Abstract
A novel approach to control strategies for integrated blood-feeding parasite management is in high demand, including the use of biological control agents. The present study aims to determine the efficacy of optimized crude extract of actinomycetes strain LK1 as biological control agent against the fourth-instar larvae of Anopheles stephensi and Culex tritaeniorhynchus (Diptera: Culicidae) and adults of Haemaphysalis bispinosa, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae), and Hippobosca maculata (Diptera: Hippoboscidae). Antiparasitic activity was optimized using the Plackett–Burman method, and the design was developed using the software Design-Expert version 8.0.7.1. The production of the optimized crude actinomycetes LK1 strain extract was performed using response surface methodology to optimize the process parameters of protease inhibitor activity of marine actinobacteria for the independent variables like pH, temperature, glucose, casein, and NaCl at two levels (−1 and +1). The potential actinomycetes strain was identified as Saccharomonas spp., and the metamodeling surface simulation procedure was followed. It was studied using a computer-generated experimental design, automatic control of simulation experiments, and sequential optimization of the metamodels fitted to a simulation response surface function. The central composite design (CCD) used for the analysis of treatment showed that a second-order polynomial regression model was in good agreement with the experimental results at R 2 = 0.9829 (p < 0.05). The optimized values of the variables for antioxidant production were pH 6.00, glucose 1.3 %, casein 0.09 %, temperature 31.23 °C, and NaCl 0.10 %. The LK1 strain-optimized crude extract was purified using reversed-phase high-pressure liquid chromatography, and the isolated protease inhibitor showed antiparasitic activity. The antiparasitic activity of optimized crude extract of LK1 was tested against larvae of A. stephensi (LC50 = 31.82 ppm; r 2 = 0.818) and C. tritaeniorhynchus (LC50 = 26.62 ppm; r 2 = 0.790) and adults of H. bispinosa (LC50 = 106.58 ppm; r 2 = 0.871), R. (B.) microplus (LC50 = 92.96 ppm; r 2 = 0.913), and H. maculata (LC50 = 84.90 ppm; r 2 = 0.857).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a major cause of morbidity and mortality, infecting 300 to 500 million and killing an appraised two million people annually. The difficulty of obtaining an antimalarial drug along traditional lines, because of the highly adaptive character of the malarial parasite, prompts a ceaseless need for new drugs (Laurent and Pietra 2006). Marine organisms have been explored recently. Marine natural products research is a multidisciplinary field where chemists, pharmacologists, and biologists work together to extract, test, and understand the bountiful molecules found in the oceans. Three approaches to control the most important malarial parasite, Plasmodium falciparum, are vaccine development, vector control, and chemotherapy. In mosquito larvae, a proteolytic enzyme is present in the gut which acts in an alkaline medium. After blood meal ingestion, proteases are active only in the posterior midgut. Trypsin is the major primary hydrolytic protease and is secreted into the posterior midgut lumen without activation in the posterior midgut epithelium (Billingsley and Hecker 1991).
Mosquitoes are important vectors of diseases, especially in the tropics. Regulation of mosquito populations to reduce the incidence of diseases like malaria, filariasis, and several arboviruses is important from the public health viewpoint. (Karunaratne and Hemingway 2000). Filariasis is endemic in 17 states and six union territories of India, with about 553 million people at risk of infection (Raju et al. 2010). However, chronic manifestations, such as lymphedema (elephantiasis) and hydrocele, are debilitating and estimated by the World Health Organization to account for nearly five million disability-adjusted life years (WHO 2001).
Japanese encephalitis (JE) is the most important cause of viral encephalitis in Eastern and Southeast Asia. Up to 50,000 cases and 15,000 deaths annually are due to JE especially in the rural areas (Tsai 1997; Solomon 1997). It is highly endemic in few districts of Tamil Nadu, Southern India (Libraty et al. 2002), and the annual incidence and mortality estimates for JE are 30,000–50,000 and 10,000, respectively (Solomon 2004). Anopheles stephensi is the primary vector of malaria in India and other West Asian countries, and malaria remains one of the most prevalent diseases in the tropical world (Govindarajan 2010). Culex tritaeniorhynchus is an important vector of JE in India and Southeast Asian countries (Suman et al. 2008).
