Abstract
Coccidiosis is one of the most important protozoan diseases and inflicts severe economic losses on the poultry industry. The aim of this study was to evaluate the capacity of Bacillus Calmette–Guerin (BCG) to deliver apical membrane antigen1 (AMA1) of Eimeria maxima to stimulate specific cellular and humoral immune responses in chickens. Day-old birds were immunized twice with rBCG/pMV261-AMA1, rBCG/pMV361-AMA1, or BCG via oral, intranasal, and subcutaneous routes and then orally challenged with homologous E. maxima sporulated oocysts. Gain of body weight, fecal oocyst output, lesion scores, serum antibody responses, numbers of splenocyte CD4+ and CD8+ T cells, and gut cytokine transcript levels were assessed as measures of protective immunity. Challenge experiments demonstrated that rBCG vaccination via intranasal or subcutaneous routes could increase weight gain, decrease intestinal lesions, and reduce fecal oocyst shedding, and the subcutaneous and intranasal routes were superior to the oral route based on the immune effects. Furthermore, intranasal rBCG immunization could also lead to a significant increase in serum antibody, the percentage of CD4+ and CD8+ T lymphocyte cells, and the levels of IL-1β, IFN-γ, IL-15, and IL-10 mRNAs compared with the control group. These results suggested that intranasal rBCG immunization could induce a strong humoral and cellular response directed against homologous E. maxima infection. This study provides data for the use of rBCG to develop a prophylactic vaccine against coccidiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chicken coccidiosis, one of the most important poultry enteric diseases caused by members of the genus Eimeria, has an enormous impact on worldwide poultry production because of the morbidity, mortality, and reduced body weight gain caused by infection (Beattie et al. 2001; Xu et al. 2008; Shirley et al. 2004; Del Cacho et al. 2012). The current control strategy against coccidiosis in poultry is dominated by prophylactic application of anticoccidial drugs, and, to a certain extent, live vaccines (Dalloul and Lillehoj 2005). However, increasing government regulations and bans on the use of coccidiostats, the appearance of multidrug-resistant strains of Eimeria, concerns over drug residues in poultry products, the lack of new pipeline products, and disadvantages of live vaccines including the potential reversion to virulence, an early reduction in weight gain, and variable efficacy between batches suggest that new approaches are required (Blake et al. 2011; Basak et al. 2006; Jang et al. 2013; Lee et al. 2010a; Ding et al. 2008). Although several attempts have been made to develop subunit vaccines or DNA vaccines, there is no available vaccine against coccidiosis (Li et al. 2012; Liu et al. 2013; Song et al. 2010). A recent approach using live bacteria as carriers to deliver and express Eimeria antigens with the long-term aim of controlling avian coccidiosis shows great potential for large-scale control of infectious diseases in the livestock industry (McDonald and Shirley 2009; Yang et al. 2010).
The mechanism of protective immunity against Eimeria infection is largely dependent on cell-mediated immunity (Rose and Hesketh 1982; Dalloul and Lillehoj 2005; Lillehoj 1998; Ma et al. 2011), indicating that vaccines should preferentially elicit cellular immune responses. Compared with subunit vaccines, viable bacterial carriers such as Bacillus Calmette–Guerin (BCG) elicit potent Th1-mediated immune responses and require no additional adjuvant component in their formulation to evoke protective immunity, as demonstrated in several animal models of infectious disease (Santangelo et al. 2007). Mycobacterium bovis BCG, an attenuated M. bovis strain, is particularly attractive for the delivery of heterologous antigens based on its remarkable safety record and intrinsic adjuvant properties (Santangelo et al. 2007; Supply et al. 1999; Stover et al. 1991; Dietrich et al. 2003; Bastos et al. 2009). Since both M. bovis BCG and Eimeria spp. are intracellular microorganisms, we rationalized that recombinant BCG would be appropriate for the development of a vaccine against coccidiosis (Supply et al. 1999).
