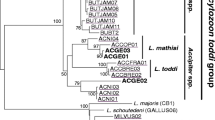
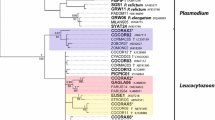
Abstract
Blood and ectoparasitic ticks were collected from migratory seabirds in New Zealand, including Australasian gannets (n = 13) from two sites and red-billed gulls (n = 9) and white-fronted terns (n = 2) from a third location. Blood smears were screened for parasite presence by microscopy, while DNA from blood samples was subjected to PCR for the presence of tick-transmitted protozoan haemoparasites belonging to the order Piroplasmida. Parasites were identified by comparing small subunit ribosomal RNA (18S rDNA) gene sequences to related sequences on GenBank. Analyses indicated that nine birds were infected with unknown variants of a Babesia poelea-like parasite (recorded as genotypes I and II), while four harboured a piroplasm that was genetically similar to Babesia kiwiensis. There was no parasite stratification by bird species; both the gannets and gulls were positive for all three parasites, while the terns were positive for the B. kiwiensis-like and the B. poelea-like (genotype I) parasites. The B. kiwiensis-like parasite found in the birds was also found in two species of ticks: Carios capensis and Ixodes eudyptidis. This represents the first report of Babesia-positive ticks parasitising seabirds in New Zealand. The lack of host specificity and evidence of wide ranging distributions of the three piroplasm genotypes suggests there is a high degree of haemoparasite transmission occurring naturally between New Zealand seabird populations and species.


Similar content being viewed by others
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552
Brocchieri L (2001) Phylogenetic inferences from molecular sequences: review and critique. Theor Popul Biol 59:27–40
Capligina V, Salmane I, Keiss O, Vilks K, Japina K, Baumanis V, Ranka R (2014) Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick Borne Dis 5:75–81
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469
Dietrich M, Gomez-Diaz E, McCoy KD (2011) Worldwide distribution and diversity of seabird ticks: implications for the ecology and epidemiology of tick-borne pathogens. Vector Borne Zoonotic Dis 11:453–470
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinforma 5:113
Franke J, Meier F, Moldenhauer A, Straube E, Dorn W, Hildebrandt A (2010) Established and emerging pathogens in Ixodes ricinus ticks collected from birds on a conservation island in the Baltic Sea. Med Vet Entomol 24:425–432
Hasle G, Leinaas HP, Roed KH, Oines O (2011) Transport of Babesia venatorum-infected Ixodes ricinus to Norway by northward migrating passerine birds. Acta Vet Scand 53
Heath ACG, Palma RL, Cane RP, Hardwick S (2011) Checklist of New Zealand ticks (Acari: Ixodidae, Argasidae). Zootaxa, 55–63
Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E (2010) The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis 1:105–107
Hunfeld KP, Hildebrandt A, Gray JS (2008) Babesiosis: recent insights into an ancient disease. Int J Parasitol 38:1219–1237
Jefferies R, Ryan UM, Irwin PJ (2007) PCR-RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet Parasitol 144:20–27
Jefferies R, Down J, McInnes L, Ryan U, Robertson H, Jakob-Hoff R, Irwin P (2008) Molecular characterization of Babesia kiwiensis from the brown kiwi (Apteryx mantelli). J Parasitol 94:557–560
Jinnai M, Kawabuchi-Kurata T, Tsuji M, Nakajima R, Fujisawa K, Nagata S, Koide H, Matoba Y, Asakawa M, Takahashi K, Ishihara C (2009) Molecular evidence for the presence of new Babesia species in feral raccoons (Procyon lotor) in Hokkaido, Japan. Vet Parasitol 162:241–247
Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A 86:6196–6200
Lack JB, Reichard MV, Van Den Bussche RA (2012) Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol 42:353–363
Merino S (1998) Babesia bennetti n. sp. from the Yellow-Legged Gull (Larus cachinnans, Aves, Laridae) on Benidorm Island, Mediterranean Sea. J Parasitol 84:422–424