Ticks transmit a greater variety of infectious organisms than any other group of bloodsucking arthropods (Daniel 2002). Ticks are responsible for severe losses due to tick worry, blood loss, hide damage, injection of toxins, and diseases transmitted by the parasite (Ducornez et al. 2005). About 40 % of cattle have been found positive for Haemaphysalis bispinosa infestation. H. bispinosa is detected in 7.6 % of examined cattle, 55.4 % of goats, and 13.2 % of pigs (Ghosh et al. 2007). Rhipicephalus (Boophilus) microplus is one of the most widely distributed tick species and constitutes a major problem for the cattle industry in tropical and subtropical regions of the world. In India, around ten million cattle are at risk for tropical theileriosis with an annual economic loss of US $800 million (Brown 1997). Hippobosca maculata is a serious pest of equines in India and is cosmopolitan in distribution (Parashar et al. 1991). Continuous and indiscriminate uses of acaricidal have led to the selection of chemical-resistant ticks along with contamination of the environment and animal products (Graf et al. 2004).
Actinobacterial metabolites have a foremost biotechnological influence from antibiotics to enzyme inhibitors and anticancer agents to various alkaloids. Actinobacteria are universally distributed in terrestrial, freshwater, and marine environments and complicated in the breakdown of organic matter. They form a distinct phylogenetic line in the 16S rDNA tree and have been of major scientific interest in the past decades, with the discovery of a large number of metabolites produced by its diverse genera. The actinomycetes are noteworthy as antibiotic and enzymatic producers; the Streptomyces are especially prolific and produce many antibiotics and other classes of biologically active secondary metabolites.
The larvicidal and pupicidal activities of spinosad from the actinomycete Saccharopolyspora spinosa were tested against A. stephensi and effectively caused larval mortality in both the laboratory and field trials (Prabhu et al. 2011). The actinomycete isolates produced strong larvicidal activity against Anopheles mosquito larvae (Dhanasekaran et al. 2010). Subramaniam et al. (2012) reported that Bacillus thuringiensis has good larvicidal properties against the potent malarial vector A. stephensi. The Bacillus cereus strain showed a significant effect against third-instar larvae of Anopheles subpictus (Chatterjee et al. 2010). The B. cereus 2362 formulation was responsible for increasing the efficacy of lyophilized toxin against the resistant larvae of Culex quinquefasciatus (Poopathi et al. 2009). The bio-efficacy of B. thuringiensis showed the highest mortality rate against fourth-instar larvae of C. quinquefasciatus (Kumar et al. 2012) and against fourth-instar larvae of A. stephensi (Kovendan et al. 2011).
In the present study, we reported a simple, low-cost, and eco-friendly green chemistry approach in optimizing the crude extract of actinomycetes strain LK1 which would be useful in promoting research aiming at the development of a new agent for the control of parasites.
Materials and methods
Insect rearing
A. stephensi and C. tritaeniorhynchus larvae were collected from a rice field and stagnant water area of Melvisharam (12°56′23″ N, 79°14′23″ E) and identified in Zonal Entomological Research Centre, Vellore (12°55′48″ N, 79°7′48″ E), Tamil Nadu, India. To start the colony, the larvae were kept in plastic and enamel trays containing tap water. They were maintained, and all the experiments were conducted, at 27 ± 2 °C and 75–85 % relative humidity under 14:10 light and dark cycles.
Larvicidal bioassay
During the preliminary screening with the laboratory trial, the larvae of A. stephensi and C. tritaeniorhynchus were collected from insect-rearing cages and identified in Zonal Entomological Research Centre, Vellore. One gram of crude extract was first dissolved in 100 mL of double-distilled water (stock solution). From the stock solution, 200 ppm was prepared with dechlorinated tap water. The larvicidal activity was assessed by the procedure of WHO (1996) with some modification and as per the method of Rahuman et al. (2000). For the bioassay test, larvae were taken in five batches of 20 in 249 mL of water and 1.0 mL of LK1 strain-optimized crude extract-desired concentration. The control was set up with distilled water. The numbers of dead larvae were counted after 24 h of exposure, and the percentage of mortality was reported from the average of five replicates. The experimental media in which 100 % mortality of larvae occurs alone were selected for dose–response bioassay.
