Abstract
Hemozoin (Hz) is considered a disposal product during the digestion of red blood cells by some blood-feeding parasites, such as Plasmodium, Schistosome, and Rhodnius. The only function of Hz that has been reported is to detoxify the free heme (Fe(III)-protoporphyrin-IX) in worms. Here we report a new role for Hz in iron transport in Schistosoma japonicum. Using transmission electron microscopy (TEM), we observed that S. japonicum hemozoin (sjHz) granules were a group of electron-dense, globe-, and comma-shaped granules. At the anterior end of female worm gut, these dark brown granules were found to be mixed with biconcave disc-shaped erythrocytes, in the middle portion of the gut these granules attached to destroyed erythrocytes and in the posterior portion of the gut no intact erythrocytes were observed except free sjHz granules. By energy dispersive spectroscopy (EDS) and Prussian blue iron staining, we found that these iron-containing sjHz granules are degraded near the microvilli adjacent to vitelline glands, resulting in the accumulation of a large amount of iron in the vitelline cells and eggs of developed S. japonicum. The accumulation of iron in vitelline glands was synchronized with the increase of sjHz granules in the gut. When S. japonicum just contained a little amount of sjHz granules in gut, hardly any accumulation of iron was detected in vitelline glands. However, when the lumen of gut filled full with sjHz granules, large amounts of iron was detected in vitelline glands. Solexa sequencing revealed that expression of iron store protein, ferritin-1 (CAX77379.1), is just significantly up-regulated in worms that contained a large amount of sjHz in gut. In contrast to the idea that sjHz granules are simply by-product of heme detoxification, we found that formation and degradation of sjHz granules in vivo likely serve for the iron transport. Our findings provide new insights into the biological significance of Hz formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the estimates by the World Health Organization, there were 216 million episodes of malaria and 537,000 to 907,000 malaria-related deaths in 2010. Likewise, over 230 million people require treatment for schistosomiasis each year (WHO 2012). The etiological agents of malaria and schistosomiasis are different, but they both produce hemozoin (Hz; Noland et al. 2003; Oliveira et al. 2000; Hempelmann 2007), a crystal composed of polymerized heme molecules (Fe III-protoporphyrin-IX). Free heme is toxic to cells (Aft and Mueller 1983, 1984; Schmitt et al. 1993), so these parasites detoxified it via sequestration by hemozoin. Hemozoin is a disposal product of the digestion of hemoglobin produced by many blood-feeding parasites, such as Echinostoma trivolvis (Pisciotta et al. 2005), Haemoproteus columbae (Chen et al. 2001), and Rhodnius prolixus (Stiebler et al. 2010), besides Plasmodium and Schistosome. The formation of hemozoin is essential for the survival of these parasites. Current therapeutic drugs, such as chloroquine and mefloquine, are thought to kill malaria parasites or Schistosoma japonicum by inhibiting hemozoin formation to prevent the detoxification of the heme (de Villiers et al. 2008; Sullivan et al. 1996; Weissbuch and Leiserowitz 2008; Xiao and Xue 2012; Ziegler et al. 2001). However, in this study, we found that these iron-containing sjHz granules were not abandoned completely, but degraded in the S. japonicum gut near the microvilli adjacent to vitelline glands, and a large amount of iron accumulated in vitelline glands. Meanwhile, Ferritin genes were up-regulated. The findings suggest that the formation and degradation of sjHz granules play a role in iron transport in worms, which is a new function besides heme detoxification. It provides new insights into the biological significance of Hz formation.
Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Regulations for the Administration of Affairs Concerning Experimental Animals of the State Science and Technology Commission. The protocol was approved by the Internal Review Board of Tongji University School of Medicine.
Chemicals and reagents
Reagents used in this study were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and Sigma-Aldrich (St. Louis, MO). The Iron Stain kit was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Milli-Q water (resistivity ≥ 18.2MΩ · cm) was used for the preparation of solutions.
Parasites and isolation of sjHz
Oncomelania hupensis snails infected with S. japonicum miracidia (Jiangsu isolate) were obtained from the Jiangsu Institute of Schistosome Diseases, Jiangsu province, China. Cercariae freshly shed by the snails were used to infect mice percutaneously with 40 female cercariae or 40 mixed-sex cercariae each. The schistosomula were recovered by perfusion 2 and 3 weeks post-infection and subjected to Prussian blue iron staining and Solexa sequencing analysis.
