection rate (MIR) of pools calculated according to Kahl et al. (1989). The concentration of DNA in the isolates was determined with the NanoDrop ND1000 Spectrophotometer (USA). The determined DNA concentrations ranged from 500 to 660 ng/μl for males and from 670 to 880 ng/μl for females of D. reticulatus and from 300 to 500 ng/μl for females, from 180 to 330 ng/μl for males, and from 20 to 80 ng/μl for nymphs of I. ricinus.
Detection of B. microti DNA by PCR and nested PCR
All tick lysates were examined for the presence of B. microti DNA using amplification by PCR and confirmatory re-amplification by nested PCR with the method described previously (Persing et al. 1992) with some modification (Wójcik-Fatla et al. 2012). The primers used in this study are specific for a gene encoding the nuclear small subunit ribosomal RNA (SS-rDNA). As a positive control, DNA extracted from the antigen of B. microti from the slide used for detection of antibodies (Fuller Laboratories, Germany) was used, while nuclease-free water was used as a negative control. The amplifications were carried out in a C1000 Thermal Cycler (BioRad, USA).
Detection of Babesia spp., B. divergens, and B. venatorum
Primers for detection of Babesia spp. including bovine Babesia: B. divergens, B. bigemina, B. major; B. venatorum; B. canis; B. odocoilei; B. ovata; B. motasi, and B. crassa—and primers for identification of B. divergens and B. venatorum were described previously by Hilpertshauser et al. (2006).
Each PCR reaction was carried out in a 25-μl reaction volume which contained the following mix of reagents: 0.625 U Taq DNA polymerase (Qiagen, USA), 1 × PCR buffer containing 15 mM MgCl2, 2.5 μl 2 mM dNTP (final concentration 0.1 mM) (Thermo Scientific, Lithuania), 1.25 μl 10 μM each of primer (Eurogentec, Seraing, Belgium), 2 μl of matrix DNA, and nuclease-free water (Applied Biosystems, USA). Tick lysates confirmed as positive for B. divergens and B. venatorum were used as positive and nuclease-free water as negative controls. The amplification was carried out in C1000 Thermal Cycler (BioRad, USA) under the following conditions: preincubation at 95 °C for 3 min, 45 cycles, each of 30 s at 94 °C (denaturation), 30 s at 61 °C (primers annealing), and 45 s at 72 °C (elongation). Final elongation was performed for 10 min at 72 °C. Products of amplification were identified in 2 % agarose gel (Prona, Basica LE), after electrophoresis in standard conditions and staining with ethidium bromide solution (2 μg/ml).
DNA sequencing
DNA sequencing of all Babesia spp. positive samples was performed with ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Inc., Foster City, CA, USA) using ABI PRISM Big Dye Terminator v. 3.1. Cycle Sequencing Kits and Big Dye XTerminator Purification Kit (Applied Biosystems). For sequencing of B. microti positive samples, the tenfold dilution of amplified DNA was used, and fivefold for Babesia spp. The results were compared with sequences in GenBank database using the BLAST software at the National Center for Biotechnology Information (Bethesda, Maryland, USA).
Statistical analysis
The obtained results were analyzed by χ 2 test and Student’s t test, using the STATISTICA v. 6.0 package (Statsoft, Tulsa, OK, USA). The value p < 0.05 was considered significant.
Results
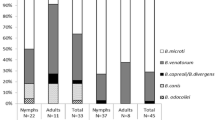
The overall incidence of Babesia strains in I. ricinus ticks collected in eastern Poland was 4.6 % (Table 1). Three species of Babesia were identified. The prevalent species was B. microti which occurred in 2.8 % of ticks, while B. venatorum, B. divergens, and unidentified Babesia species were found at the frequency of 1.2, 0.2, and 0.3 %, respectively. Altogether, B. microti constituted 61.5 % of the total strains detected in I. ricinus, B. venatorum—25.7 %, B. divergens—5.1 %, and unidentified Babesia species—7.7 %. The prevalence of Babesia species in I. ricinus did not depend significantly on locality (χ 2 = 1.885, P = 0.390) nor on the tick stage (χ 2 = 4.874, P = 0.087).
