Abstract
Bartonella henselae is the causative agent of cat scratch disease in humans, which is recognized as an emerging zoonotic disease. Ctenocephalides felis is the main vector, and transmission of B. henselae infection between cats and humans occurs mainly through infected flea feces. Control of feline infestation with this arthropod vector therefore provides an important strategy for the prevention of infection of both humans and cats. In the present study, a new challenge model is used to evaluate the efficacy of selamectin (Stronghold® spot on) in the prevention of B. henselae transmission by C. felis. In this new challenge model, domestic cats were infected by direct application of B. henselae-positive fleas. The fleas used for infestation were infected by feeding on blood that contained in vitro-cultured B. henselae. The direct application of the fleas to the animals and the use of different B. henselae strains ensured a high and consistent challenge. Two groups of six cats were randomly allocated on pre-treatment flea counts to either control (untreated cats) or the selamectin-treated group with one pipette per cat according to the label instruction. Stronghold (selamectin 6 % spot on solution) was administered on days 0 and 32. On days 3, 10, 19, 25, and 31, each cat was infested by direct application of 20 fleas that fed on blood inoculated with B. henselae. Polymerase chain reaction (PCR) on pooled fleas confirmed that the fleas were infected. Blood samples were collected from each cat on days −3 (prior to flea infestation and treatment), 9, 17, 24, 30, 37, and 44 and assayed for B. henselae antibodies using an indirect immunofluorescence (IFA), for the presence of bacteria by bacterial culture and for B. henselae DNA presence by PCR. Cats were also assessed on a daily basis for general health. There were no abnormal health observations during the study and none of the animals required concomitant treatment. None of the cats displayed any clinical signs of bartonellosis during the study. In the untreated group, all cats became bacteremic within 17 to 44 days. None of the selamectin-treated cats became positive during the study. It was concluded that Stronghold® spot on administered to cats was efficacious in the prevention of the transmission of B. henselae by fleas to cats in a high-challenge model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bartonella henselae is a Gram-negative bacteria distributed worldwide and is recognized as an emerging zoonotic agent (Chomel and Kasten 2010). It is mostly known as the causative agent of cat scratch disease (CSD) in humans (Chomel et al. 2004). The reservoir is the domestic cat, and the cat flea, Ctenocephalides felis, is believed to function as a competent vector (Chomel et al. 1996). Infected cats are usually asymptomatic but may experience relapsing bacteremia, which can last for months or even years (Breitschwerdt and Kordick 1995; Jacomo et al. 2002). Intradermal inoculation of B. henselae to kittens is followed by bacteremia, which usually appears within 1 to 3 weeks and may last for 60 days (Kordick et al. 1995; Abbott et al. 1997). In some cats, B. henselae seropositivity has been associated with lymphadenitis, gingivitis, stomatitis, or urological diseases (Glaus et al. 1997; Breitschwerdt and Kordick 2000). B. henselae has also been considered as a potential cause of anterior uveitis, myocarditis (Lappin and Black 1999; Bradbury and Lappin 2010), and endocarditis (Chomel et al. 2003).
In humans, CSD is characterized by a persistent regional lymphadenopathy, which usually resolves within 2 to 4 months in immunocompetent patients (Boulouis et al. 2005). In immunocompromised individuals (such as those with AIDS or organ transplant recipients), the infection with B. henselae can lead to a wide range of life-threatening diseases, such as bacillary angiomatosis or bacillary peliosis (Boulouis et al. 2005; Breitschwerdt 2008; Klotz et al. 2011).
The prevalence of bacteremia among cats varies from 4 to 70 % depending on the way of life of the cat (domestic or stray cat), its age, its geographical location, and the degree of flea infestation (Chomel et al. 1995; Maruyama et al. 2000; Rolain et al. 2004). More than 50 % of cats are bacteremic to B. henselae in regions where C. felis is endemic (Heller et al. 1997). The key identified vector of B. henselae transmission among cats is Ctenocephalides felis (Chomel et al. 1996), and cats are the natural reservoir for this flea. The transmitted material seems to be the infected flea feces excreted on the fur of the flea-infested cat (Foil et al. 1998). B. henselae remains viable in the environment for at least 3 days (Finkelstein et al. 2002). Bacterial DNA is still detected in the fleas and excreted feces 12 days after infection (Bouhsira et al. 2013a, b). While grooming, cats contaminate their mouth and claws with contaminated flea dejections which can also be inoculated to humans by scratching or biting (Zangwill et al. 1993; Eisen and Gage 2012). Therefore, the best strategy to prevent bartonellosis relies on the control of flea infestations.