Parasite collection
The attached adults of H. bispinosa Neumann (Acarina: Ixodidae) and the fly of H. maculata (Diptera: Hippoboscidae) were collected from the softer skin inside the thigh, flanks, abdomen, brisket, and forelegs of naturally infested cattle and sheep. H. maculata adults have a short, straight capitulum and brown-to-cream-colored body. The adult ticks of R. (B.) microplus (Acari: Ixodidae) were collected from naturally infested cows and dogs. The parasites were identified in the Department of Veterinary Parasitology, Madras Veterinary College, Tamil Nadu Veterinary and Animal Sciences University, Chennai, Tamil Nadu.
Impregnated filter paper bioassay test
The optimized crude extracts prepared from LK1 were used in impregnated filter paper tests in sealed glass jars. Ten pairs of adults of H. bispinosa, R. (B.) microplus, and H. maculata were used for the bioassay test in each concentration. A series of filter paper envelopes (Whatman filter paper no. 1, 16 × 20 cm) with micropores were treated with each concentration of extracts. Each envelope was treated with a 3-mL solution uniformly distributed with a pipette on the internal surfaces. Five envelopes were impregnated with each tested solution. The control papers were impregnated with distilled water with five replicates for each individual concentration. The impregnated paper was air-dried for 10 min. The dose–response data were subjected to probit analysis to determine the LC50 value for a 24-h exposure under constant climatic conditions (25 °C; 12:12 h, light/dark cycle) (Nyamador et al. 2010).
Dose–response bioassay
From the stock solution, different concentrations ranging from 4.69 to 500 ppm were prepared. Based on the preliminary screening results, optimized crude extracts prepared from LK1 strain were subjected to dose–response bioassay for antiparasitic activity against the fourth-instar larvae of A. stephensi and C. tritaeniorhynchus and adults of H. bispinosa, R. (B.) microplus, and H. maculata. Mortality was assessed after 24 h, and the percent mortality was reported from the average of five replicates. However, at the end of 24 h, the selected test samples turned out to be equal in their toxic potential.
Sample collection and isolation of marine actinobacteria
The marine samples were collected from the island of Nicobar (9°09′ N, 92°49′ E). The samples were collected in plastic bags and immediately transported to the laboratory. All media were supplemented with a final concentration of 50 μg/mL−1 potassium dichromate and 15 μg/mL−1 nalidixic acid to facilitate the isolation of slow-growing actinobacteria. Potassium dichromate inhibits fungal growth, whereas nalidixic acid inhibits many gram-negative bacteria. The plates were incubated at room temperature (Karthik et al. 2011).
Fermentation medium
The cultures were grown in liquid media that contained soluble starch, casein, KNO3, NaCl, KH2PO4, FeSO4, MgSO4, and CaCO3 in seawater at 50 % and distilled water at 50 %, pH 7.5, and incubated for 7 days in a rotary shaker incubator at 28 °C. After fermentation, the broth was centrifuged at 10,000 rpm for 10 min, and the filtrate was separated (Karthik et al. 2013).
Assay for protease inhibitor activity
A mixture of mercuric chloride, phosphate buffer, and protease solution was added in the reaction mixture. After adjusting the pH to 7.5, casein was added and kept for incubation at 37 °C for 20 min and incubated with 5 % of the TCA. The solution was filtered using Whatman no. 1 filter paper, and the absorbance was taken at 280 nm (Bijina et al. 2011).
Optimization using response surface methodology
Five variables and an optimal design were used in the present study to optimize the process parameters of protease inhibitor activity of marine actinobacteria. The five independent variables were pH (A), temperature (B), glucose (C), casein (D), and NaCl (E). A set of 31 experiments with five variables were conducted. Each variable was set at two levels (−1 and +1). Trace element solution was constantly maintained (Murrell and Barker 2003). The design was developed using the software Design-Expert version 8.0.7.1.