The incidence of Babesia strains in D. reticulatus ticks collected in eastern Poland was 2.7 % (Table 2). Two species of Babesia were identified. The prevalent species was also B. microti which occurred in 2.1 % of ticks, while B. canis was found in 0.7 % of ticks. Altogether, B. microti constituted 75 % of the total strains detected in D. reticulatus, while B. canis formed 25 % of the total strains. There was one D. reticulatus female co-infected with B. canis and B. microti. The frequency of the occurrence of Babesia species in D. reticulatus did not depend significantly on locality (χ 2 = 0.463, P = 0.793). The difference between the prevalence of Babesia in males and females of D. reticulatus was insignificant (P = 0.0954); nymphs were not found. However, the difference between the diversity of Babesia species among I. ricinus and D. reticulatus ticks proved to be significant (P < 0.05).
A total of 54 positive samples were sequenced (one sample was co-infected). Twenty-four samples from I. ricinus ticks showed a high level of similarity to B. microti (accession numbers: KM051833.1, KM051836.1). B. venatorum was confirmed in ten samples (accession numbers: JQ929917.1, KM244044.1). Two samples (accession numbers: KC465977.2, AY572456.1) were identified as B. divergens. In three cases, the sequencing failed and Babesia species remained unidentified.
In most cases, isolates obtained from D. reticulatus ticks showed 100 % similarity to B. microti (accession numbers: AB085191.1, AB366158.1). Three samples were defined as B. canis (accession numbers: AY072926.1, KM111283.1), and in one isolate, two sequences of B. canis and B. microti (accession numbers: AY072926.1 and AB085191.1) were found.
Discussion
The presented results demonstrate that 4.6 % of I. ricinus ticks collected on the territory of the Lublin region (eastern Poland) are infected with Babesia hemoprotozoans, which confirms that in Poland, there is a potential risk of babesiosis from exposure to the bite of this very common tick species, a competent vector of the disease. Values similar to the presented study, ranging from 3.5 to 4.1 % were recorded in ticks of this species in Germany by Eshoo et al. (2014) and Silaghi et al. (2012), respectively. Studies in other European countries revealed a lower prevalence of Babesia in I. ricinus compared to the current study, with values ranging from 0.3 % in Hungary (Egyed et al. 2012) to 2.7 % in Belgium (Lempereur et al. 2011). Higher values ranging from 6.1 to 51.7 % were reported from France (Halos et al. 2005; Cotté et al. 2010), Germany (Franke et al. 2011), the Netherlands (Tijsse-Klasen et al. 2011), and Austria (Blaschitz et al. 2008).
B. microti distinctly prevailed among Babesia species detected in the current study in I. ricinus ticks, amounting to 61.5 % of the total count. A similar or higher prevalence of this species in I. ricinus was reported from Slovenia (Duh et al. 2001), from Germany (Silaghi et al. 2012; Eshoo et al. 2014), and from Belarus (Reye et al. 2013). All but one Babesia species were identified as B. microti in the Netherland (96.4 % of all positive Babesia spp. ticks) (Tijsse-Klasen et al. 2011).
Different results were obtained by many other authors, mostly from Western and Northern Europe and, less frequently, from central and eastern Europe, who reported the dominancy of B. venatorum among Babesia species determined in I. ricinus ticks. The dominance of B. divergens in I. ricinus ticks was confirmed by Overzier et al. (2013), Otranto et al. (2014).