Lately, the use of ectoparasitic treatment as a tool in the prevention of pathogen transmission has been advocated (Beugnet and Franc 2012). Therefore, available formulations against ticks and fleas in cats and dogs need to be tested for their efficacy in preventing the transmission of arthropod-borne pathogens such as B. henselae. Two recent studies indeed demonstrated the efficacy of imidacloprid/moxidectin and imidacloprid/flumethrin combinations in preventing B. henselae transmission among cats via natural transfer of fleas (Bradbury and Lappin 2010; Lappin et al. 2013). In the present study, a new challenge model using experimentally infected fleas was used to evaluate the efficacy of selamectin in protecting cats from B. henselae transmission through infected fleas.
Materials and methods
Ethics statement
Animals were handled in strict accordance with good animal practice as defined by the relevant European standards of welfare for animals in research. The animal study was performed at the École Nationale Vétérinaire de Toulouse (ENVT), Toulouse, France, and was reviewed and approved by the ethic committee of the Midi-Pyrénées region and by the Zoetis Ethical Review Assessment Committee.
Animals
Twelve healthy specific pathogen-free (SPF) domestic cats from ENVT, weighing between 3.2 and 5.2 kg and aged 2 years (11 cats) and 4 years (1 cat), were used in this study. Before inclusion, all cats had been vaccinated against feline viral rhinotracheitis, feline calicivirus, feline distemper virus, and rabies virus and were FeLV antigen and FIV antibody negative. The absence of Bartonella spp. in cat blood was confirmed by polymerase chain reaction (PCR) and bacterial cultures (see below); results for serum antibody against Bartonella spp. were also negative. Cats were flea free and had not received any ectoparasitic treatments in the 3 months prior to the study treatment.
Cats were housed in an indoor cattery with natural environmental lighting and acclimatized to the study conditions for 2 months prior to inclusion. Cats were kept by treatment group in two separate pens. The pen environment was enriched by shelves, perches, toys, and wooden claw-sharpening boards. Water and dry cat food appropriate for the cats’ age were provided ad libitum. The cattery and litter trays were cleaned daily. Animals were handled and monitored daily for any physiological abnormalities. Once a week, ocular and gingival mucosae were inspected for signs of anemia.
Treatment administration
On days 0 and 32, six cats were treated with selamectin (Stronghold® spot on for cats, 1 pipette administered topically) according to the manufacturer’s instruction on the approved European label of the product. The actual dose varied between 8.7 and 14.1 mg/kg bodyweight. The other six cats were left untreated and served as an untreated control group.
Bacterial strains and growth conditions
Strains of B. henselae 16S rRNA genotypes I and II isolated from cats were used. The B. henselae strains were grown on brain heart infusion (BHI) agar (Difco Laboratories, Detroit, USA) supplemented with 5 % of fresh rabbit blood in humidified atmosphere at 35 °C in 5 % carbon dioxide atmosphere. For flea infection procedures, bacteria were collected after 5 days growth on BHI agar plates and suspended in phosphate-buffered saline (PBS) buffer. The bacterial suspension was diluted with PBS to obtain approximately 2 × 108 bacteria/ml as adjusted by turbidimetry.
Fleas and artificial feeding
The C. felis strain was obtained from a laboratory-reared colony originating from a wild strain harvested from a cat and maintained on cats under laboratory conditions since 1990 at ENVT. Prior to use for this study, fleas were ascertained to be negative for Bartonella spp. infection by PCR. Canine blood used to feed fleas was obtained from three healthy 6-year-old beagle dogs correctly vaccinated. Blood smears and the microhematocrit of the three dogs were checked before their inclusion in the study, and none of the dogs showed any abnormalities. The absence of Bartonella spp. in dog blood was confirmed by PCR. Blood samples obtained by venipuncture were placed in lithium heparin-coated Vacutainer tubes (Venosafe®, Laboratoires Terumo France, Guyancourt, France). Blood functional complement was deactivated by storing blood samples at room temperature for 1 h after the blood was drawn and before storage at 4 ° C. Blood samples were kept at 4 °C for no longer than 24 h.