Reversed-phase high-pressure liquid chromatography fractionation of the entire extract
Lyophilized marine actinobacteria extract (2 mg) was dissolved and subjected to reversed-phase high-pressure liquid chromatography (RP-HPLC) on a RP-18-5 (250 × 4.6 mm) column using a linear gradient from 10 to 50 % acetonitrile in 0.1 % trifluoroacetic acid at a flow rate of 0.5 mL/min for 60 min with a UV detector.
Statistical analysis
The average larval mortality data were subjected to probit analysis for calculating LC50 and other statistics at 95 % fiducial limits of upper confidence limit and lower confidence limit, and Chi-square values were calculated using the software developed by Reddy et al. (1992). Results with p < 0.05 were considered statistically significant.
Results
Larvicidal activity of mosquito
In the present study, mosquito larvae were exposed to varying concentrations of crude extracts of LK1 for 24 h. The fourth-instar larval percent mortality of crude extracts of LK1 showed 25, 39, 66, 88, and 100 and 33, 46, 71, 92, and 100 at 12.5, 25, 50, 100, and 200 ppm against A. stephensi and C. tritaeniorhynchus, respectively. The larvicidal activity of optimized crude extracts of LK1 against A. stephensi (LC50 = 31.82 ppm; r 2 = 0.818) and C. tritaeniorhynchus (LC50 = 26.62 ppm; r 2 = 0.790) at the concentration of 200, 100, 50, 25, and 12.5 mg/L, respectively. The control distilled water showed nil mortality in the concurrent assay (Table 1).
Bioassay test for hematophagous parasites
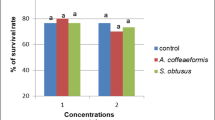
The percent mortality observed in optimized crude extracts of LK1 were 100, 71, 58, 33, 19; 100, 79, 56, 39, 24, and 100, 82, 57, 42, and 32 against H. bispinosa, R. (B.) microplus, and H. maculata, respectively. The crude extracts of LK1 showed maximum activity against the larvae of H. bispinosa (LC50 = 106.58 ppm; r 2 = 0.871), R. (B.) microplus (LC50 = 92.96 ppm; r 2 = 0.913), and H. maculata (LC50 = 84.90 ppm; r 2 = 0.857) at the concentration of 500, 250, 125, 62.5, and 31.2 mg/L, respectively. The control distilled water showed nil mortality in the concurrent assay (Fig. 1).
In the present study, the sediment sample harbored the highest number of actinomycetes population. Thus, the sediment in the crude extracts of LK1 is a good source for enumerating the actinomycetes population. From the total number of actinomycetes, 20 different types of actinomycetes were isolated. All the 20 isolated actinomycetes were tested for protease inhibitor activities whereas three isolates were found to produce protease inhibitor. Out of the three, LK1 showed maximum activity against A. stephensi, C. tritaeniorhynchus, H. bispinosa, R. (B.) microplus, and H. maculata. The protease inhibitor was partially purified (Fig. 2). The entire extract of marine actinobacteria was fractionated by means of RP-HPLC. This is a favorable technique for resolving proteins and peptides with low molecular weight. Most of the serine protease inhibitors described until now are peptides and small extracellular proteins with compact structures, rich in disulfide bonds and resistant to harsh conditions (high amount of acetonitrile and low pH) of RP-HPLC (Yanes et al. 2005). The protease inhibitor was shown at 200 and 119 % against trypsin and chymotrypsin, respectively. The protease inhibitor was partially purified by ion exchange chromatography using DEAE-Sepharose matrix. The potential strain was identified as Saccharomonas spp.
Experimental design for optimization study
A 23-factorial central composite design of response surface methodology with three factors leading to a total of 20 sets per experiment was formulated to optimize the independent variables, temperature, initial pH, and yeast extract. The variables were coded according to the following equation:
where X i is the coded value of an independent variable, X i is the real value of an independent variable, X 0 is the real value of an independent variable at the center point, and ΔX i is the step change value.