It is evident from the above presented results that with only a few exceptions, the I. ricinus ticks living in eastern Europe, including Poland, harbor mostly B. microti, while those living in western and northern Europe harbor mostly B. venatorum. Germany is a transitory area where ticks of this species harbor, usually in almost equal parts, B. microti and B. venatorum and/or B. divergens. This regularity could probably be explained by the fact that in the countries of eastern Europe, the prevalence of B. microti in rodents is 10–20 %, which is distinctly higher compared to the countries of western Europe. In consequence, higher infection rates of ticks with B. microti could be determined in eastern Europe (Siński et al. 2006; Hartelt et al. 2008). The common occurrence of B. microti in I. ricinus ticks living on the territory of Poland and other countries of eastern Europe has also been shown by a number of earlier studies where only B. microti was determined (Rudolf et al. 2005; Wójcik-Fatla et al. 2006).
The prevalence of Babesia spp. in D. reticulatus ticks noted in this study was 2.7 %, being lower compared to that found in I. ricinus. Similar to I. ricinus, also in D. reticulatus, the B. microti strains prevailed. In Poland, the presence of B. microti in adult D. reticulatus ticks collected from vegetation has been detected so far only by Wójcik-Fatla et al. (2012) with frequency of 4.5 %. The present study is the first confirmation of these findings, with the slightly lower incidence. To date, the authors of other studies performed in Belgium (Cochez et al. 2012), Germany (Silaghi et al. 2012; Najm et al. 2014), France (Bonnet et al. 2013), Belarus (Reye et al. 2013), and Slovakia (Švehlová et al. 2014) have detected neither the presence of B. microti nor other Babesia species pathogenic for humans in adult D. reticulatus ticks.
The repeatedly found occurrence of B. microti in the adult D. reticulatus ticks stated in the current study suggests that this species should be considered as a potential vector of human babesiosis, although its role needs an experimental confirmation (Hildebrandt et al. 2013). So far, Walter (1982) has not been successful in the experimental transmission of B. microti into golden hamsters by infected D. reticulatus nymphs. However, to solve unequivocally the problem of potential risk, such an experiment should be repeated with the adult ticks which are known to feed on humans and large animals. A small percent of the D. reticulatus ticks (0.7 %) examined in the presented study harbored B. canis, an important causative agent of babesiosis in dogs. D. reticulatus is a known vector of this pathogen, and its presence in ticks from eastern Poland is in accordance with the results of Adaszek et al. (2011) that canine babesiosis occurs more often in eastern Poland than in other parts of the country.
Genus Babesia spp., as a tick-borne protozoan parasite developing in erythrocytes, could led to rare but potentially life-threatening parasitic disease, which is confirmed by reported clinical cases of babesiosis. The first two cases of this disease in Poland was described by Welc-Falęciak et al. (2010) as a co-infection with Lyme borreliosis, caused by a parasite with a homology of 98.9 % to B. divergens or B. venatorum. In Europe, before the aforementioned study, only three clinical cases caused by B. microti were described, which is a minority of the circa. Fifty cases of human babesiosis recorded in continental Europe, caused mostly by B. divergens and, to a less extent, by B. venatorum in immunocompromised individuals (Hildebrandt and Hunfeld 2014). The number of babesiosis cases caused by B. microti in Europe forms only a small fraction of those reported from North America (Yabsley and Shock 2012). The reason for this discrepancy remains unclear.
Conclusion
In conclusion, the current study reveals that the population of I. ricinus, a competent vector of human babesiosis occurring on the territory of eastern Poland, is infected with a relatively marked frequency with three species of Babesia pathogenic for humans, which creates the risk of babesiosis in persons exposed to tick bite. The population of D. reticulatus, another tick species inhabiting this territory, is also infected with B. microti, and its potential role in spreading the disease should be considered and further investigated by experimental studies.