Apical membrane antigen1 (AMA1), secreted by micronemes, was first identified as a conserved antigenic protein in the malaria parasite Plasmodium knowlesi and has been widely proposed as an anti-apicomplexan vaccine candidate (Richie and Saul 2002; Zhang et al. 2007; Zhang et al. 2010; Remarque et al. 2008). AMA1 from Plasmodium falciparum induced protective immunity against parasite challenge in animal models (Yoshida et al. 2010), and Neospora caninum AMA1, encapsulated in liposomes, induced a parasite-specific Th1 immune response in pregnant mice and decreased offspring mortality (Zhang et al. 2010). Immunization using E. maxima AMA1 as a DNA vaccine in the eukaryotic expression vector pcDNA3.1(+) or as a bacterially expressed recombinant protein induced significant immune protection against subsequent challenge by E. maxima (Blake et al. 2011). Here, we constructed two recombinant (r)BCG strains (pMV261-AMA1 and pMV361-AMA1) expressing AMA1 gene of E. maxima and evaluated their protective efficacy for resistance to homologous challenge.
Materials and methods
Bacteria, chickens, and parasites
M. bovis BCG were cultured in Middlebrook 7H9 (Difco Laboratories, Detroit, MI, USA) liquid medium supplemented with 0.5 % glycerol, 0.05 % Tween-80, and 10 % ADC (same as OADC) or on solid Middlebrook 7H10 medium supplemented with OADC enrichment. One-day-old specific pathogen-free chickens were obtained from the Center of Laboratory Animals in Jilin Province, reared in a coccidian-free environment in cages, and provided with feed and water ad libitum. All experimental procedures were approved by the ethics committee on the use and care of animals, Jilin University (Changchun, China). The wild-type strain of E. maxima, kindly provided by Dr Jianping Tao, College of Veterinary Medicine, Yangzhou University, China, was maintained in our laboratory (Fig. S1). Sporulated oocysts were purified from feces and enumerated using the McMaster method before experimental infection (Ding et al. 2004).
Construction of pMV261-AMA1 and pMV361-AMA1 plasmid
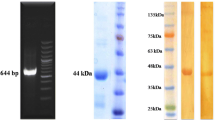
AMA1 coding nucleotides 70–1,338 were amplified from E. maxima sporozoite cDNA by PCR with primers 261 F/R and 361 F/R (Table 1) and cloned into the expression vector pMV261 and pMV361 (Stover et al. 1991) using BamHI/HindIII and MunI/HindIII restriction sites, respectively. The plasmids pMV261-AMA1 and pMV361-AMA1 were transformed into BCG by electroporation and selected by kanamycin (Sigma-Aldrich, USA).
Expression of recombinant AMA1 protein in BCG
Cultivation and induced expression of rBCG were performed as described previously (Miyaji et al. 2001; Wang et al. 2007; Wang et al. 2009). Briefly, after transformation, 800 μl of 7H9 liquid medium containing 10 % OADC was added to the mixture followed by incubation at 37 °C for 3 h. The bacteria were then plated in 7H10 solid medium supplemented with 10 % OADC and 20 μg/ml of kanamycin. After 3–4 weeks, individual colonies were transferred to liquid medium for verification of the viral protein expression. M. bovis BCG was harvested at mid-log phase by centrifugation at 5000×g for 10 min. For expression analysis, rBCG cells were inducted at 45 °C for 2 h, and disrupted on ice with ultrasound sonication. Total cellular lysate was electrophoresed in a 12 % SDS-polyacrylamide gel. Subsequently, the proteins were transferred to nitrocellulose filters and probed with mouse anti-E. maxima polyclonal antibody (1:1,000) and goat–anti-mouse IgG HRP conjugated antibody(1:2,000; Dingguo Changsheng Biotech Co., Ltd., Beijing).
Immunization and parasite-challenge infection
The experimental design is summarized in Table 1 and Fig. 1. At 7 days of age, birds were randomly divided into 11 groups (25 birds/group) and orally, intranasally, or subcutaneously immunized with rBCG pMV261-AMA1 (groups 6–8), rBCG pMV361-AMA1 (groups 9–11), or BCG (groups 3–5). Control birds were orally immunized with phosphate-buffered saline (PBS) (groups 1–2). At 7 days post-immunization, animals were given a booster immunization identical to the primary immunization. At 7 days post-secondary immunization, all birds except the unchallenged control group were challenged orally with 5 × 104 E. maxima sporulated oocysts. Unchallenged control chickens were administered PBS orally.
Body weight gain, fecal oocyst shedding, and lesion scores
Body weight gain in each group was assessed between 0 and 10 days post-challenge infection with or without E. maxima oocysts. For determination of fecal parasite shedding, fecal samples were collected daily between 5 and 10 days postinfection, and oocysts were individually enumerated using a McMaster counting chamber as previously described (Ding et al. 2004). Lesion scores were observed and recorded at 6 days postinfection on a scale of 0 (none) to 4 (high) in a blinded fashion by two independent observers as previously described (Johnson and Reid 1970).