Response surface methodology
In the ANOVA for a response surface quadratic model, the pH, temperature, glucose, casein, and NaCl were indicated as A, B, C, D, and E, respectively.
In the present work, all the linear, interactive effects of AB, AC, AD, AE, and DE were significant for protease inhibitor activity. The interaction effects of independent variables on the protease inhibitor were studied by plotting 3D surface curves. The 3D curves of the calculated protease inhibitor activity for the interactions between the variables are shown in Fig. 3a–e. The optimized values of the variables for antioxidant production were shown to be pH 6.00, glucose 1.3 %, casein 0.09 % temperature −31.23 °C, and NaCl −0.10 %. Thus, the above results prove that the optimization of the extract leads to far better larvicidal activity.
Out of three, LK-1 showed maximum activity against A. stephensi, C. tritaeniorhynchus, H. bispinosa, H. maculata, and R. (B.) microplus. The protease inhibitor was partially purified (Fig. 2). The entire extract of marine actinobacteria was fractionated by means of RP-HPLC. This is a favorable technique for resolving low molecular weight proteins and peptides. Most of the serine protease inhibitors described until now are peptides and small extracellular proteins with compact structures, rich in dissolved bonds and resistant to the harsh conditions (high amount of acetonitrile and low pH) of RP-HPLC (Yanes et al. 2005).
Discussion
It is essential to control mosquito population so that people can be protected from mosquito-borne diseases. Therefore, biological control can thus provide an effective and environmentally friendly approach, which can be used as an alternative to minimize the mosquito population. Actinomycetes have provided many important bioactive compounds of high commercial value and continue to be routinely screened for new bioactive substances. These searches have been remarkably successful, and approximately two thirds of naturally occurring antibiotics, including those with medical importance, have been isolated from actinomycetes. Vijayakumar et al. (2010) reported the larvicidal effect of actinobacteria extract tested in different concentrations of the extracts of the isolates CC11 and SH22 highly inhibited the larvae (20 %) followed by CC110 and SH23 (16 %), SH15 (12 %), CC19 and S22 (8 %), and S21 (4 %) for 3 h of inoculation against Anopheles mosquito. The larvicidal effect of actinobacteria extracts was tested in different concentration level such as 2 %. The CC11 showed high inhibited activity (13.3 %) followed by CC17 (6.6 %), CC19, and SM13 (6.6 %) after 3 h of inoculation (Dhanasekaran et al. 2010).
The marine actinobacterial extracts showed moderate to high larvicidal effects after 24 h of exposure at 1,000 ppm, and the highest larval mortality was found in the extract of LK3 against the larvae of Culex gelidus (LC50 = 108.08 ppm) and C. tritaeniorhynchus (LC50 = 146.24 ppm) (Karthik et al. 2011). Singh and Prakash (2012) reported the culture filtrates of Streptomyces citreofluorescens showed the LC50 values of 122.6 and 60.0 μL/mL, respectively, against the fourth-instar larvae of A. stephensi and C. quinquefasciatus. The larvicidal activity of spinosad from the actinomycete S. spinosa was tested against the fourth-instar larvae of A. stephensi with LC50 value of 0.036 ppm (Prabhu et al. 2011). The synthesized gold nanoparticles using Aspergillus niger were tested against the fourth-instar larvae of A. stephensi (LC50 = 12 ppm) and Aedes aegypti (LC50 = 30 ppm), respectively (Soni and Prakash 2012). The two fungal isolates, Paecilomyces fumosoroseus and Beauveria bassiana, were evaluated against C. quinquefasciatus at different spore concentration such as 103 to 108 and 103 to 107, respectively; 100 % mortality was found on the 2nd day of treatment at 107 spore concentration by B. bassiana against C. quinquefasciatus, whereas 97.73 % as the highest mortality was found in P. fumosoroseus at the concentration of 108 on the 8th day (Gayathri et al. 2010).