Adult female schistosomes were recovered by perfusion 42 to 45 days post-infection (double sex infection), and washed thrice with sterile 0.15 mol/l NaCl solution (normal saline; Hu et al. 2003). The worms were placed in a 1.5-ml tube with sterile normal saline solution, and cut into small pieces to release sjHz from their gut. Then, the worm suspensions were collected and centrifuged at 1,500×g for 30 s, and the worm pellet was discarded. The supernatant was re-centrifuged at 12,000×g for 10 min. The dark-brown pellets were washed thrice with sterile normal saline and stored at 4 °C until use.
Sample preparation for transmission electron microscopy (TEM)
The worms recovered from each group were rinsed three times with ice-cold normal saline and fixed in 2.5 % glutaraldehyde-phosphate buffer (0.2 mol/l, pH 7.4). Post-fixation was done in buffered osmium tetraoxide, followed by dehydration before embedding in spur resin. Ultrathin sections of the schistosomes were stained with uranyl acetate and lead citrate. Finally, the adult schistosome specimens were examined under a JEOL EW-1230 scanning electron microscope at an accelerating voltage of 80 kV, and images were acquired using a digital photo-documentation system (Gatan Bioscan Camera, model 792).
Observation of sjHz granules by field-emission gun environmental scanning electron microscopy (FEG-ESEM)
The sjHz specimens collected from the gut of adult female worms or from the aforementioned cell incubations were fixed with 2.5 % glutaraldehyde–PBS buffer (pH 7.4) at 4 °C for 1 to 2 h, then rinsed and fixed with 1 % to 4 % osmium tetroxide at 4 °C for 1 to 2 h, and sequentially dehydrated in graded ethanol series. These steps were followed by critical-point drying in CO2 and sputter-coating with gold-platinum using standard techniques. FEG-ESEM can be used for high-resolution imaging. It can be switched between three vacuum modes, thus enabling the investigation of conductive, non-conductive, and high-vacuum incompatible materials. The high vacuum mode (typically at 10–5 mbar) is called the FEG mode in this study.
Analysis by energy dispersive spectroscopy (EDS)
The TEM sections of S. japonicum female worms were analyzed using energy dispersive (X-ray) spectroscopy (Oxford INCA, Lyford, OX, UK) in a JEM-2010 transmission electron microscope at an accelerating voltage of 200 kV as previously described (Jones et al. 2007). The microscope was set up to produce a probe of approximately 3–5 nm in diameter to acquire spectra of the sample through 60 live seconds. The Oxford INCA software package was used to carry out X-ray analysis for determining the elemental composition of the sample and for generating its characteristic spectrum.
Prussian blue Iron Stain assay
The Iron Stain Kit was used to identify the ferric iron deposits in tissue samples under light microscopy (LM). This kit is composed of two ready-to-use dispenser packs of 10 % potassium ferrocyanide and 20 % hydrochloric acid. Before use, equal parts of 20 % hydrochloric acid and 10 % potassium ferrocyanide solution were mixed. Fresh worms were fixed with 2 % glutaraldehyde or 10 % formalin in a 1.5-ml Eppendorf tube. Before staining, the fixative solution was discarded and the worms were washed once with distilled water. Equal amounts of hydrochloric acid and potassium ferrocyanide solution were then added to the tube, and the worms were immersed in the solution at room temperature overnight. The worms were washed in distilled water three times and dehydrated through a series of 95 % alcohol and two changes of 100 % alcohol. They were then cleared in two changes of xylene for 5 min each. Finally, the worms were transferred to a glass slide and covered with a cover slip in resinous mounting medium.