Serum antibody levels
Blood was collected from the wing vein of birds (five/group) of groups immunized intranasally with rBCG, BCG, or PBS (Table 1) at 0, 1, 2, 3, and 4 weeks after primary immunization. Sera were collected by low-speed centrifugation and anti-AMA1 antibodies were measured by ELISA in sera, as described previously (Lillehoj et al. 2005). Briefly, microtiter plates were coated with E. maxima antigen (10 mg/ml) overnight at 4–8 °C, washed with PBS containing 0.05 % Tween-20, and blocked with 10 % nonfat dry milk in PBS. Serial dilutions of sera were added, incubated with continuous shaking, the plates were washed, and bound antibody detected with peroxidase-conjugated rabbit anti-chicken IgG (Sigma-Aldrich, USA) and peroxidase-specific substrate. After color development, the reaction was stopped by addition of 2 M sulfuric acid, and optical density values at 450 nm (OD450) were determined by ELISA microplate reader (Bio-Rad, USA).
Flow cytometric analysis
The evaluation of cellular immunity was analyzed as previously described (Wang et al. 2009; Liu et al. 2013). Birds (five/group) in groups immunized intranasally with rBCG, BCG, or PBS were euthanized randomly at 7 days post-secondary immunization and their spleens removed aseptically. Single splenocyte suspensions (1 × 106 cells/ml) were dually stained with R-phycoerythrin-conjugated mouse anti-chicken CD4 antibody (0.1 mg/ml) and fluorescein-conjugated mouse anti-chicken CD8α antibody (0.5 mg/ml; Southern Biotech Associates, Inc.) for 30 min at room temperature in the dark. The percentages of splenocyte CD4+ and CD8+ cells, resuspended in a fluorescence preservative fluid, were analyzed by flow cytometry (BD Biosciences, USA).
Quantification of cytokine mRNA levels
Intestinal tissues from birds (three/group) in groups immunized intranasally with rBCG, BCG, or PBS were collected between the jejunum and the ileum at 7 days post-secondary immunization, cut longitudinally, and washed three times with ice-cold Hank's balanced salt solution. Total RNA was extracted from the intestinal tissues using Trizol™ reagent (Invitrogen, USA). After DNase I treatment, RNAs were reverse-transcribed using PrimeScript™ RT-PCR kit (Takara, China) according to the manufacturer's instructions. Quantitative RT-PCR oligonucleotide primers for chicken interleukin (IL)-1β, IL-15, IL-10, interferon (IFN)-γ, and GAPDH control (Table 2) were designed as described (Hong et al. 2006). PCR amplification and detection were performed using equivalent amounts of total RNA using the ABI7500 system and SYBR premix EX Taq™ (Takara, China). Each analysis was performed in triplicate. Relative expression levels for each target gene were determined by the comparative cycle threshold (CT) method (using the formula 2_∆∆CT, ∆∆CT = (CT.Target − CT.GAPDH)Treatment − (CT.Target − CT.GAPDH)Control) (Pfaffl 2001).
Statistical analysis
Statistical analysis was performed by SPSS 15.0 software. Data were expressed as mean ± standard error, and Duncan's multiple range test was used to analyze differences between the mean values. Differences between groups were considered statistically significant when p values were less than 0.05.
Results
Expression of AMA1 in BCG
The plasmids, pMV261-AMA1 and pMV361-AMA1, were verified by restriction enzyme digestion and PCR and were then electrotransfected into BCG. The individual colonies of M. bovis BCG on the solid medium were typical (Fig. S2). rBCG expressing AMA1 were subjected to Western blotting and probed with polyclonal mouse antibodies. Specific immunoreactive proteins were detected in the cell lysates of rBCG/pMV261-AMA1 and rBCG/pMV361-AMA1, whereas they were absent in BCG (Fig. 2). Thus, the AMA1 protein was successfully expressed in rBCG/pMV261-AMA1 and rBCG/pMV361-AMA1.