Saurav et al. (2013) reported the impurified marine actinobacterial compound DMBPO showed LC50 and r 2 values against the larvae of R. microplus (84.31 ppm, 0.889), A. stephensi (88.97 ppm, 0. 817), and C. tritaeniorhynchus (74.95 ppm, 0.781), respectively. Thenmozhi et al. (2013) reported the compounds 1, 2, and 3 and the ethyl acetate extract of Streptomyces VITSTK7 spp. against the larvae of H. bispinosa (LC50 = 1,573.36, 1,333.09, 1,073.29, and 409.71 ppm; r 2 = 0.0.990, 0.934, 0.935, and 0.908), R. microplus (LC50 = 1,877.86, 815.83, 1,631.14, and 441.54 ppm; r 2 = 0.981, 0.926, 0.0970, and 0.915), A. subpictus (LC50 = 273.89, 687.69, 464.75, and 223.83 ppm; r 2 = 0.758, 0.924, 0.841, and 0.902), and C. quinquefasciatus (LC50 = 430.06, 881.59, 777.0, and 195.70 ppm; r 2 = 0.839, 0.859, 0.870, and 0.882), respectively.
The potential activity of three varieties of B. thuringiensis (Dipel 2× (kurstaki), Vectobac (israeliensis), and HD 703 (thuringiensis)) against the soft ticks of Argas persicus with LC50 values of 214.8, 441.6, and 667.4 L g/mL and against the hard ticks of Hyalomma dromedarii with LC50 values of 1,199.9 L, 1,462.0 L, and 1,502.4 g/mL, respectively (Hassanain et al. 1997).
The acaricidal activity of fungal strains Metarhizium anisopliae, Beauveria bassiana, and Lecanicillium psalliotae tested against larvae of Boophilus annulatus showed mortality of 71.5–86.0, 50–96.5, and 70–80 %, respectively (Pirali-kheirabadi et al. 2007). M. anisopliae, a filamentous fungus isolated from the M5 and E6S1 strain, showed mortality of 79 and 53 %, respectively, against B. microplus (Frazzon et al. 2000). M. anisopliae and B. bassiana strains were most virulent to engorged females of B. annulatus and caused 85–100 % mortality within 7–10 days postinoculation (Gindin et al. 2001).
In conclusion, the present investigation clearly reveals the biodiversity and distribution of actinomycetes in marine sources and their larvicidal potentials of LK1 strain. To exploit these findings for human welfare, it is necessary to conduct field trails and strategies for the optimization of large-scale production of both cell biomass and larvicidal compounds. This investigation explores the importance of larivicidal actinomycetes as a valuable resource for the discovery of a novel insecticide.
References
Bijina B, Chellappan S, Krishna JG, Basheer SM, Elyas K, Bahkali AH, Chandrasekaran M (2011) Protease inhibitor from Moringa oleifera with potential for use as therapeutic drug and as seafood preservative. Saudi J Bio Sci 18(3):273–281
Billingsley PF, Hecker H (1991) Blood digestion in the mosquito, Anopheles stephensi Liston (Diptera: Culicidae): activity and distribution of trypsin, aminopeptidase, and alpha-glucosidase in the midgut. J Med Entomol 28(6):865–871
Brown CG (1997) Dynamics and impact of tick-borne diseases of cattle. Trop Anim Health Prod 29(4):1–3
Chatterjee S, Ghosh TS, Das S (2010) Virulence of Bacillus cereus as natural facultative pathogen of Anopheles subpictus Grassi (Diptera: Culicidae) larvae in submerged rice-fields and shallow ponds. Af J Biotechnol 9(41):6983–6987
Daniel S (2002) Ticks. In: Encyclopedia of pest management (Print). CRC Press, Boca Raton. doi:10.1201/NOE0824706326.ch395
Dhanasekaran D, Sakthi V, Thajuddin N, Panneerselvam A (2010) Preliminary evaluation of Anopheles mosquito larvicidal efficacy of mangrove actinobacteria. IJABPT 1(2):374–381
Ducornez S, Barre N, Miller RJ, Garine-Wichatitsky M (2005) Diagnosis of amitraz resistance in Boophilus microplus in New Caledonia with the modified Larval Packet Test. Vet Parasitol 130(4):285–292