On days 2, 9, 18, 24, and 30, 500 freshly emerged adult unfed fleas were placed in a Plexiglas box in contact with a glass feeder closed at the bottom with a thin Parafilm® membrane (Parafilm® 3M, Pechiney Plastic Packaging, IL, USA). Fleas had not received any blood meal before the start of the study. To stimulate the flea to blood feed, a constant temperature of 38.5 °C, mimicking the host’s body temperature, was maintained by a water jacket circulation system through the glass feeder. The fleas were fed on 5 ml of blood mixed with 500 μl of B. henselae in suspension in PBS at a concentration of approximately 2 × 108 bacteria/ml. After 24 h of feeding, fleas were removed and identified as having fed successfully by observation of distension of the abdomen with the naked eye. The engorged fleas were then divided into 12 batches of 20 fleas each. A 13th batch of 20 fleas was reserved weekly for B. henselae PCR analysis, in order to check the proper contamination of fleas with the bacterium. The twelve cats were infested weekly with 20 engorged fleas on days 3, 10, 19, 25, and 31. Fleas were not removed by combing from cats until the end of the study.
Sample collection
Blood samples of cats were collected via ante-brachial venipuncture by use of a 22-gauge needle on study days −3 (prior to flea infestation and treatment) 9, 17, 24, 30, 37, and 44. Blood was distributed into two sterilized, DNA/RNA-free and EDTA-treated tubes (Venosafe® plastic tubes, Laboratoires Terumo France, Guyancourt, France) to avoid contaminations (1 ml dedicated to PCR and 1 ml to bacterial culture) and in one untreated tube (1 ml blood for serum) (Venosafe® plastic tubes, Laboratoires Terumo France, Guyancourt, France). The EDTA blood samples were immediately frozen at −18 °C until PCR assays and bacterial culture. The untreated tubes were kept at ambient temperature for 1 h to help the blood clot before serum preparation. They were then centrifuged at 500 g at 4 °C for 10 min and stored at −18 °C until determination of IgG titers against Bartonella spp.
Blood culture for Bartonella
The EDTA tubes were thawed and spun at 1800g for 75 min at room temperature, and the supernatant was discarded. The pellet was suspended in 250 μl of inoculation medium (Schneider’s Drosophila medium 1×, l-glutamine, Gibco, France) and the volume recorded. Then, 250 μl of the suspension was inoculated onto BHI agar supplemented with 5 % defibrinized fresh rabbit blood. The remaining suspension was inoculated onto a second plate. The plates were incubated for 1 month at 35.5 °C with 5 % CO2 in a humid incubator and checked regularly for bacterial growth. Colonies were identified to Bartonella by their aspect, time of colony appearance, and Gram staining. At least one colony per cat/day was subcultured, harvested, and frozen at −70 °C in 100 % fetal calf serum. Identification of B. henselae was performed on subculture of one colony by PCR targeting the citrate synthase (gltA) gene (Norman et al. 1995).
PCR assays
Two-hundred microliters of EDTA blood was extracted by Nucleospin Blood Quickpure Macherey Nagel Kit (Macherey-Nagel, Hoerdt, France). Then, two PCRs were carried out: a PCR targeting the citrate synthase (gltA) gene followed by a nested PCR targeting the ITS gene (Rampersad et al. 2005). A cat blood sample was regarded as positive to B. henselae DNA when at least one PCR result among the two performed PCR assays (gltA gene and ITS gene) was positive.
From each batch of fleas used for the weekly infestations of the cats, a pool of five fleas was extracted by Nucleospin Tissue Quickpure Macherey Nagel Kit (Macherey-Nagel, Hoerdt, France) and a PCR targeting the Bartonella gltA gene was carried out.
Serology/IFA
One of the B. henselae strains used in this assay was passed from agar-grown cultures into Vero cells. Heavily infected cell cultures were spotted onto 12-well Teflon-coated slides, incubated 18 h in a CO2 incubator, acetone fixed, and stored frozen. B. henselae antibodies were determined after traditional IFA practices. Fluorescein-conjugated goat anti-cat IgG (Bethyl Lab, Montgomery, USA) was used at the dilution of 1/50 in PBS. Serum samples were diluted in phosphate-buffered saline solution. Cat sera were screened at a dilution of 1:64 (cutoff titer). All sera that remained reactive at a titer of 1:64 were further tested with twofold dilutions out to a final dilution of 1:1024.
Results
Clinical findings
No abnormal health event was observed in any of the cats throughout the study. Pruritus or hair loss was not observed. No abnormal behavior, anorexia, weakness, or anemia was detected, and no clinical signs of bartonellosis were observed after the start of the flea infestation.