Effect of rBCG-AMA1 vaccination on body weight gain, fecal oocyst shedding, and lesion scores
The challenged control group exhibited significantly reduced weight gain between 0 and 10 days postinfection compared with the unchallenged control group indicative of active intestinal disease (p < 0.05, Table 1). The challenged control group exhibited significantly reduced weight gain compared with groups immunized with rBCG (except for the group vaccinated orally with rBCG/pMV261-AMA1) (p < 0.05), but identical weight gain to groups immunized with BCG (p > 0.05). Birds immunized with rBCG via intranasal or subcutaneous routes exhibited similar increased body weight gain, both greater compared with groups immunized with BCG (p < 0.05). The weight gain in groups immunized with rBCG by intranasal or subcutaneous routes were higher than in groups immunized orally with rBCG. No difference was noted between rBCG intranasal immunization group and rBCG subcutaneous immunization group (p > 0.05).
The oocyst counts of birds in all groups vaccinated with rBCG were decreased and were significantly lower in groups vaccinated with rBCG via intranasal or subcutaneous routes than the challenged control or BCG immunization groups (p < 0.05, Table 1). The lowest fecal oocyst shedding was recorded in the group vaccinated subcutaneously with rBCG/pMV261-AMA1. Numbers of fecal oocysts in groups vaccinated with BCG showed no obvious differences compared with the challenged control (p > 0.05). No differences were observed between the rBCG intranasal immunization group and rBCG subcutaneous immunization group.
Although all rBCG formulations decreased intestinal lesions, this was only significant in groups vaccinated with rBCG/pMV261-AMA1 by intranasal or subcutaneous routes or the group vaccinated with rBCG/pMV361-AMA1 via intranasal route when compared with the challenged control or BCG immunization groups (p < 0.05, Table 1). Surprisingly, animals vaccinated with rBCG/pMV361-AMA1 via subcutaneous route demonstrated little reduction in fecal oocyst shedding.
Effect of rBCG-AMA1 vaccination on anti-AMA1 antibody responses
The humoral immune response to AMA1 was monitored by ELISA at 0, 1, 2, 3, and 4 weeks after primary immunization (Fig. 3). No obvious antibody response was detected at 0 and 1 week post-primary immunization with rBCG. However, a significant increase in mean absorbance values was observed from the second week, in birds immunized with rBCG/pMV261-AMA1 compared with other groups (p < 0.05). The antibody levels of birds immunized with rBCG /pMV361-AMA1 increased slower compared with birds immunized with rBCG/pMV261-AMA1 at 2 weeks post-primary immunization, but there was no significant difference between rBCG/pMV261-AMA1 and pMV361-AMA1 groups (p < 0.05). The antibody titers of rBCG/pMV261-AMA1 and rBCG/pMV361-AMA1 groups gradually increased during weeks 2–4. From 0–4 weeks, no significant differences in antibody levels were noted between the BCG and control groups.
Effect of vaccination with rBCG/pMV261-AMA1 and pMV361-AMA1 on AMA1 antibody levels. Birds were intranasally immunized with BCG, rBCG/pMV261-AMA1, rBCG/pMV361-AMA1, or PBS (Table 1), and sera were collected and analyzed by ELISA at 0, 1, 2, 3, and 4 weeks post-primary immunization. Each bar represents the mean ± SE value (n = 5). Bars not sharing the same letters are significantly different according to Duncan's multiple range test (p < 0.05)
Effect of rBCG-AMA1 vaccination on cellular immune reactions
One week after the secondary immunization, splenocytes from birds vaccinated with rBCG or BCG and controls were analyzed for a phenotype. The proportions of splenocyte subsets in each group are shown in Table 3. Compared with the control group (CD4+ 14.98 % ± 0.39 and CD8+ 24.85 % ± 0.93), all vaccinated groups (BCG and rBCG) induced significantly greater percentages of CD4+ and CD8+ cells (p < 0.05), especially in the rBCG/pMV261-AMA1 group.
Effect of rBCG-AMA1 vaccination on intestinal cytokine transcript levels
The levels of transcripts encoding proinflammatory (IL-1β), Th1 (IFN-γ and IL-15), or Th2 (IL-10) cytokines in intestinal tissues between the jejunum and the ileum were measured following rBCG immunization in noninfected birds. All four cytokines showed significantly higher mRNA levels in the two groups immunized with rBCG compared with the controls (p < 0.05). The levels of transcripts encoding IL-1β in the two rBCG immunization groups were increased more than threefold compared with other groups (rBCG/pMV261-AMA1, 4.14 ± 0.55-fold and rBCG/pMV361-AMA1, 4.29 ± 0.22-fold; p < 0.05; Fig. 4a). IFN-γ mRNA transcript levels were 3.40–4.83-fold increased in the two rBCG immunization groups (Fig. 4b). IL-15 transcript levels were significantly upregulated (4.48–5.37-fold) following rBCG immunization (Fig. 4c). IL-10 mRNA transcripts increased (2.13–2.49-fold) following rBCG immunization (Fig. 4d).