Analysis of differentially expressed genes via Solexa sequencing
Two-week-old and 3-week-old female schistosomula from single or double sex infections were isolated and their total RNA was extracted using Trizol reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. RNA concentration and purity were evaluated spectrophotometrically at 260 and 280 nm, using a NanoDrop ND1000 spectrophotometer and an Agilent 2100 Bioanalyzer (Agient Technologics, Palo Alto, CA). RNA samples were stored at −80 °C. The Illumina Gene Expression Sample Prep Kit and Solexa Sequencing Chip (flowcell) were used, and the main instruments were Illumina Cluster Station and Illumina HiSeq™ 2000 Systemat in the Beijing Genomics Institute (Beijing, China). All identical sequences were identified and eliminated from the initial data set. The resulting set of unique sequences with associate read counts was referred to as sequence tags. Unique reads were mapped to the S. japonicum genome (http://www.chgc.sh.cn/japonicum/Resources.html) and the Schistosoma mansoni genome (ftp://ftp.sanger.ac.uk/pub/pathogens/Schistosoma/mansoni/genome/gene_predictions/GeneDB_Smansoni_Genes.v4.0h.gz). The analysis was performed using the method described in previous papers (Huang et al. 2009; Wang et al. 2010).
Results/discussion
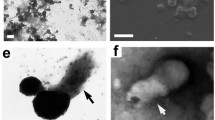
Under LM, sjHz granules were observed to be characteristic dark brown granules in the gut lumen of S. japonicum. The granules possessed the same spectroscopic features as malarial Hz (Fig. 1), as reported by previous studies (Oliveira et al. 2000). Under TEM and FEG-ESEM, those dark brown and electron-dense granules were found to be globular or comma shaped (Figs. 2 and 3). The energy dispersive spectrum (EDS) analysis revealed the existence of iron in the electron dense granules under TEM, clearly indicating sjHz granules are iron-containing granules (Fig. 4).
SjHz granules exhibited the same absorption peak at 400 nm as malarial hemozoin. Fig. 2 The FEG-ESEM image, showing globular (arrow a) and comma shaped sjHz granules (arrow b) in the gut of female S. japonicum. Scale bar = 1 μm. Fig. 3 The TEM image, showing electron-dense globular and comma-shaped sjHz granules. Scale bar = 1 μm. Fig. 4 The EDS analysis, showing that sjHz granules possessed a characteristic iron peak
In the gut lumen of S. japonicum, the erythrocytes near the anterior end of female worms retained their typical biconcave disc shape (Fig. 5). In the middle part of the gut, nearly all erythrocytes were “surrounded” by dark brown sjHz granules, and few intact erythrocytes with typical shapes were detected (Fig. 6). In the posterior part of the schistosome gut adjacent to the vitelline glands, few intact erythrocytes were observed, but full of free sjHz granules (Fig. 7). Under TEM, electron-dense iron-containing sjHz granules or their parts were easily recognized because electron density of erythrocytes and their debris were far lower than that of sjHz granules (Fig. 3). These electron-dense iron-containing sjHz granules were degraded near the microvilli adjacent to the vitelline glands (Figs. 8, 9, 10, and 11). The electron-dense sjHz granules near the microvilli were first degraded into partly electron-dense granules, then into completely electron-lucent lipid-like granules (Figs. 12 and 13). Finally, these whole granules were completely degraded. Using EDS analysis of the degrading granules, the electron-dense granules exhibited a characteristic iron peak (Fig. 14), suggesting that this part contains iron. Within the partly electron-dense granules, only the electron-dense regions displayed the characteristic iron peak (Fig. 14), whereas no iron peak was observed in the electron-lucent granules or any other tissues of guts (Fig. 15). These results clearly revealed a complete degradation process of an iron-containing sjHz granule in the Schistosome gut (Fig. 16) . Thus, it is interesting to investigate where the iron in the degraded sjHz granules goes.
