Abstract
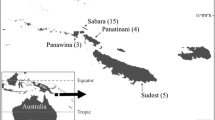
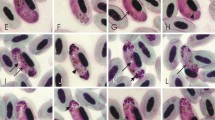
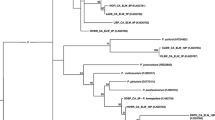
Avian Haemoproteus (Haemosporida) parasites occur in birds on all continents apart from Antarctica. Molecular screening techniques have uncovered previously unforeseen levels of Haemoproteus lineage diversity; however, fewer than 20 % of genetic parasite lineages have been linked to morphological descriptions. The process of linking morphological descriptions to DNA barcodes for Haemoproteus spp. is important for the study of host-parasite interactions and the potential for cryptic speciation. Here, we describe cytochrome-b barcodes and morphological diagnostics for the identification of Haemoproteus (Parahaemoproteus) ptilotis, a systematically confusing parasite found in Australian honeyeaters (family Meliphagidae). We characterised infections from the original type host (Lichenostomus chrysops; Family Meliphagidae) as well as from four co-occurring meliphagid species in southeast Queensland, Australia, to investigate intraspecific variation in morphology and lineage identity. We recorded eight lineages that grouped into a well-supported monophyletic group, supporting the linkage of the described lineages to H. ptilotis. However, comparisons of diagnostics between the type host and co-occurring meliphagid hosts revealed high genetic diversity and variable morphology that could be indicative of cryptic speciation. This study highlights that morphological descriptions alongside molecular characterisation remain crucial if we are to gain an understanding of the true diversity and host specificity of protozoan parasites in Australia and elsewhere.


Similar content being viewed by others
References
Adlard RD, Peirce MA, Lederer R (2002) New species of Leucocytozoon from the avian families Otidae, Podargidae and Threskiornithidae. J Nat Hist 36:1261–1267
Adlard RD, Peirce MA, Lederer R (2004) Blood parasites of birds from south-east Queensland. Emu 104:191–196
Beadell JS, Gering E, Austin J, Dumbacher JP, Peirce MA, Pratt TK, Atkinson CT, Fleischer RC (2004) Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol Ecol 13:3829–3844
Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins SL, Graves GR, Fleischer RC (2009) Host associations and evolutionary relationships of avian blood parasites from West Africa. Int J Parasitol 39:257–266
Bennett GF, Squires-Parsons D, Poldmaa T (1994) The species of Haemoproteus, Leucocytozoon and Trypanosoma of the Australian honeyeater family Meliphagidae (Aves: Passeriformes). Mem Queensl Mus 37:13–18
Bensch S, Pérez-Tris J, Waldenström J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: Multiple cases of cryptic speciation? Evolution 58:1617–1621
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Res 9:1353–1358
Clark NJ, Clegg SM (2014) The influence of vagrant hosts and weather patterns on the colonization and persistence of blood parasites in an island bird. J Biog. doi:10.1111/jbi.12454
Clark NJ, Adlard RD, Clegg SM (2014a) First evidence of avian malaria in Capricorn Silvereyes (Zosterops lateralis chlorocephalus) on Heron Island. Sunbird 44:1–11
Clark NJ, Clegg SM, Lima MR (2014b) A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data. Int J Parasitol 44:329–338
Clarke MF, Schipper C, Boulton R, Ewen J (2003) The social organization and breeding behaviour of the Yellow-faced Honeyeater Lichenostomus chrysops-a migratory passerine from the Southern Hemisphere. Ibis 145:611–623
Cleland JB, Johnston TH (1909) Descriptions of new haemoprotozoa from birds in New South Wales, with a note on the resemblance between the spermatozoa of certain honeyeaters (Fam. Meliphagidae) and spirochaete-trypanosomes. J R Soc New South Wales 43:75–96
Dimitrov D, Zehtindjiev P, Bensch S, Ilieva M, Iezhova T, Valkiūnas G (2014) Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst Parasitol 87:135–151