Effect of rBCG/pMV261-AMA1 and pMV361-AMA1 vaccination on intestinal cytokine transcript levels. Birds were immunized intranasally with BCG, rBCG/pMV261-AMA1, rBCG /pMV361-AMA1, or PBS (Table 1). a IL-1β, b IFN-γ, c IL-15, and d IL-10 mRNA levels were determined by quantitative RT-PCR at 7 days post-secondary immunization. Each bar represents the mean ± SE value from triplicate samples/bird (n = 3). Bars not sharing the same letters are significantly different according to Duncan's multiple range test (p < 0.05)
Discussion
Despite advances in controlling coccidiosis, it remains one of the leading causes of morbidity and mortality of poultry (Zhang et al. 2012; Awad et al. 2013). The development of a protective vaccine against this disease, although challenging, is desirable for decreasing its negative economic impact and for improving the welfare of poultry (Konjufca et al. 2008). For a successful vaccine, an effective antigen delivery system is essential. The use of live recombinant BCG as a platform for vaccine development against parasitic diseases is highly attractive, given its proven ability to induce robust immune responses to numerous antigens from apicomplexan parasites (Santangelo et al. 2007; Wang et al. 2009; Supply et al. 1999; Wang et al. 2007). In the present study, we designed and transformed BCG using an extrachromosomal vector pMV261 and an integrated vector pMV361 to evaluate the immunogenicity of an E. maxima antigen, AMA1, expressed in rBCG. The results demonstrated that (1) the subcutaneous and intranasal immunization routes were superior to the oral route for stimulating protective immunity against experimental E. maxima infection based on weight gain, fecal oocyst shedding, and intestinal lesions, and (2) birds in the intranasally rBCG immunized group developed a strong humoral and cellular response directed against E. maxima infection.
The measured parameters of resistance to avian coccidiosis were augmented weight gain, reduced fecal oocyst shedding, and decreased intestinal lesions in coccidia-infected birds. Weight gain has been shown to be the most useful criterion for evaluating the efficacy of anticoccidial drugs during the acute phase of infection (Li et al. 2005). We observed a significant increase in weight gain, post-challenge, among birds vaccinated with rBCG via intranasal or subcutaneous routes compared with the challenged control, BCG immunization groups, or rBCG oral immunization groups (p < 0.05). The positive effect on body weight gain by subcutaneous or intranasal immunization with rBCG indicated the mode of action of rBCG vaccine might include influencing intestinal physiology, increasing nutrient absorption, and/or preventing Eimeria cytotoxicity (Lee et al. 2008; Lee et al. 2010b). The oocyst counts in groups vaccinated subcutaneously or intranasally with rBCG were significantly decreased compared with the challenge control or BCG controls (p < 0.05). Birds vaccinated with rBCG via the intranasal and subcutaneous route (except for the rBCG/pMV361-AMA1 subcutaneously vaccinated group) had decreased intestinal lesions compared with the challenged control or BCG controls (p < 0.05). According to the three measured parameters, we compared the immune effects of the three immunization routes and found that the subcutaneous and intranasal immunization routes were superior to the oral route. The slight effect of oral immunization may partly correlate with the small amounts of rBCG taken up by intestinal cells that could not efficiently activate immune defense mechanisms against coccidiosis, possibly due to the effect of gastric acidity and enzymatic lysis on rBCG. Because the intranasal immunization route with rBCG elicited disease protection similar to that by subcutaneous vaccination in this study, intranasal immunization would offer a practical means for large-scale commercial vaccination efforts (Jang et al. 2011).
