Abstract
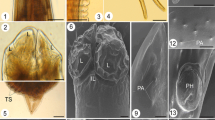
Haemonchus longistipes is a gastrointestinal abomasal nematode which is one of the most prevalent and pathogenic parasites infesting the stomach of ruminants. On the basis of light and ultrastructural data, the objective of the present study was to introduce a first identification of the cameline haemonchosis caused by H. longistipes. Abomasa of 42 Egyptian camels Camelus dromedarius (Artiodactyla: Camelidae) were collected monthly from September 2013 to April 2014 from the main slaughter house of Cairo, Egypt. Adult male and female nematode worms were recovered from 26 (62 %) specimens of the examined abomasa. The parasites were of yellow color; the body was filiform (slender) tapered towards the anterior end in male and towards both ends in female. Buccal capsules absent, the buccal cavity was small with a conspicuous dorsal lancet extended from dorsal wall. The cervical papillae were prominent and spine-like. The body length of the female worm was 16.6–20.5 (18.5 ± 0.3) mm. The anterior end to the cervical papillae was 3.19–4.30 (4.12 ± 0.5) mm. The vulva of the female had a linguiform process or flap, the tail is without a spine, and the anal pore at the posterior end of the body had a simple dorsal rim. The body of male was 10.4–14.7 (13.9 ± 2.0) mm in length. The male bursa had elongated lobes supported by long, slender rays. The small dorsal lobe was asymmetrical with Y-shaped dorsal rays. The spicules were long with a length of 0.52–0.54 (0.53 ± 0.05) mm, each provided with a small barb and pore near its extremity. Synlophe was bilaterally and dorsoventrally symmetrical; it extended from cephalic expansion over anterior 50 % of prebursal or prevulvar body and consisted of a maximum of 42 ridges. The described species herein was compared with the three morphologically similar species Haemonchus mitchelli, Haemonchus okapiae, and H. longistipes with their synlophes consist of 42 ridges distributed over the anterior half of the body. These species can be separated by unique structural characteristics of their synlophes, spicules, and copulatory bursa. The most morphologically similar species to the recovered worm was H. longistipes. Also, some of the parameters with regard to morphology and morphometry of this parasite were described for the first time.



Similar content being viewed by others
References
Almedia JL (1941) Revisao de genera Haemonchus Cobb, (1898). (Nematoda: Trichostrongylidae). Mem Inst Osw Cruz 30:57–114
Al-Rofaai A, Rahman WA, Abdulghani M (2013) Sensitivity of two in vitro assays for evaluating plant activity against the infective stage of Haemonchus contortus strains. Parasitol Res 112(2):893–898
Altaif M, Issa K (1983) Abomasal and intestinal nematodes of sheep. J Anim Health Prod 38:31–36
Bahirathan M, Miller JE, Barras SR, Kearney MT (1996a) Susceptibility of Suffolk and Gulf Coast Native suckling lambs to naturally acquired strongylate nematode infections. Vet Parasitol 65:259–268
Bahirathan M, Miller JE, Barras SR, Kearney MT (1996b) Susceptibility of Suffolk and Gulf Coast Native suckling lambs to naturally acquired strongylate nematode infection. Vet Parasitol 65(3–4):259–268
Boomker J, Horak IG, Ramsay KA (1989) Helminth and arthropod parasites of indigenous goats in the Northern Transvaal. Onderstepoort J Vet Res 61:13–20
Das KM, Whitlock JH (1960) Subspeciation in Haemonchus contortus (Rudolphi, 1803) Nematoda, Trichostrongyloidae. Cornell Vet 50:182–197
Dinaburg AG (1939) Helminth parasites collected from deer, Odocoileus virginianus, in Florida. Proc Helminthol Soc Wash 6:102–104
Domke AV, Chartier C, Gjerde B, Höglund J, Leine N, Vatn S, Stuen S (2012) Prevalence of anthelmintic resistance in gastrointestinal nematodes of sheep and goats in Norway. Parasitol Res 111(1):185–193
Donald AD (1963) The occurrence of Haemonchus similis Travassos, 1914 in cattle in Fiji. J Helminthol 37:179–184
Doss MA, Rayburn JD, Shaw JH, Kirby MD (1985) Nematoda and nematode diseases. Supergenera, genera, species and subspecies: F-M. Index Catalogue of medical and veterinary zoology. Special Publication No. 6, Part 3, Issued July 1985, U.S. Department of Agriculture, Agricultural Research Service, U.S. Government Printing Office, Washington, D.C., 556 p.
