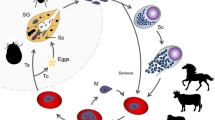
Abstract.
The phylogenetic relationship of Theileria and Babesia species infecting sheep and goats on the basis of their 18S RNA gene structure was addressed in the present study. For this purpose, the complete sequences of the small ribosomal RNA genes of a panel of sheep and goat piroplasm isolates, including T. lestoquardi, T. ovis, T. separata, B. ovis, B. motasi, B. crassa and several novel species, were sequenced and compared. The classification based on the established phylogenetic tree corresponded with traditional systematics and revealed that sheep/goat piroplasm species are of polyphyletic origin. The independent evolution of almost all sheep/goat piroplasms suggests that speciation may have occurred after transfer of the piroplasm-transmitting tick from a primal wild ruminant host to domestic sheep and goats. In accordance with recent reports, our study confirms the existence of at least two additional sheep/goat piroplasm species, designated Theileria sp. 1 (China) and Theileria sp. 2 (China). The recently reported pathogenic sheep/goat Theileria sp. 1 (China) seems to be identical with a Theileria sp. isolated from Japanese serow. Furthermore, our results suggest that T. ovis represents a single species.

Similar content being viewed by others
References
Ahmed J, Yin H, Schnittger L Jongejan F (2002) Ticks and tick-borne diseases in Asia with special emphasis on China. Parasitol Res 88:S51–S55
Alani AJ, Herbert IV (1988) Morphology and transmission of Theileria recondita (Theileriidae: Sporozoa) isolated from Haemaphysalis punctata from North Wales. Vet Parasitol 28:283–291
Allsopp MT, Cavalier-Smith T, De Waal DT, Allsopp BA (1994) Phylogeny and evolution of the piroplasms. Parasitology 108:147–152
Atteson K (1999) The performance of the neighbor-joining methods of phylogenetic reconstruction. Algorithmica 25:251–278
Bai Q, Liu G, Liu D, Ren J, Li X (2002) Isolation and preliminary characterization of a large Babesia sp. from sheep and goats in the eastern part of Gansu Province, China. Parasitol Res 88:S16–S21
Chae JS, Allsopp BA, Waghela SD, Park JH, Kakuda T, Sugimoto C, Allsopp MT, Wagner GG, Holman PJ (1999) A study of the systematics of Theileria spp based upon small-subunit ribosomal RNA gene sequences. Parasitol Res 85:877–883
Gubbels MJ, Yin H, Weide M van der, Qi B, Nijman IJ, Guangyuan L, Jongejan F (2000) Molecular characterisation of the Theileria buffeli/orientalis group. Int J Parasitol 30:943–952
Gubbels MJ, Yin H, Bai Q, Liu G, Nijman IJ, Jongejan F (2002) The phylogenetic position of the Theileria buffeli group in relation to other Theileria species. Parasitol Res 88:S28–S32
Herwaldt BL, Kjemtrup AM, Conrad PA, Barnes RC, Wilson M, McCarthy MG, Sayers MH, Eberhard ML (1997) Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J Infect Dis 175:1259–1262
Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH (2000) Babesiosis. Clin Microbiol Rev 13:451–469
Hooshmand-Rad P, Hawa NJ (1973) Transmission of Theileria hirci in sheep by Hyalomma anatolicum anatolicum . Trop Anim Health Prod 5:100–109
Katzer F, McKellar S, Kirvar E, Shiels B (1998) Phylogenetic analysis of Theileria and Babesia equi in relation to the establishment of parasite populations within novel host species and the development of diagnostic tests. Mol Biochem Parasitol 95:33–44
Leemans I, Hooshmand-Rad P, Uggla A (1997) The indirect fluorescent antibody test based on schizont antigen for study of the sheep parasite Theileria lestoquardi. Vet Parasitol 69:9–18
Luo J, Yin H (1997) Theileriosis of sheep and goats in China. Trop Anim Health Prod 29:8S–10S
Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499
Mehlhorn H, Schein E (1984) The piroplasms: life cycle and sexual stages. Adv Parasitol 23:37–103
Mehlhorn H, Schein E, Ahmed JS (1994) Theileria. In: Kreier JP (ed) Parasitic protozoa, vol 7. Academic Press, San Diego, pp 217–304