References
Adaszek Ł, Martinez AC, Winiarczyk S (2011) The factors affecting the distribution of babesiosis in dogs in Poland. Vet Parasitol 181:160–165
Altay K, Dumanli N, Aktas M (2012) A study on ovine tick-borne hemoprotozoan parasites (Theileria and Babesia) in the East Black Sea Region of Turkey. Parasitol Res 111:149–153
Aydin MF, Aktas M, Dumanli N (2015) Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitol Res 114:65–69
Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Stanek G, Walochnik J (2008) Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol 74:4841–4846
Bonnet S, de la Fuente J, Nicollet P, Liu X, Madani N, Blanchard B, Maingourd C, Alongi A, Torina A, Fernández de Mera IG, Vicente J, George JC, Vayssier-Taussat M, Joncour G (2013) Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis 13:226–236
Cochez C, Lempereur L, Madder M, Claerebout E, Simons L, De Wilde N, Linden A, Saegerman C, Heyman P, Losson B (2012) Foci report on indigenous Dermacentor reticulatus populations in Belgium and a preliminary study of associated babesiosis pathogens. Med Vet Entomol 26:355–358
Cotté V, Bonnet S, Cote M, Vayssier-Taussat M (2010) Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis 10:723–730
Duh D, Petrovec M, Avsic-Zupanc T (2001) Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J Clin Microbiol 39:3395–3397
Egyed L, Elő P, Sréter-Lancz Z, Széll Z, Balogh Z, Sréter T (2012) Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis 3:90–94
Eshoo MW, Crowder CD, Carolan HE, Rounds MA, Ecker DJ, Haag H, Mothes B, Nolte O (2014) Broad-range survey of tick-borne pathogens in Southern Germany reveals a high prevalence of Babesia microti and a diversity of other tick-borne pathogens. Vector Borne Zoonotic Dis 14:584–591
Franke J, Hildebrandt A, Meier F, Straube E, Dorn W (2011) Prevalence of Lyme disease agents and several emerging pathogens in questing ticks from the German Baltic coast. J Med Entomol 48:441–444
Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis HJ, Vayssier-Taussat M (2005) Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res 36:79–87
Hamel D, Silaghi C, Lescai D, Pfister K (2012) Epidemiological aspects on vector-borne infections in stray and pet dogs from Romania and Hungary with focus on Babesia spp. Parasitol Res 110:1537–1545
Hartelt K, Pluta S, Oehme R, Kimmig P (2008) Spread of ticks and tick-borne diseases in Germany due to global warming. Parasitol Res 103(Suppl 1):S109–S116
Hildebrandt A, Hunfeld KP (2014) Human babesiosis—a rare but potentially dangerous zoonosis. Dtsch Med Wochenschr 139:957–962 (in German)
Hildebrandt A, Gray JS, Hunfeld KP (2013) Human babesiosis in Europe: what clinicians need to know. Infection 41:1057–1072
Hilpertshauser H, Deplazes P, Schnyder M, Gern L, Mathis A (2006) Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl Environ Microbiol 72:6503–6507
Hunfeld KP, Brade V (2004) Zoonotic Babesia: possibly emerging pathogens to be considered for tick-infested humans in central Europe. Int J Med Microbiol 293(Suppl 37):93–103
Kahl O, Schmidt K, Schönberg A, Laukamm-Josten U, Knülle W, Bienzle U (1989) Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Berlin (West). Zbl Bakt Hyg A 270:434–440
Katargina O, Geller J, Vasilenko V, Kuznetsova T, Järvekülg L, Vene S, Lundkvist Å, Golovljova I (2011) Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector Borne Zoonotic Dis 11:923–928
Lempereur L, De Cat A, Caron Y, Madder M, Claerebout E, Saegerman C, Losson B (2011) First molecular evidence of potentially zoonotic Babesia microti and Babesia sp. EU1 in Ixodes ricinus ticks in Belgium. Vector Borne Zoonotic Dis 11:125–130
Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, Jouglin M, Chauvin A, De Briel D (2011) Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis 17:114–116