El Bihari S, Kawasmeh ZA (1980) Occurrence and seasonal variation of some gastrointestinal helminths of the dromedary, Camelus dromedarius, in Saudi Arabia. Proc Saudi Biol Soc 4:297–304
Franklin K (1935) Prevalence of gastrointestinal nematode parasites of sheep in Gurtuk, USSR. J Parasit Dis 23:241–245
Gelaye E, Wossene AS (2003) Small ruminant Haemonchosis: morphology and prolificacy study in Eastern Ethiopia. Bull Anim Health Prod Afr 51:6773
Gibbons LM (1979a) Revision of the genus Haemonchus Cobb, 1898 (Nematoda: Trichostrongylidae). Syst Parasitol 1:3–24
Gibbons LM (1979b) Revision of the genus Haemonchus Cobb, 1898 (Nematoda: Trichostrongylidae). Syst Parasitol 1:3–24
Gillian S, Behnke JM, Buttle DJ, Duce LR (2004) Natural plant cysteine proteinases as anthelmintics? Trends Parasitol 20:322–327
Hussein SH, Hussein MF (1985) The prevalence and pathology of Haemonchus longistipes infection in Saudi Arabian camels (Camelus dromedarius). Proc Saudi Biol Soc 8:247–257
Jacquiet P, Humbert JF, Comes AM, Cabaret J, Thiam A, Cheikh D (1995) Ecological, morphological, and genetic characterization of sympatric Haemonchus spp. parasites of domestic ruminants in Mauritania. Parasitology 110:483–492
Jacquiet P, Cabaret J, Cheikh D, Thiam E (1997) Identification of Haemonchus species in domestic ruminants based on morphometrics of spicules. Parasitol Res 83:82–86
Kaplan RM, Vidyashankar AN (2012) An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol 186:70–77
Ksull NP (1939) Anoplocephalate infection in sheep. Vet Moscow USSR 3:40–41
Kuchai JA, Chishti MZ, Zaki MM, Javid A, Dar SA, Muzaffar R, Tak H (2011) Epidemiology of helminth parasites in small ruminants of Ladakh, India. Online J Anim Feed Res 1:239242
Kuchai JA, Tak H, Chishti MZ, Lone BA (2012) Faecal examination of Pashmina goats of Ladakh for nematode infections. Glob J Sci Front Res 12:37–40
Kumsa B, Tolera A, Abebe R (2008) Vulvar morphology and sympatry of Haemonchus species in naturally infected sheep and goats of Ogaden region, eastern Ethiopia. Vet Arhiv 78(4):331–342
LE Jambre LF, Whitlock JH (1968) Seasonal fluctuation in linguiform morphs of Haemonchus contortus cayugensis. J Parasitol 54:827–830
Lichtenfels JR, Pilitt PA, Le Jambre LF (1986) Cuticular ridge patterns of Haemonchus contortus and Haemonchus placei (Nematoda: Trichostrongyloidea). Proc Helminthol Soc Wash 53:94–101
Lichtenfels JR, Patricia AP, Lynda MG, Eric PH (2002) Redescriptions of Haemonchus mitchelli and Haemonchus okapiae (Nematoda: Trichostrongyloidea) and description of a unique synlophe for the Haemonchinae. J Parasitol 88(5):947–960
Lins de Almeida J (1935) Revisio do genero Haemonchus Cobb, 1898. (Nematoda: Trichostrongylidae). Mem Inst Oswaldo Cruz 30:57–114
Masud SA, Jamil M (1887) Prevalence of gastrointestinal nematodes in small ruminants of Kingort district of Tanzania. Ir J Vet Helminthol 17:58–62
Menkir MS, Uggla A, Waller PJ (2006) Epidemiology and seasonal dynamics of gastrointestinal nematode infections of sheep in a semi-arid region of eastern Ethiopia. Vet Parasitol 143:311–321
Miller JE, Bahirathan M, Lemarie SL, Hembry FG, Kearney MT, Barras SR (1998) Epidemiology of gastrointestinal nematode parasitism in Suffolk and Gulf Coast Native sheep with special emphasis on relative susceptibility to Haemonchus contortus infection. Vet Parasitol 74(1):55–74
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mehlhorn H, Quraishy SA, El-Mahdi M, Al-Ghamdi A, Mostafa N (2012) First record of anisakid juveniles (Nematoda) in the European seabass Dicentrarchus labrax (family: Moronidae), and their role as bio-indicators of heavy metal pollution. Parasitol Res 110(3):1131–1138
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mostafa N (2013) New host and locality records of two nematode parasites Dujardinnascaris mujibii (Heterocheilidae) and Hysterothylacium aduncum (Anisakidae) from the common seabream Pagrus pagrus: a light and scanning electron microscopic study. Parasitol Res 112(2):807–815
Mulugeta HS, Getachew T, Taffesse M, Getache WM, Kinfe G, TeshomeY (1989) The significance of helminth in livestock production. The 2nd national livestock improvement conference pp: 49-53.