Bartonella spp. PCR assays and blood culture
In the selamectin-treated group, none of the cats became positive at any time during the study.
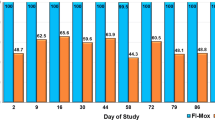
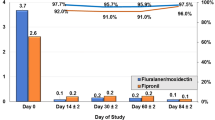
In the untreated group, all cats became PCR positive during the study. The PCR-positive samples from the control cats were also positive by blood culture. The interval to a positive PCR assay or culture result ranged from 14 to 41 days of flea exposure with a median of 21 days. The first amplification of B. henselae DNA was obtained on two cats (cats 5 and 6) after 14 days of exposure to fleas. Bacteremia in these two cats lasted for at least 4 weeks, the duration of the study. Cats 2, 3, and 4 had positive PCR and culture results 3 weeks after the first flea infestation and 1 week after the second infestation. These three cats were bacteremic for at least 3 weeks. On study day 44 (41 days after the first flea infestation), the sixth cat in the negative control group (cat 1) was also bacteremic and PCR positive. (Fig. 1)
All the pools of fleas tested were PCR positive for the gltA gene except the pool used before the treatment for the allocation.
Serology
In the untreated group, five out of the six cats developed detectable anti-B. henselae IgG titers, with interval to seroconversion ranging from 21 to 41 days of the first fleas exposure. Titers ranged from 1:64 to 1:512, and once positive titers were detected, cats remained seropositive for the duration of the study. None of the selamectin-treated cats seroconvert during the study. (Fig. 1)
Discussion
Recent studies investigated the efficacy of imidacloprid formulations in preventing the transmission of B. henselae by fleas in cats (Bradbury and Lappin 2010; Lappin et al. 2013). In these studies, cats in one group were inoculated by intravenous inoculation of B. henselae and were infested on a regular basis with fleas in order to mimic the natural transmission pattern. This group of inoculated cats was housed in a pen next to pens with either treated or untreated cats. The pens were separated from each other by a mesh, preventing cats in different groups from licking or scratching each other, yet allowing fleas to infest animals in neighboring pens. In the different pens, carpets allowed fleas to complete their life cycle.
In the Bradburry and Lappin (2010) study, the cats in the B. henselae-infected group became bacteremic within 21 days after intravenous inoculation. The untreated control cats became bacteremic within 42 to 70 days after the first flea infestation and had on average less than 6 fleas. The Lappin et al. (2013) study was a long-term study, and cats were examined for 252 days after the application of the collar in the treated group. The inoculated cats became bacteremic within 39 days after intravenous injection of B. henselae and were infested with 100 C. felis on day 0 and then monthly afterwards. The mean flea counts on infected and control cats were very low: <2 in the control group and <8 in the infected group. Bacteremia and seroconversion were detected in only four out of seven cats in the control group, while the treated group remained negative, suggesting an overall low infection pressure.
The challenge model used in the present study is based on direct infestation of cats with B. henselae-infected fleas and provides several advantages: the amount of fleas used for infestation is standardized and does not depend on the migration of fleas from one group of cats to the other. As such, each cat is equally challenged at the same time, and the challenge model provides a consistent and high infection pressure.
In the abovementioned studies (Bradbury and Lappin 2010; Lappin et al. 2013), the migration of fleas between the different groups was generally low, suggesting an inconsistent infection pressure. Moreover, the fleas detected on the cats could have originated from adults newly emerged from the environment. These young fleas are potentially not infected with B. henselae as the transmission does not seem to occur transovarially in the flea (Bouhsira et al. 2013a) and as they did not yet feed on the inoculated cats.
Furthermore, the use of bacteremic cats as infection source for B. henselae leads to inconsistency in the infection pressure, as the bacteremia in cats varies in function of the individual cat, the Bartonella strain, and the route of inoculation (Kordick and Breitschwerdt 1997; Bradbury and Lappin 2010; Lappin et al. 201). After an intradermal inoculation, cats became bacteremic within 12 days (unpublished data), whereas after an intravenous inoculation, cats became bacteremic within 21 to 39 days (Bradbury and Lappin 2010; Lappin et al. 2013).