In the current study, AMA1-reactive antibodies, numbers of splenocyte CD4+ and CD8+ T cells, and intestinal cytokine transcript levels were used as indicators of acquired immunity following experimental E. maxima infection. T cell immunity is thought to play a dominant role in protection against Eimeria infection (Lillehoj and Lillehoj 2000). However, Eimeria-specific antibodies might neutralize Eimeria during the extracellular stages of its life cycle (Lillehoj 1987). We observed that antibody levels of chickens immunized with rBCG gradually increased after 2 weeks post-primary immunization and were higher than the other groups, similar to that observed by Wang et al. (2009). Considering that Eimeria AMA1 was identified in all four different developmental stages of Eimeria tenella, we suspect that anti-AMA1 antibodies might inhibit cell invasion and replication by binding to sporozoite, merozoite, and other extracellular stages of parasites (Jiang et al. 2012). In our study, all vaccinated groups (BCG and rBCG) induced significantly greater percentages of CD4+ and CD8+ cells, compared with the control group (p < 0.05). These observations provided strong evidence demonstrating that rBCG could induce cell-mediated and humoral immunity in birds.
Gut cytokines synthesized and secreted following Eimeria infection, including proinflammatory Th1-type, and Th2-type cytokines, have important regulatory roles in cellular responses (Hong et al. 2006; Lillehoj et al. 2004). IL-1β is a proinflammatory cytokine secreted by many different cell types including macrophages, monocytes, and dendritic cells. Changes in IL-1β mRNA production observed in this study are similar to those reported for Salmonella infection (Withanage et al. 2004). IFN-γ is a dominant cytokine elicited early during infection with Eimeria parasites, which exerts a direct inhibitory effect on intracellular parasite development (Hong et al. 2006). In the current study, IFN-γ gene transcripts were significantly increased in birds vaccinated with rBCG compared with controls (p < 0.05) and is in agreement with a previous report indicating the absence of a positive association between endogenous IFN-γ production in gut epithelia and improved body weight gain and decreased oocyst shedding in Eimeria-infected birds (Lillehoj et al. 2005). IL-15 is a structurally homologous Th1 or Th1-related cytokine produced by mononuclear phagocytes and other cell types in response to viral or protozoan infection. In the current study, transcription of IL-15 was upregulated (4.48–5.37-fold) following rBCG vaccination. Elevated IFN-γ and IL-15 mRNA levels indicate that host T lymphocyte responses are augmented at earlier time points following rBCG vaccination. Of note, rBCG vaccination also increased the transcripts levels of IL-10, a suppressor cytokine produced by a novel subset of T cells that inhibits the synthesis of proinflammatory cytokines, which could prevent tissue damage as a consequence of uncontrolled intestinal inflammation later in infection (Rothwell et al. 2004). Therefore, elevated transcripts levels of IFN-γ, IL-15, IL-1β, and IL-10 following rBCG immunization appear to maintain the natural balance of pro- and antiinflammatory pathways in the gut that are vital for effective cellular immune responses against invading parasites, while maintaining tissue homeostasis (Jang et al. 2013).
In conclusion, this study demonstrated humoral and cellular immune responses that were elicited by rBCG vaccine carrying the AMA1 gene and provided encouragement for continued research into the development of rBCG as a convenient and practical method to control coccidiosis. It is worth noting that in common with all other live recombinant vaccine systems, a number of technical aspects, including optimal immunization routes, doses, times, underlying immune mechanisms, and bio-safety of rBCG, have to be reassessed to enhance the protective efficacy against infection.
References
Awad AM, El-Nahas AF, Abu-Akkada SS (2013) Evaluation of the protective efficacy of the anticoccidial vaccine coccivac-B in broilers when challenged with Egyptian field isolates of E. Tenella. Parasitol Res 112:113–121
Basak SC, Lee S, Barta JR, Fernando MA (2006) Differential display analysis of gene expression in two immunologically distinct strains of Eimeria maxima. Parasitol Res 99:28–36