The LM image of anterior end of gut in a female worm, showing free sjHz granules and biconcave disc-shaped erythrocytes. Scale bar = 5 μm. Fig. 6 The LM image of mid portion of gut in a female worm, showing the destroyed erythrocytes attached to or surrounded by dark brown sjHz granules. Scale bar = 5 μm. Fig. 7 The LM image of posterior portion of gut in a female worm, not showing any intact erythrocytes except for numerous free sjHz granules. Scale bar = 5 μm. Fig. 8 A schematic diagram of a female Schistosoma japonicum, showing the sampling spots for Figs. 5–11. Figs. 9–11 Near the microvilli in the gut, electron-dense sjHz granules were degraded to electron-lucent granules (arrow), as observed under different magnifications. Scale bar = 5 μm
The TEM image of microvilli in the gut lumen, showing electron-dense globe-shaped sjHz granules degraded to electron–lucent granules (arrow) near by the microvilli. Scale bar = 1 μm. Fig. 13 The TEM image of microvilli in the gut, showing partly degraded sjHz granules. Scale bar = 1 μm. Fig. 14 EDS analysis showed that the electron-dense granules or electron-dense regions of electron–lucent granules exhibited a characteristic iron peak. Fig. 15 EDS analysis showed that electron–lucent granules and regions or any other electron–lucent tissues did not show a iron peak. Fig. 16 A schematic representing the degradation process of electron-dense sjHz granules: a a sjHz granule composed of lipids at the core and outer layer of sjHz crystals, b dissolution of sjHz crystal layer in initial degradation, c–e then digestion of the lipid-like part within a granule until eventual disappearance
To investigate the presence of iron in the tissues, whole worms, including schistosomula and adult worms of S. japonicum, were examined using Prussian Blue Iron Stain. In 2-week-old or younger schistosomula, sjHz granules were either absent or present only in small quantities in the gut. No tissues were stained iron positive, indicating the absence of iron in them (Fig. 17). In schistosomula that were 2–3 weeks old, the quantity of sjHz granuels in the gut gradually increased. Accordingly, some parts of the vitelline gland started to be stained light Prussian blue, but none of the other tissues were stained (Fig. 18). These results suggested that with increase in the quantity of sjHz in the gut, vitelline glands start to accumulate small amounts of iron. In schistosomula more than 3 weeks old or in adult female worms, along with the gut filled with a large amount of sjHz granules, the vitelline glands were strongly stained with Prussian blue, indicating that the tissues contain large amounts of iron (Fig. 19). Furthermore, the vitelline duct and the eggs in the ootype and uterus were also stained (Fig. 20). These results revealed that iron is mainly accumulated in vitelline cells and the structures that contain vitelline cells, such as vitelline duct, ootype, and eggs in adult female worms.
The LM image of a 2-week-old female schistosomulum with a little amount of sjHz in the gut (arrow), showing negative staining with Prussian blue in vitelline glands. vit vitelline glands. Scale bar = 100 μm. Fig. 18 The LM image of a female schistosomulum less than 3 weeks old with more amount of sjHz granules in the gut (arrow), showing partially staining with Prussian blue in vitelline glands. vit vitelline glands. Scale bar = 100 μm. Fig. 19 The LM image of a 3-week-old or older female schistosomulum or adult female worm with a large amount of sjHz granules, showing strong positive staining with Prussian blue in vitelline glands (arrow). vit vitelline glands. Scale bar = 100 μm. Fig. 20 The LM image of stained vitelline ducts (vitelline cells, black arrow) and stained eggs in the ootype and uterine (white arrows) in adult female worms. duc vitelline ducts, oot ootype. Scale bar = 100 μm. Fig. 21 The LM image of stained ootype (white arrow) and gut wall of female worms (black arrow). oot ootype, gut gut wall. Scale bar = 100 μm. Fig. 22 The LM image of a 2-week-old female schistosomulum from single sex infection with a little amount of sjHz in the gut, showing negative staining with Prussian blue in the whole worm. Scale bar = 100 μm. Fig. 23 The LM image of a 3-week-old female schistosomulum from single sex infection with a little amount of sjHz in the gut, showing negative staining with Prussian blue in the whole worm. Scale bar = 100 μm. Fig. 24 The LM image of a male schistosomulum with a little amount of sjHz in the gut, showing negative staining with Prussian blue in the whole worm. Scale bar = 100 μm. Fig. 25–26 The LM images of male adult worms, showing negative staining with Prussian blue in worm tissues except for the gut wall. male male adult worm, female female adult worm, gut gut wall. Scale bar = 100 μm
It is known that females from single-sex infections do not produce a large number of sjHz granules. To further investigate the relationship between the number of sjHz granules in guts and the accumulation of iron in vitelline cells, we analyzed the abundance and distribution of iron in these worms from single-sex infections using Prussian blue stain. The result showed that both 2- and 3-week-old females just contained a small number of sjHz granules in guts. Meanwhile, almost no iron could be detected in their tissues (Figs. 22 and 23). This indicated that with the development of females, neither does the amount of sjHz granules significantly increase in guts, nor the iron accumulated in tissues is detectable. The same result was also observed in males. The male worms contained less sjHz granules in their guts, and we hardly detected iron in most of their tissues, except their gut walls (Figs. 24, 25 and 26). Therefore, it is clear that the formation and abundance of sjHz granules are closely related to the accumulation of iron in worm tissues.