Driskell AC, Christidis L (2004) Phylogeny and evolution of the Australo-Papuan honeyeaters (Passeriformes, Meliphagidae). Mol Phy Evol 31:943–960
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7. doi: 10.1186/1471-2148-1187-1214
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A (2011) Geneious v5.4
Fecchio A, Lima MR, Svensson LME, Marini MA, Ricklefs RE (2013) Structure and organization of an avian haemosporidian assemblage in a Neotropical savanna in Brazil. Parasitology. doi:10.1017/S0031182012001412
Hart BL (1990) Behavioral adaptations to pathogens and parasites: five strategies. Neurosci Biobehav Rev 14:273–294
Hellgren O, Križanauskienė A, Valkiūnas G, Bensch S (2007) Diversity and phylogeny of mitochondrial cytochrome B lineages from six morphospecies of avian Haemoproteus (Haemosporida : Haemoproteidae). J Parasitol 93:889–896
Ishtiaq F, Guillaumot L, Clegg SM, Phillimore AB, Black RA, Owens IPF, Mundy NA, Sheldon BC (2008) Avian haematozoan parasites and their associations with mosquitoes across Southwest Pacific Islands. Mol Ecol 17:4545–4555
Ishtiaq F, Clegg SM, Phillimore AB, Black RA, Owens IPF, Sheldon BC (2010) Biogeographical patterns of blood parasite lineage diversity in avian hosts from southern Melanesian islands. J Biog 37:120–132
Joseph L, Toon A, NyáriÁS LNW, Rowe K, Haryoko T, Trueman J, Gardner JL (2014) A new synthesis of the molecular systematics and biogeography of honeyeaters (Passeriformes: Meliphagidae) highlights biogeographical and ecological complexity of a spectacular avian radiation. Zool Scr 43:235–248
Knowles SC, Wood MJ, Alves R, Sheldon BC (2013) Dispersal in a patchy landscape reveals contrasting determinants of infection in a wild avian malaria system. J Anim Ecol 83:429–439
Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G (2006) Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome B gene sequences. J Parasitol 92:1319–1324
Križanauskienė A, Pérez J, Palinauskas V, Hellgren O, Bensch S, Valkiūnas G (2010) Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp nov. Parasitology 137:217–227
Laird M, Laird E (1960) Culicidae and haematozoa from 'Bellona and Rennell. The natural history of Rennell Island, British Solomon Islands: scientific results of the Danish Rennell Expedition, 1951, and the British Museum (Natural History) Expedition, 1953 2:213.
Laurance SG, Jones D, Westcott D, Mckeown A, Harrington G, Hilbert DW (2013) Habitat fragmentation and ecological traits influence the prevalence of avian blood parasites in a tropical rainforest landscape. Plos One 8:e76227
Lederer R (2000) Studies on avian haematozoa in Australian Birds. The University of Queensland, Brisbane
Levin II, Zwiers P, Deem S, Geest E, Higashiguchi J, Iezhova T, Jiménez-Uzcátegui KD, Morton J, Perlut N, Renfrew R, Sari E, Valkiūnas G, Parker PG (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Cons Biol 27:1366–1377
Lima MR, Simpson S, Fecchio A, Kyaw C (2010) Low prevalence of haemosporidian parasites in the introduced house sparrow (Passer domesticus) in Brazil. Acta Parasitol 55:297–303
Mackerras MJ, Mackerras IM (1960) The Haematozoa of Australian birds. Aust J Zool 8:226–260
Medeiros MC, Hamer GL, Ricklefs RE (2013) Host compatibility rather than vector-host-encounter rate determines the host range of avian Plasmodium parasites. Proc R Soc Lond Ser B Biol Sci 280:doi:10.1098/rspb.2012.2947
Moran C, Catterall C, Green RJ, Olsen MF (2004) Functional variation among frugivorous birds: implications for rainforest seed dispersal in a fragmented subtropical landscape. Ocologica 141:584–595
O'Donoghue P, Adlard RD (2000) Catalogue of protozoan parasites recorded in Australia. Mem Queensl Mus 45:1–163
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida : Plasmodiidae). Zootaxa 39–50