Bastos RG, Borsuk S, Seixas FK, Dellagostin OA (2009) Recombinant mycobacterium bovis BCG. Vaccine 27:6495–6503
Beattie SE, Fernando MA, Barta JR (2001) A comparison of sporozoite transport after homologous and heterologous challenge in chickens immunized with the Guelph strain or the Florida strain of Eimeria maxima. Parasitol Res 87:116–121
Blake DP et al (2011) Genetic mapping identifies novel highly protective antigens for an apicomplexan parasite. PLoS Pathog 7:e1001279
Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Disease 49:1–8
Del Cacho E et al (2012) Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infect Immun 80:1909–1916
Dietrich G, Viret JF, Hess J (2003) Novel vaccination based on recombinant Mycobacterium bovis BCG. Int J Med Microbiol 292:441–451
Ding J, Bao W, Liu Q, Yu Q, Abdille MH, Wei Z (2008) Immunoprotection of chickens against Eimeria acervulina by recombinant alpha-Tubulin protein. Parasitol Res 103:1133–1140
Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP (2004) Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun 72:6939–6944
Hong YH, Lillehoj HS, Lillehoj EP, Lee SH (2006) Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet Immunol Immunopathol 114:259–272
Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Bertrand F, Dupuis L, Deville S (2011) Montanide™ IMS 1313 N VG PR nanoparticle adjuvant enhances antigen-specific immune responses to profilin following mucosal vaccination against Eimeria acervulina. Vet Parasitol 182:163–170
Jang SI, Kim DK, Lillehoj HS, Lee SH, Lee KW, Bertrand F, Dupuis L, Deville S, Ben Arous J, Lillehoj EP (2013) Evaluation of montanide™ ISA 71 VG adjuvant during profilin vaccination against experimental coccidiosis. PLoS One 8:e59786
Jiang LL, Lin JJ, Hong Y, Han HD, Zhao QP, Zhu SH, Huang B (2012) Identification and characterization of Eimeria tenella apical membrane antigen-1 (AMA1). PLoS One 7:e41115
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Exp Parasitol l28:30–36
Konjufca V, Jenkins M, Wang S, Juarez-Rodriguez MD, Curtiss R 3rd (2008) Immunogenicity of recombinant attenuated Salmonella enterica serovar typhimurium vaccine strains carrying a gene that encodes Eimeria tenella antigen SO7. Infect Immun 76:5745–5753
Lee SH, Lillehoj HS, Cho SM, Park DW, Hong YH, Lillehoj EP (2008) Protective effects of dietary safflower (carthamus tinctorius) on experimental coccidiosis. J Poult Sci 46:155–162
Lee SH, Lillehoj HS, Jang SI, Lee KW, Yancey RJ, Dominowski P (2010a) The effects of novel adjuvant complex/Eimeria profilin vaccine on intestinal host immune responses against live E. acervulina challenge infection. Vaccine 28:6498–6504
Lee SH, Lillehoj HS, Jang SI, Hong YH, Min W, Lillehoj EP, Yancey RJ, Dominowski P (2010b) Embryo vaccination of chickens using a novel adjuvant formulation stimulates protective immunity against Eimeria maxima infection. Vaccine 28:7774–7778
Li GQ, Kanu S, Xiao SM, Xiang FY (2005) Responses of chickens vaccinated with a live attenuated multi-valent ionophore-tolerant Eimeria vaccine. Vet Parasitol 129:179–186
Li J, Zheng J, Gong P, Zhang X (2012) Efficacy of Eimeria tenella rhomboid-like protein as a subunit vaccine in protective immunity against homologous challenge. Parasitol Res 110:1139–1145
Liu Y, Zheng J, Li J, Gong P, Zhang X (2013) Protective immunity induced by a DNA vaccine encoding Eimeria tenella rhomboid against homologous challenge. Parasitol Res 112:251–257
Lillehoj HS (1987) Effects of immunosuppression on avian coccidiosis: cyclosporin a but not hormonal bursectomy abrogates host protective immunity. Infect Immun 55:1616–1621
Lillehoj HS (1998) Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 28:1071–1081
Lillehoj HS, Lillehoj EP (2000) Avian coccidiosis.a review of acquired intestinal immunity and vaccination strategies. Avian Dis 44:408–425
Lillehoj HS, Min W, Dalloul RA (2004) Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult Sci 83:611–623
Lillehoj HS, Ding X, Marco AQ, Bevensee E, Lillehoj EP (2005) Resistance to intestinal coccidiosis following DNA immunization with the cloned 3–1E Eimeria gene plus IL-2, IL-15, and IFN-γ. Avian Dis 49:112–117
Ma D, Ma C, Pan L, Li G, Yang J, Hong J, Cai H, Ren X (2011) Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp Parasitol 127:208–214
McDonald V, Shirley MW (2009) Past and future: vaccination against Eimeria. Parasitology 136:1477–1489