Movila A, Reye AL, Dubinina HV, Tolstenkov OO, Toderas I, Huebschen JM, Muller CP, Alekseev AN (2011) Detection of Babesia Sp. EU1 and Members of Spotted Fever Group Rickettsiae in Ticks Collected from Migratory Birds at Curonian Spit, North-Western Russia. Vector Borne Zoonotic Dis 11:89–91
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Inc., New York
Paparini A, Ryan UM, Warren K, McInnes LM, de Tores P, Irwin PJ (2012) Identification of novel Babesia and Theileria genotypes in the endangered marsupials, the woylie (Bettongia penicillata ogilbyi) and boodie (Bettongia lesueur). Exp Parasitol 131:25–30
Peirce MA (2000) A taxonomic review of avian piroplasms of the genus Babesia Starcovici, 1893 (Apicomplexa: Piroplasmorida: Babesiidae). J Nat Hist 34:317–332
Peirce MA (2005) A checklist of the valid avian species of Babesia (Apicomplexa : Piroplasmorida), Haemoproteus, Leucocytozoon (Apicomplexa : Haemosporida), and Hepatozoon (Apicomplexa : Haemogregarinidae). J Nat Hist 39:3621–3632
Peirce MA, Parsons NJ (2012) Babesia ugwidiensis, a new species of avian piroplasm from Phalacrocoracidae in South Africa. Parasite J Soc Fr Parasitol 19:375–379
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Quillfeldt P, Arriero E, Martinez J, Masello JF, Merino S (2011) Prevalence of blood parasites in seabirds—a review. Frontiers in Zoology 8
Sandor AD, Marcutan DI, D’Amico G, Gherman CM, Dumitrache MO, Mihalca AD (2014) Do the ticks of birds at an important migratory hotspot reflect the seasonal dynamics of Ixodes ricinus at the migration initiation site? A case study in the Danube Delta. PLoS ONE 9
Schnittger L, Yin H, Gubbels M, Beyer D, Niemann S, Jongejan F, Ahmed J (2003) Phylogeny of sheep and goat Theileria and Babesia parasites. Parasitol Res 91:398–406
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA (2012) Babesia: a world emerging. Infect Genet Evol 12:1788–1809
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tavaré S (1986) Some probabilistic and statistical problems in the analysis of DNA sequences, American Mathematical Society: lectures on mathematics in the life sciences. Amer Mathematical Society, pp. 57–86
Tompkins DM, Paterson R, Massey B, Gleeson DM (2010) Whataroa virus four decades on: emerging, persisting, or fading out? J R Soc N Z 40:1–9
Tsiodras S, Kelesidis T, Kelesidis I, Bauchinger U, Falagas ME (2008) Human infections associated with wild birds. J Infect 56:83–98
Wilkinson DA, Dietrich M, Lebarbenchon C, Jaeger A, Le Rouzic C, Bastien M, Lagadec E, McCoy KD, Pascalis H, Le Corre M, Dellagi K, Tortosa P (2014) Massive infection of seabird ticks with bacterial species related to Coxiella burnetii. Appl Environ Microbiol 80:3327–3333
Work TM, Rameyer RA (1997) Description and epizootiology of Babesia poelea n. sp. in brown boobies (Sula leucogaster (Boddaert)) on Sand Island, Johnston Atoll, Central Pacific. J Parasitol 83:734–738
Yabsley MJ, Murphy SM, Cunningham MW (2006a) Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J Wildl Dis 42:366–374
Yabsley MJ, Work TM, Rameyer RA (2006b) Molecular phylogeny of Babesia poelea from brown boobies (Sula leucogaster) from Johnston Atoll, Central Pacific. J Parasitol 92:423–425
Yabsley MJ, Greiner E, Tseng FS, Garner MM, Nordhausen RW, Ziccardi MH, Borjesson DL, Zabolotzky S (2009) Description of novel Babesia species and associated lesions from common murres (Uria aalge) from California. J Parasitol 95:1183–1188
Acknowledgments
The authors wish to acknowledge Ms. Frances Brigg and Dr. Dave Berryman from the Western Australian State Agricultural Biotechnology Centre (SABC) at Murdoch University for the technical support and Dr. Richard Jakob-Hoff from the New Zealand Centre for Conservation Medicine (NZCCM) at Auckland Zoo for the field assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andrea Paparini and Linda M McInnes contributed equally to this article.
Rights and permissions
About this article
Cite this article
Paparini, A., McInnes, L.M., Di Placido, D. et al. Piroplasms of New Zealand seabirds. Parasitol Res 113, 4407–4414 (2014). https://doi.org/10.1007/s00436-014-4118-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4118-z