Palinauskas V, Dolnik OV, Valkiūnas G, Bensch S (2010) Laser microdissection microscopy and single cell PCR of avian haemosporidians. J Parasitol 96:420–424
Palinauskas V, Iezhova TA, Križanauskienė A, Markovets MY, Bensch S, Valkiūnas G (2013) Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): A pathogenic avian parasite. Parasitol Int 62:358–363
Palinauskas V, Žiegytė R, Ilgūnas M, Iezhova TA, Bernotienė R, Bolshakov C, Valkiūnas G (2014) Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int J Parasitol 45:51–62
Pérez-Tris J, Bensch S (2005) Dispersal increases local transmission of avian malarial parasites. Ecol Let 8:838–845
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Poulin R (2014) Parasite biodiversity revisited: frontiers and constraints. Int J Parasitol 44:581–589
Poulin R, Mouillot D (2003) Parasite specialization from a phylogenetic perspective: a new index of host specificity. Parasitology 126:473–480
Richardson D, Jury F, Blaakmeer K, Komdeur J, Burke T (2001) Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol Ecol 10:2263–2273
Schodde R, Mason IJ (1999) Directory of Australian birds: passerines: Passerines. CSIRO Publishing
Sehgal RNM, Hull AC, Anderson NL, Valkiūnas G, Markovets MJ, Kawamura S, Tell LA (2006) Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal raptors. J Parasitol 92:375–379
Valkiūnas G (2005) Avian malaria parasites and other Haemosporida. CRC Press, Boca Raton
Valkiūnas G, Krizanauskiene A, Iezhova TA, Hellgren O, Bensch S (2007) Molecular phylogenetic analysis of circumnuclear hemoproteids (Haemosporida : haemoproteidae) of sylviid birds, with a description of Haemoproteus parabelopolskyi sp nov. J Parasitol 93:680–687
Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Sehgal RNM, Bensch S (2008a) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401
Valkiūnas G, Iezhova TA, Loiseau C, Chasar A, Smith TB, Sehgal RNM (2008b) New species of haemosporidian parasites (Haemosporida) from African rainforest birds, with remarks on their classification. Parasitol Res 103:1213–1228
Valkiūnas G, Kazlauskiene R, Bernotiene R, Palinauskas V, Iezhova TA (2013) Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res 1–11
Valkiūnas G, Palinauskas V, Ilgunas M, Bukauskaite D, Dimitrov D, Bernotiene R, Zehtindjiev P, Ilieva M, Iezhova TA (2014) Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 1–13
Ventim R, Morais J, Pardal S, Mendes L, Ramos JA, Pérez-Tris J (2012) Host-parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology 139:310–316
Waldenström J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
Zamora-Vilchis I, Williams SE, Johnson CN (2012) Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: implications for disease in a warming climate. Plos One 7:e39208
Zehtindjiev P, Krizanauskiene A, Bensch S, Palinauskas V, Asghar M, Dimitrov D, Scebba S, Valkiūnas G (2012) A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction-based protocols for amplification of the cytochrome b gene. J Parasitol 98:657–665
Acknowledgments
We are thankful to S. Olsson-Pons and numerous volunteers for helping with field sampling. The project was supported by BirdLife and Birds Queensland grants to N. Clark and a Griffith University New Researcher Grant to S. M. Clegg. Fieldwork was completed under Queensland Department of Environment and Resource Management (DERM) permit WISP10823212, Griffith University Ethics Approval ENV/01/12/AEC and a project licence from the Australian Bird and Bat Banding Authority to S. M. Clegg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, N.J., Adlard, R.D. & Clegg, S.M. Molecular and morphological characterization of Haemoproteus (Parahaemoproteus) ptilotis, a parasite infecting Australian honeyeaters (Meliphagidae), with remarks on prevalence and potential cryptic speciation. Parasitol Res 114, 1921–1928 (2015). https://doi.org/10.1007/s00436-015-4380-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4380-8