In addition, we demonstrated that the 3-week-old female schistosomula from double-sex infections contained more abundant sjHz granules than 2-week-old female schistosomula. The expression of ferritin-1, an isoform of the iron storage protein preferentially expressed in vitelline cells (Dietzel et al. 1992; Glanfield et al. 2007; Jones et al. 2007; Schussler et al. 1995), was significantly up-regulated in the 3-week-old female schistosomula from double-sex infections compared with their 2-week-old counterparts (Table 1). As for worms from single-sex infections, we did not detect the differential expression of ferritin-1 heavy chain between 2- and 3-week-old female schistosomula (Table 1). However, the 3-week-old female schistosomula from double-sex infections significantly up-regulated the expression of ferritin-1 heavy chain, compared with those from single-sex infections (Table 1). The results showed that the expression of ferritin-1 was in accordance with the accumulation of iron in vetilline cells and the abundance change of sjHz granules in guts. This suggests that ferritin-1 participants in iron store and transport in vitelline glands. In addition, as for another isoform of the iron storage protein in schistosome, ferritin-2, which were detected in diverse tissues in S. mansoni (Schussler et al. 1995), we found that in S. japonicum from double- or single-sex infections, the expression of ferritin light chain (XP_002569939.1), i.e. ferritin-2, were up-regulated in 3-week-old females, compared with 2-week-old females. However, no differential expression of ferritin light chain was detected in 3-week-old females from single- versus double-sex infections. This indicated that the expression of this iron storage protein is not closely associated with the accumulation of sjHz granules, which differs from ferritin-1. It is possible that ferritin-2 serve for routine iron requirement, whereas ferritin-1 for special requirement. Since eggs, especially eggshells, were reported to store abundant iron (Glanfield et al. 2007; Jones et al. 2007), the role of up-regulation of ferritin-1 expression in vitelline cells has been suggested. It is possible that the accumulation of iron in vitelline cells with the help of ferritin-1 is used to meet the need of iron for egg formation. Therefore, iron-containing sjHz granules, derived from heamoglobin degradation and heme detoxification, actually play a vital role in transporting iron from erythrocytes to schistosoma eggs. Notably, the gut walls of males and females, rather than the tegument, were stained Prussian blue (Figs. 21, 22, 23, 24, 25, and 26), indicating that the gut walls contain iron, whereas the tegument and its adjacent tissues lack detectable iron. This result suggested that iron in vitelline cells could be transported from gut lumen to vitelline cells via gut walls, rather than via tegument. Therefore, iron-containing sjHz granules in guts probably serve as a source of iron. Interestingly, Hz was also found in Garnham bodies of Plasmodium falciparum gametocytes (Orjih 2012). It is difficult to understand the biological significance of the transport of the disposal product (Hz) from the parasite’s food vacuole to G-bodies, unless the process plays a role in gametocytogenesis. Thus, whether iron is transported to G-bodies by Hz to participates in gametocytogenesis in P. falciparum is need to be investigated.
Conclusions
SjHz has been considered to be a disposal product formed by blood-feeding organisms to detoxify free heme. However, degradation of iron-containing sjHz granules and accumulation of iron in vitelline cells and eggs suggest that sjHz probably functions as a form of storage or transport of iron. Therefore, schistosomes may digest erythrocytes for obtaining iron.