The use of an artificial feeding system to infect the fleas in the new challenge model allowed the infection of fleas within 24 h only, synchronizing the start of the exposure to B. henselae in this study. Furthermore, the infection status of the fleas can be confirmed on a pool of fleas prior to the infestation of the cats, either by PCR or indirectly on the excreted feces by quantitative PCR (Bouhsira et al. 2013b). In this study, all flea pools used for infestation were confirmed to be positive for B. henselae.
In the previously used model, the transmission of infection to other cats is prone to variation, as the fleas placed on the inoculated cats have to (1) acquire the bacteria from the inoculated cats and (2) to infest another host before being able to transmit the infection. Bradburry and Lappin (2010) obtained bacteremia in the control cats within 42 to 70 days with seroconversion occurring between 63 and 84 days. In the Lappin (2013) study, not all cats became bacteremic and those that did occurred within 28 to 140 days. Cats seroconverted within 56 to 154 days. In the present study using direct infestation of the cats with infected fleas, bacteremia occurred in 100 % of the cats within 14 to 41 days, and all cats seroconverted within 21 to 44 days. These results are in accordance with previous studies in which SPF kittens were infested with fleas harvested from contaminated cats (Chomel et al. 1996).
The use of bacteremic cats as the source of B. henselae can be questioned in a long-term study, as it is uncertain if cats remain bacteremic throughout the study. In the Bradburry and Lappin (2010) study, the inoculated cats were not bacteremic anymore by day 84, and in the Lappin et al. (2013) study, only one control cat was bacteremic by that time, and all cats were negative by day 252, making it impossible to truly evaluate the efficacy of the ectoparasiticide.
This new challenge model allows regular infestations of cats with a standardized number of B. henselae-infected fleas. This challenge model has an additional benefit as it decreases the number of animals included as contaminated cats are not used as source of bacteria. The demonstration of an ectoparasiticidal product efficacy can therefore be performed using two groups of cats, as in the present study.
In this study, all untreated cats were successfully infected with B. henselae after infestation with infected fleas. In contrast, none of the selamectin-treated cats became positive during the study, even under the high and continuous infection pressure of the new challenge model. The high efficacy of selamectin in the current challenge model is due to the high efficacy of the product against adult fleas (McTier et al. 2000; Dryden et al. 2005), larvae, and eggs (Shanks et al. 2000).
The new challenge model confirmed that Stronghold® spot on is highly efficacious in preventing the transmission of B. henselae by fleas to cats, under high infection pressure.
References
Abbott RC, Chomel BB, Kasten RW, Floyd-Hawkins KA, Kikuchi Y, Koehler JE, Pedersen NC (1997) Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis 20:41–51
Beugnet F, Franc M (2012) Insecticide and acaricide molecules and/or combinations to prevent pet infestation by ectoparasites. Trends Parasitol 28:267–279
Bouhsira E, Ferrandez Y, Liu M, Franc M, Boulouis HJ, Biville F (2013a) Ctenocephalides felis an in vitro potential vector for five Bartonella species. Comp Immunol Microbiol Infect Dis 36:105–111
Bouhsira E, Franc M, Boulouis HJ, Jacquiet P, Raymond-Letron I, Liénard E (2013b) Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl Environ Microbiol 79:7439–7444
Boulouis HJ, Chang CC, Henn JB, Kasten RW, Chomel BB (2005) Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res 36:383–410
Bradbury CA, Lappin MR (2010) Evaluation of topical application of 10% imidacloprid-1% moxidectin to prevent Bartonella henselae transmission from cat fleas. J Am Vet Med Assoc 236:869–873
Breitschwerdt EB (2008) Feline bartonellosis and cat scratch disease. Vet Immunol Immunopathol 123:167–171
Breitschwerdt EB, Kordick DL (1995) Bartonellosis. J Am Vet Med Assoc 206:1928–1931
Breitschwerdt EB, Kordick DL (2000) Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 13:428–438
Chomel BB, Kasten RW (2010) Bartonellosis, an increasingly recognized zoonosis. J Appl Microbiol 109:743–750
Chomel BB, Abbott RC, Kasten RW, Floyd-Hawkins KA, Kass PH, Glaser CA, Pedersen NC, Koehler JE (1995) Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteraemia and antibody titers. J Clin Microbiol 33:2445–2450
Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield AN, Abbott RC, Pedersen NC, Koehler JE (1996) Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 34:1952–1956
Chomel BB, Wey AC, Kasten RW, Stacy BA, Labelle P (2003) Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat. J Clin Microbiol 41:5337–5339
Chomel BB, Boulouis HJ, Breitschwerdt EB (2004) Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc 224:1270–1279
Dryden MW1, Smith V, Payne PA, McTier TL. (2005) Comparative speed of kill of selamectin, imidacloprid, and fipronil-(S)-methoprene spot-on formulations against fleas on cats. Vet Ther. 2005 Fall;6(3):228–36
Eisen RJ, Gage KL (2012) Transmission of flea-borne zoonotic agents. Annu Rev Entomol 57:61–82
Finkelstein JL, Brown TP, O’Reilly KL, Wedincamp J Jr, Foil LD (2002) Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J Med Entomol 39:915–919
Foil L, Andress E, Freeland RL, Roy AF, Rutledge R, Triche PC, O’Reilly KL (1998) Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol 35:625–628
Glaus T, Hofmann-Lehmann R, Greene C, Glaus B, Wolfensberger C, Lutz H (1997) Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol 35:2883–2885
Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y (1997) Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol 35:1327–1331
Jacomo V, Kelly PJ, Raoult D (2002) Natural history of Bartonella infections (an exception to Koch’s postulate). Clin Diagn Lab Immunol 9:8–18
Klotz SA, Ianas V, Elliott SP (2011) Cat-scratch disease. Am Fam Physician 83:152–155
Kordick DL, Breitschwerdt EB (1997) Relapsing bacteraemia after blood transmission of Bartonella henselae to cats. Am J Vet Res 58:492–497
Kordick DL, Wilson KH, Sexton DJ, Hadfield TL, Berkhoff HA, Breitschwerdt EB (1995) Prolonged Bartonella bacteraemia in cats associated with cat-scratch disease patients. J Clin Microbiol 33:3245–3251
Lappin MR, Black JC (1999) Bartonella spp infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc 214:1205–1207
Lappin MR, Davis WL, Hawley JR, Brewer M, Morris A, Stanneck D (2013) A flea and tick collar containing 10% imidacloprid and 4.5% flumethrin prevents flea transmission of Bartonella henselae in cats. Parasit Vectors 6:26
Maruyama S, Nakamura Y, Kabeya H, Tanaka S, Sakai T, Katsube Y (2000) Prevalence of Bartonella henselae, Bartonella clarridgeiae and the 16S rRNA gene types of Bartonella henselae among pet cats in Japan. J Vet Med Sci 62:273–279
McTier TL, Jones RL, Holbert MS, Murphy MG, Watson P, Sun F, Smith DG, Rowan TG, Jernigan AD (2000) Efficacy of selamectin against adult flea infestations (Ctenocephalides felis felis and Ctenocephalides canis) on dogs and cats. Vet Parasitol 91(3–4):187–199
Norman AF, Regnery R, Jameson P, Greene C, Krause DC (1995) Differentiation of Bartonella like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33:1797–1803
Rampersad JN, Watkins JD, Samlal MS, Deonanan R, Ramsubeik S, Ammons DR (2005) A nested-PCR with an internal amplification control for the detection and differentiation of Bartonella henselae and Bartonella clarridgeiae: an examination of cats in Trinidad. BMC Infect Dis 5:63
Rolain JM, Locatelli C, Chabanne L, Davoust B, Raoult D (2004) Prevalence of Bartonella clarridgeiae and Bartonella henselae in domestic cats from France and detection of the organisms in erythrocytes by immunofluorescence. Clin Diagn Lab Immunol 11:423–425
Shanks DJ, Rowan TG, Jones RL, Watson P, Murphy MG, Smith DG, Jernigan AD (2000) Efficacy of selamectin in the treatment and prevention of flea (Ctenocephalides felis felis) infestations on dogs and cats housed in simulated home environments. Vet Parasitol 91(3–4):213–222
Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cartter ML, Wenger JD (1993) Cat-scratch disease in Connecticut: epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 329:8–13
Acknowledgments
This study was funded in part by a grant from Zoetis International Services, Animal Health (Zaventem, Belgium). The authors are grateful to Martine Roques and Sonia Gounaud (ENVT, Toulouse, France), for their help with the handling of the cats and for the flea colony maintenance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bouhsira, E., Franc, M., Lienard, E. et al. The efficacy of a selamectin (Stronghold®) spot on treatment in the prevention of Bartonella henselae transmission by Ctenocephalides felis in cats, using a new high-challenge model. Parasitol Res 114, 1045–1050 (2015). https://doi.org/10.1007/s00436-014-4271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4271-4