Najm NA, Meyer-Kayser E, Hoffmann L, Herb I, Fensterer V, Pfister K, Silaghi C (2014) A molecular survey of Babesia spp. and Theileria spp. in red foxes (Vulpes vulpes) and their ticks from Thuringia, Germany. Ticks Tick Borne Dis 5:386–391
Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Cascio A, Cazzin S, Ravagnan S, Montarsi F, Zanzani SA, Manfredi MT, Capelli G (2014) Ticks infesting humans in Italy and associated pathogens. Parasit Vectors 7:328–336
Overzier E, Pfister K, Thiel C, Herb I, Mahling M, Silaghi C (2013) Diversity of Babesia and Rickettsia species in questing Ixodes ricinus: a longitudinal study in urban, pasture, and natural habitats. Vector Borne Zoonotic Dis 13:559–564
Peirce MA (2000) A taxonomic review of avian piroplasms of the genus Babesia Starcovici, 1893 (Apicomplexa: Piroplasmorida: Babesiidae). J Nat Hist 34:317–332
Persing DH, Mathiesen D, Marshall WF, Telford SR, Apielman A, Thomford JW, Conrad PA (1992) Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol 30:2097–2103
Reye AL, Stegniy V, Mishaeva NP, Velhin S, Hübschen JM, Ignatyev G, Muller CP (2013) Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One 8(1), e54476. doi:10.1371/journal.pone.0054476
Rijpkema S, Golubic D, Moelkenboer M, Verbeek-De Kruif N, Schellekens J (1996) Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp Appl Acarol 20:23–30
Rudolf I, Golovchenko M, Sikutová S, Rudenko N, Grubhoffer L, Hubálek Z (2005) Babesia microti (Piroplasmida: Babesiidae) in nymphal Ixodes ricinus (Acari: Ixodidae) in the Czech Republic. Folia Parasitol (Praha) 52:274–276
Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M (2012) Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents-analyzing the host-pathogen-vector interface in a metropolitan area. Parasit Vectors 5:191–204
Siński E, Bajer A, Welc R, Pawełczyk A, Ogrzewalska M, Behnke JM (2006) Babesia microti: prevalence in wild rodents and Ixodes ricinus ticks from the Mazury Lakes District of North-Eastern Poland. Int J Med Microbiol 296(Suppl 40):137–143
Švehlová A, Berthová L, Sallay B, Boldiš V, Sparagano OA, Špitalská E (2014) Sympatric occurrence of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks and Rickettsia and Babesia species in Slovakia. Ticks Tick Borne Dis 5:600–605
Tijsse-Klasen E, Jacobs JJ, Swart A, Fonville M, Reimerink JH, Brandenburg AH, van der Giessen JW, Hofhuis A, Sprong H (2011) Small risk of developing symptomatic tick-borne diseases following a tick bite in The Netherlands. Parasit Vectors 4:17–24
Walter G (1982) Transmission of Babesia microti by nymphs of Dermacentor marginatus, D. reticulatus, Haemaphysalis punctata, Rhipicephalus sanguineus and Ixodes hexagonus. Z Parasitenkd 66:353–354 (in German)
Welc-Falęciak R, Hildebrandt A, Siński E (2010) Co-infection with Borrelia species and other tick-borne pathogens in humans: two cases from Poland. Ann Agric Environ Med 17:309–313
Wójcik-Fatla A, Cisak E, Chmielewska-Badora J, Zwoliński J, Buczek A, Dutkiewicz J (2006) Prevalence of Babesia microti in Ixodes ricinus ticks from Lublin region (eastern Poland). Ann Agric Environ Med 13:319–322
Wójcik-Fatla A, Bartosik K, Buczek A, Dutkiewicz J (2012) Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis 12:841–843
Yabsley MJ, Shock BC (2012) Natural history of Zoonotic Babesia: role of wildlife reservoirs. Int J Parasitol Parasites Wildl 2:18–31
Acknowledgments
This study was funded by the National Science Centre (grant number N N40404267640).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wójcik-Fatla, A., Zając, V., Sawczyn, A. et al. Babesia spp. in questing ticks from eastern Poland: prevalence and species diversity. Parasitol Res 114, 3111–3116 (2015). https://doi.org/10.1007/s00436-015-4529-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4529-5