Miyaji EN, Mazzantini RP, Dias WO, Nascimento AL, Marcovistz R, Matos DS, Raw I, Winter N, Gicquel B, Rappuoli R, Leite LC (2001) Induction of neutralizing antibodies against diphtheria toxin by priming with recombinant Mycobacterium bovis BCG expressing CRM(197), a mutant diphtheria toxin. Infect Immun 69:869–874
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Remarque EJ, Faber BW, Kocken CH, Thomas AW (2008) Apical membrane antigen 1: a malaria. vaccine candidate in review. Trends Parasitol 24:74–84
Richie T, Saul A (2002) Progress and challenges for malaria vaccines. Nature 415:694–701
Rose ME, Hesketh P (1982) Immunity to coccidia in chickens: adoptive transfer with peripheral blood lymphocytes and spleen cells. Parasite Immunol 4:171–185
Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P (2004) Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173:2675–2682
Santangelo MP, McIntosh D, Bigi F, Armôa GR, Campos AS, Ruybal P, Dellagostin OA, McFadden J, Mendum T, Gicquel B, Winter N, Farber M, Cataldi A (2007) Mycobacterium bovis BCG as a delivery system for the RAP-1 antigen from Babesia bovis. Vaccine 25:1104–1113
Shirley MW, Ivens A, Gruber A, Madeira AM, Wan KL (2004) The Eimeria genome projects: a sequence of events. Trends Parasitol 20:199–201
Song H, Yan R, Xu L, Song X, Shah MA, Zhu H, Li X (2010) Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol 126:224–231
Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF et al (1991) New use of BCG for recombinant vaccine. Nature 351:456–460
Supply P, Sutton P, Coughlan SN, Bilo K, Saman E, Trees AJ, Cesbron Delauw MF, Locht C (1999) Immunogenicity of recombinant BCG producing the GRA1 antigen from Toxoplasma gondii. Vaccine 17:705–714
Wang H, Liu Q, Liu K, Zhong W, Gao S, Jiang L, An N (2007) Immune response induced by recombinant Mycobacterium bovis BCG expressing ROP2 gene of Toxoplasma gondii. Parasitol Int 56:263–268
Wang Q, Li J, Zhang X, Liu Q, Liu C, Ma G, Cao L, Gong P, Cai Y, Zhang G (2009) Protective immunity of recombinant Mycobacterium bovis BCG expressing rhomboid gene against Eimeria tenella challenge. Vet Parasitol 160:198–200
Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, Brooks H, Barrow P, Smith A, Maskell D, McConnell I (2004) Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar typhimurium. Infect Immun 72:2152–2159
Xu JH, Qin ZH, Liao YS, Xie MQ, Li AX, Cai JP (2008) Characterization and expression of an actin-depolymerizing factor from Eimeria tenella. Parasitol Res 103:263–270
Yang G, Wang C, Hao F, Zhao D, Zhang Y, Li Y (2010) Studies on construction of a recombinant Eimeria tenella SO7 gene expressing Escherichia coli and its protective efficacy against homologous infection. Parasitol Int 59:517–523
Yoshida S, Nagumo H, Yokomine T, Araki H, Suzuki A, Matsuoka H (2010) Plasmodium berghei circumvents immune responses induced by merozoite surface protein 1- and apical membrane antigen 1-based vaccines. PLoS One 5:e13727
Zhang H, Compaore MK, Lee EG, Liao M, Zhang G, Sugimoto C, Fujisaki K, Nishikawa Y, Xuan X (2007) Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol Biochem Parasitol 151:205–212
Zhang H, Nishikawa Y, Yamagishi J, Zhou J, Ikehara Y, Kojima N, Yokoyama N, Xuan X (2010) Neospora caninum: application of apical membrane antigen 1 encapsulated in the oligomannose-coated liposomes for reduction of offspring mortality from infection in BALB/c mice. Exp Parasitol 125:130–136
Zhang DF, Sun BB, Yue YY, Zhou QJ, Du AF (2012) Anticoccidial activity of traditional Chinese herbal Dichroa febrifuga lour. Extract against Eimeria tenella infection in chickens. Parasitol Res 111:2229–2233
Acknowledgments
This study was supported by the National Hi-Tech Research and Development Program of China (863 program) under grant number 2011AA10A215 and Key Discipline Construction Program of Anhui Science and Technology University (AKXK20101-2). We declare that the experiments comply with the current laws of China when they were performed.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 148 kb)
Rights and permissions
About this article
Cite this article
Li, WC., Zhang, Xk., Du, L. et al. Eimeria maxima: efficacy of recombinant Mycobacterium bovis BCG expressing apical membrane antigen1 against homologous infection. Parasitol Res 112, 3825–3833 (2013). https://doi.org/10.1007/s00436-013-3570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3570-5