References
Aft RL, Mueller GC (1983) Hemin-mediated DNA strand scission. J Biol Chem 258:12069–12072
Aft RL, Mueller GC (1984) Hemin-mediated oxidative degradation of proteins. J Biol Chem 259:301–305
Chen MM, Shi LR, Sullivan DJ (2001) Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and beta-hematin. Mol Biochem Parasit 113:1–8
de Villiers KA, Marques HM, Egan TJ (2008) The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J Inorg Biochem 102:1660–1667
Dietzel J, Hirzmann J, Preis D, Symmons P, Kunz W (1992) Ferritins of Schistosoma mansoni: sequence comparison and expression in female and male worms. Mol Biochem Parasitol 50:245–254
Glanfield A, McManus DP, Anderson GJ, Jones MK (2007) Pumping iron: a potential target for novel therapeutics against schistosomes. Trends Parasitol 23:583–588
Hempelmann E (2007) Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Res 100:671–676
Hu W, Yan Q, Shen DK, Liu F, Zhu ZD, Song HD, Xu XR, Wang ZJ, Rong YP, Zeng LC, Wu J, Zhang X, Wang JJ, Xu XN, Wang SY, Fu G, Zhang XL, Wang ZQ, Brindley PJ, McManus DP, Xue CL, Feng Z, Chen Z, Han ZG (2003) Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat Genet 35:139–147
Huang J, Hao P, Chen H, Hu W, Yan Q, Liu F, Han ZG (2009) Genome-wide identification of Schistosoma japonicum microRNAs using a deep-sequencing approach. PLoS One 4:e8206
Jones MK, McManus DP, Sivadorai P, Glanfield A, Moertel L, Belli SI, Gobert GN (2007) Tracking the fate of iron in early development of human blood flukes. Int J Biochem Cell Biol 39:1646–1658
Noland GS, Briones N, Sullivan DJ (2003) The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol Biochem Parasit 130:91–99
Oliveira MF, D’avila JC, Torres CR, Oliveira PL, Tempone AJ, Rumjanek FD, Braga CMS, Silva JR, Dansa-Petretski M, Oliveira MA, de Souza W, Ferreira ST (2000) Haemozoin in Schistosoma mansoni. Mol Biochem Parasit 111:217–221
Orjih AU (2012) Hemozoin accumulation in Garnham bodies of Plasmodium falciparum gametocytes. Parasitol Res 111:2353–2359
Pisciotta JM, Ponder EL, Fried B, Sullivan D (2005) Hemozoin formation in Echinostoma trivolvis rediae. Int J Parasitol 35:1037–1042
Schmitt TH, Frezzatti WA, Schreier S (1993) Hemin-induced lipid-membrane disorder and increased permeability—a molecular-model for the mechanism of cell-lysis. Arch Biochem Biophys 307:96–103
Schussler P, Potters E, Winnen R, Bottke W, Kunz W (1995) An isoform of ferritin as a component of protein yolk platelets in Schistosoma mansoni. Mol Reprod Dev 41:325–330
Stiebler R, Timm BL, Oliveira PL, Hearne GR, Egan TJ, Oliveira MF (2010) On the physico-chemical and physiological requirements of hemozoin formation promoted by perimicrovillar membranes in Rhodnius prolixus midgut. Insect Biochem Mol Biol 40:284–292
Sullivan DJ Jr, Gluzman IY, Russell DG, Goldberg DE (1996) On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA 93:11865–11870
Wang Z, Xue X, Sun J, Luo R, Xu X, Jiang Y, Zhang Q, Pan W (2010) An “in-depth” description of the small non-coding RNA population of Schistosoma japonicum schistosomulum. PLoS Negl Trop Dis 4:e596
Weissbuch I, Leiserowitz L (2008) Interplay between malaria, crystalline hemozoin formation, and antimalarial drug action and design. Chem Rev 108:4899–4914
World Health Organization (2012) Schistosomiasis. Fact sheet No. 115. http://www.who.int/mediacentre/factsheets/fs115/en/index.html. Accessed 26 August 2012
Xiao SH, Xue J (2012) Study progress on mefloquine against schistosomes and other helminths. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi 30:131–138
Ziegler J, Linck R, Wright DW (2001) Heme aggregation inhibitors: antimalarial drugs targeting an essential biomineralization process. Curr Med Chem 8:171–189
Acknowledgements
We would like to thank Pan W.Q. for his valuable advice. We are grateful for discussions and comments from Xiao S.H., Feng Z., Zhu J., Xue H.C. and Shan Y.F.. We thank Ni B., Du X.L. and Sha J.H. for their technical assistance in SEM and TEM, Xia C.H. for his assistance in FEG-ESEM. This research was supported by the National Natural Science Foundation of China (No.81071383).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, J., Hu, W. & Li, C. Beyond heme detoxification: a role for hemozoin in iron transport in S. japonicum . Parasitol Res 112, 2983–2990 (2013). https://doi.org/10.1007/s00436-013-3470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3470-8