Nery PS, Nogueira FA, Oliveira NJ, Martins ER, Duarte ER (2012) Efficacy of extracts of immature mango on ovine gastrointestinal nematodes. Parasitol Res 111(6):2467–2471
Roberts FHS, Turner HN, Mckevett (1954) On the specific distinctness of the ovine and bovine strains of Haemonchus contortus (Rudolphi) Cobb (Nematoda: Trichostrongylidae). Aust J Zoo 2:2753–2795
Rose JH (1966) The vulval flap formula of Haemonchus contortus from sheep in South east England. Res Vet Sci 7:480–48
Rudolphi GA (1802) Fortesetzing der Beobachtingen uber die Eingewel dewurmer. Arch Zool U Zoot 2:23–25
Sahai BN, Deo PG (1964) Studies on the Haemonchus contortus (Rudalphi 1803) Cobb 1898 and Haemonchus (Molin, 1860) Railliet and Henry (1909) with a note on the synonym of Haemonchus placel (place, 1893) with Haemonchus bispinosus. Ind J Helminthol 16:5–11
Silverman PH, Patterson JE (1960) Histotrophic (parasite) stages of Haemonchus contortus. Nature (London) 185:54–55
Soulsby EJL (1982) Helminths, arthopods and protozoa of domesticated animals. Bailliere, Tindal and Cassel, London, 809 spp
Soulsby EJL (1986) Helminths, arthropods and protozoa of domesticated animals, 7th edn. Bailliere Tindall, England
Tembely S, Lahlou-Kassi A, Rege JE, Sovani S, Diedhiou ML, Bake RL (1997) The epidemiology of nematode infections in sheep in a cool tropical environment. J Vet Parasitol 70:129–141
Thomas NS, Teshale B, Kumsa (2007) Abomasal nematodes of sheep and goats slaughtered in Awassa (Ethiopia): Species composition, prevalence and vulvar morphology. Helminthologia 44:70–75
Tod EM (1965) On the morphology of Haemonchus contortus (Rudolphi) Cobb (Nematoda: Trichostrogylidae) in sheep and cattle. Aust J Zool 13:773–781
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996) Veterinary parasitology, 2nd edn. Blackwell Science, United Kingdom, p 307
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (2006) Veterinary parasitology. Longman Scientific and Technical, England
Zaros LG, Neves MR, Benvenuti CL, Navarro AM, Sider LH, Coutinho LL, Vieira LS (2014) Response of resistant and susceptible Brazilian Somalis crossbreed sheep naturally infected by Haemonchus contortus. Parasitol Res 113(3):1155–1161
Acknowledgments
The authors extend appreciations to the Faculty of Science, Cairo University, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsy, K., Bashtar, AR., Fol, M. et al. Haemonchus longistipes Railliet & Henry, 1909 (Nematoda, Trichostrongylidae) from the Egyptian dromedary, Camelus dromedarius (Artiodactyla: Camelidae), first identification on the basis of light and ultrastructural data. Parasitol Res 113, 4579–4585 (2014). https://doi.org/10.1007/s00436-014-4148-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4148-6