Frazzon APG, Junior ISV, Masuda A, Schrank A, Vainstein MH (2000) In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Vet Parasitol 94:117–125
Gayathri G, Balasubramanian C, Moorthi PV, Kubendran T (2010) Larvicidal potential of Beauveria bassiana (Balsamo) Vuillemin and Paecilomyces fumosoroseus (Wize,) Brown and Smith on Culex quinquefasciatus (Say). J Biopest 3(1):147–151
Ghosh S, Bansal GC, Gupta SC, Ray D, Khan MQ, Irshad H, Shahiduzzaman M, Seitzer U, Ahmed JS (2007) Status of tick distribution in Bangladesh, India and Pakistan. Parasitol Res 101(2):207–216
Gindin G, Samish M, Alekseev E, Glazer I (2001) The susceptibility of Boophilus annulatus (Ixodidae) ticks to entomopathogenic fungi. Biocontrol Sci Technol 11(1):111–118
Govindarajan M (2010) Larvicidal and repellent activities of Sida acuta Burm. F. (Family: Malvaceae) against three important vector mosquitoes. Asian Pac J Trop Med 1:691–695
Graf JF, Gogolewski R, Leach-Bing N, Sabatini GA, Molento MB, Bordin EL, Arantes GJ (2004) Tick control: an industry point of view. Parasitol 129:427–442
Hassanain MA, el Garhy MF, Abdel-Ghaffar FA, el-Sharaby A, Abdel Megeed KN (1997) Biological control studies of soft and hard ticks in Egypt. I. The effect of Bacillus thuringiensis varieties on soft and hard ticks (Ixodidae). Parasitol Res 83(3):209–213
Karthik L, Gaurav K, Rao KV, Rajakumar G, Rahuman AA (2011) Larvicidal, repellent, and ovicidal activity of marine actinobacteria extracts against Culex tritaeniorhynchus and Culex gelidus. Parasitol Res 108(6):1447–1455
Karthik L, Kumar G, Bhaskara Rao KV (2013) Antioxidant activity of newly discovered lineage of marine actinobacteria. Asian Pac J Trop Med 6(4):325–332
Karunaratne SH, Hemingway J (2000) Insecticide resistance spectra and resistance mechanisms in populations of Japanese encephalitis vector mosquitoes, Culex tritaeniorhynchus and Cx. gelidus, in Sri Lanka. Med Vet Entomol 14(4):430–436
Kovendan K, Murugan K, Vincent S, Kamalakannan S (2011) Larvicidal efficacy of Jatropha curcas and bacterial insecticide, Bacillus thuringiensis, against lymphatic filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 109(5):1251–1257
Kumar MP, Murugan K, Kovendan K, Subramaniam J, Amaresan D (2012) Mosquito larvicidal and pupicidal efficacy of Solanum xanthocarpum (Family: Solanaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 110(6):2541–2550
Laurent D, Pietra F (2006) Antiplasmodial marine natural products in the perspective of current chemotherapy and prevention of malaria: a review. Mar Biotechnol, New York 8(5):433–447
Libraty DH, Nisalak A, Endy TP, Suntayakorn S, Vaughn DW, Innis BL (2002) Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg 96(2):173–178
Murrell A, Barker SC (2003) Synonymy of Boophilus Curtice, 1891 with Rhipicephalus Koch, 1844 (Acari: Ixodidae). Syst Parasitol 56(3):169–172
Nyamador WS, Ketoh GK, Amévoin K, Nuto Y, Koumaglo HK, Glitho IA (2010) Variation in the susceptibility of two Callosobruchus species to essential oils. J Stor Prod Res 46(1):48–51
Parashar BD, Gupta GP, Rao KM (1991) Control of the haematophagous fly Hippobosca maculata, a series pest of equines, by deltamethrin. Med Vet Entomol 5(3):363–367
Pirali-kheirabadi KH, Haddadzadeh HR, Razzaghi-Abyaneh M, Zare R, Ranjbar-Bahadori SH, Rahbari S, Nabian S, Rezaeian M (2007) Preliminary study on virulence of some isolates of entomopathogenic fungi in different developmental stage of Boophilus annulatus in Iran. Iranian J Vet Res 62:113–118
Poopathi S, Ramesh N, Sundaravadivelu K, Samuel PP, Tyagi BK (2009) Larvicidal efficacy of various formulations of Bacillus sphaericus against the resistant strain of Culex quinquefasciatus (Diptera: Culicidae) from southern India. Trop Biomed 26(1):23–29