Persing DH, Kobayashi J M, Juranek DD, Conrad PA (1993) Babesiosis in Washington State: a new species of Babesia? Ann Intern Med 119:284–290
Persing DH, Herwaldt BL, Glaser C, Lane RS, Thomford JW, Mathiesen D, Krause PJ, Phillip DF, Conrad PA (1995) Infection with a Babesia-like organism in northern California. N Engl J Med 332:298–303
Quick RE, Herwaldt BL, Thomford JW, Garnett ME, Eberhard ML, Wilson M, Spach DH, Dickerson JW, Telford SR, Steingart KR, Pollock R, Persing DH, Kobayashi JM, Juranek DD, Conrad PA (1993) Babesiosis in Washington State: a new species of Babesia? Ann Intern Med 119:284–290
Saitou N, Nei M (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schnittger L, Yin H, Jianxun L, Ludwig W, Shayan P, Rahbari S, Voss-Holtmann A, Ahmed JS (2000a). Ribosomal small-subunit RNA gene-sequence analysis of Theileria lestoquardi and a Theileria species highly pathogenic for small ruminants in China. Parasitol Res 86:352–358
Schnittger L, Yin H, Jianxun L, Ludwig W, Shayan P, Rahbari S, Voss-Holtmann A, Ahmed JS (2000b). Phylogenetic analysis by rRNA comparison of the highly pathogenic sheep-infecting parasites Theileria lestoquardi and a Theileria species identified in China. Ann NY Acad Sci 916:271–275
Stoltsz WH, Dunsterville MT (1992) In vitro establishment and cultivation of a Cytauxzoon sp. (Theileria sp.) from a sable antelope (Hippotragus niger Harris, 1838). J S Afr Vet Med Assoc 63:176–186
Takahashi K, Kubota S, Kawai S, Hagiwara K, Kurosawa T, Tajima M, Sonoda M, Maede Y (1992) Babesia and Theileria protozoa detected from wild deer (Cervus nippon yesoensis) in Hokkaido. J Protozool Res 2:158–164
Thomas SE, Wilson DE, Mason TE (1982) Babesia, Theileria and Anaplasma spp infecting sable antelope, Hippotragus niger (Harris, 1838), in southern Africa. Onderstepoort J Vet Res 49:163–166
Thomford JW, Conrad PA, Telford SR, Mathiesen D, Bowman BH, Spielmann A, Eberhard ML, Herwaldt B, Quick RE, Persing DH (1994) Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis 169:1050–1056
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tibayrenc M (1998) Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int J Parasitol 28:85–104
Uilenberg G (1981) Theileria species of domestic livestock. In: Irvin AD, Cunningham MP, Young AS (eds) Advances in the control of theileriosis. Nijhoff, The Hague, pp 4–37
Uilenberg G, Andreasen MP (1974) Haematoxenus separatus sp. n. (Sporozoa, Theileriidae) a new blood parasite of domestic sheep in Tanzania. Rev Elev Med Vet Pays Trop 27:459–465
Yin H, Luo J, Guan G, Gao Y, Lu B, Zhang Q, Ma M, Lu W, Lu C, Yuan Z, Guo S, Wang B, Du H, Schnittger L, Ahmed JS, Jongejan F (2002) Transmission of an unidentified Theileria species to small ruminants by Haemaphysalis qinghaiensis ticks collected in the field. Parasitol Res 88: S25–S27
Yin H, Luo J, Lu B, Beyer D, Ma M, Guan G, Bai Q, Schnittger L, Lu C, Ahmed J (2003) Phylogenetic analysis of Theileria species transmitted by Haemaphysalis qinghaiensis. (in preparation)
Acknowledgements.
This work was supported by European Union INCO-DEV programmes: (1) contract ICA4-CT2000-300028 entitled ″Molecular and immunological characterisation of merozoite antigens and their encoding genes of a Theileria species highly pathogenic for small ruminants in China: application for the development of diagnostics and vaccine″, (2) contract ICB1CT-2000-80004 entitled ″Theileria China antigens: application for the development of diagnostics and vaccines for small ruminants in China. Bursary for Y.H.″ and (3) contract ICA4-CT-2000-30006 entitled ″International consortium on ticks and tick-borne diseases (ICTTS-2)″.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schnittger, L., Yin, H., Gubbels, M.J. et al. Phylogeny of sheep and goat Theileria and Babesia parasites. Parasitol Res 91, 398–406 (2003). https://doi.org/10.1007/s00436-003-0979-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0979-2