Prabhu K, Murugan K, Nareshkumar A, Bragadeeswaran S (2011) Larvicidal and pupicidal activity of spinosad against the malarial vector Anopheles stephensi. Asian Pacif J Trop Med 610–613
Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B (2000) Effect of Feronia limonia on mosquito larvae. Fitoterapia 71(5):553–555
Raju K, Jambulingam P, Sabesan S, Vanamail P (2010) Lymphatic filariasis in India: epidemiology and control measures. J Postgrad Med 56(3):232–238
Reddy PJ, Krishna D, Murty US, Jamil K (1992) A microcomputer FORTRAN program for rapid determination of lethal concentrations of biocides in mosquito control. Comput Appl Biosci 8(3):209–213
Saurav K, Rajakumar G, Kannabiran K, Rahuman AA, Velayutham K, Elango G, Kamaraj C, Zahir AA (2013) Larvicidal activity of isolated compound 5-(2,4-dimethylbenzyl) pyrrolidin-2-one from marine Streptomyces VITSVK5 sp. against Rhipicephalus (Boophilus) microplus, Anopheles stephensi, and Culex tritaeniorhynchus. Parasitol Res 112(1):215–226
Singh G, Prakash S (2012) Lethal effect of Streptomyces citreofluorescens against larvae of malaria, filaria and dengue vectors. Asian Pac J Trop Med 5(8):594–597
Solomon T (1997) Viral encephalitis in southeast Asia. Neurol Infec Epidemiol 2:191–199
Solomon T (2004) Flavivirus encephalitis. N Engl J Med 351(4):370–378
Soni N, Prakash S (2012) Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Rep Parasitol 2:1–7
Subramaniam J, Murugan K, Kovendan K (2012) Larvicidal and pupicidal efficacy of Momordica charantia leaf extract and bacterial insecticide, Bacillus thuringiensis against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). J Biopest 5:163–169
Suman DS, Shrivastava AR, Parashar BD, Pant SC, Agrawal OP, Prakash S (2008) Scanning electron microscopic studies on egg surface morphology and morphometrics of Culex tritaeniorhynchus and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 104:173–176
Thenmozhi M, Gopal JV, Kannabiran K, Rajakumar G, Velayutham K, Rahuman AA (2013) Eco-friendly approach using marine actinobacteria and its compounds to control ticks and mosquitoes. Parasitol Res 112(2):719–729
Tsai T (1997) Factors in the changing epidemiology of Japanese encephalitis and West Nile fever. In: Factors in the emergence of arbovirus diseases. Elsevier, Paris, pp 179–189
Vijayakumar R, Murugesan S, Cholarajan A, Sakthi V (2010) Larvicidal Potentiality of Marine Actinomycetes Isolated from Muthupet Mangrove, Tamilnadu, India. Inter J Microbiol Res 1(3):179–183
WHO (1996) Report of the WHO informal consultation on the evaluation and testing of insecticides. WHO/HQ, Geneva
WHO (2001) Lymphatic filariasis. Weekly Epidemiological Record 20(76):149–156
Yanes O, Villanueva J, Querol E, Aviles FX (2005) Functional screening of serine protease inhibitors in the medical leech Hirudo medicinalis monitored by intensity fading MALDI-TOF MS. Mol Cell Proteomics 4(10):1602–1613
Acknowledgments
The authors wish to thank the management of VIT University for providing the necessary facilities required to perform this study. The authors are grateful to C. Abdul Hakeem College management, the Principal, and HOD of the Zoology Department for their help and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Loganathan, K., Kumar, G., Kirthi, A.V. et al. Entomopathogenic marine actinomycetes as potential and low-cost biocontrol agents against bloodsucking arthropods. Parasitol Res 112, 3951–3959 (2013). https://doi.org/10.1007/s00436-013-3585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3585-y