Abstract
Several DNA-based and serological tests have been established for the detection of Theileria annulata infection, including polymerase chain reaction, reverse line blot and loop-mediated isothermal amplification, indirect enzyme-linked immunosorbent assay (ELISA), and competitive ELISA. In this study, we have applied knowledge from the development and application of a recombinant protein-based indirect ELISA and competitive ELISA to establish a rapid test for point-of-care diagnosis of T. annulata infection in the field to be used by the veterinarian. For the development of a lateral flow test, the recombinantly expressed T. annulata surface protein (TaSP) was applied as the test antigen and anti-TaSP antiserum as the control line. TaSP antigen conjugated to colloidal gold particles was used as the detection system for visualization at the test line for the binding of anti-TaSP antibody present in the serum of infected animals. The developed test specifically detected antibodies in the serum of animals experimentally infected with T. annulata and showed no cross-reactivity with serum from animals infected with other tested bovine pathogens (Trypanosoma brucei, Anaplasma marginale, Babesia bigemina, Babesia bovis, and Theileria parva). Testing of field samples was compared to results obtained by other serological tests, resulting in a sensitivity and specificity of 96.3% and 87.5% compared to indirect fluorescence antibody test, 98.7% and 81.8% compared to indirect ELISA, and 100% and 47.6% compared to competitive ELISA. In conclusion, a rapid test for the detection of T. annulata infection (T. annulata lateral flow device, Ta-LFD) has been developed, which is easy to perform, delivers results to be read by the naked eye within 10 min, and is suitable for the detection of infection in field samples.
Similar content being viewed by others
Introduction
Theileria annulata causes tropical theileriosis in cattle, which is transmitted by ticks of the genus Hyalomma (Uilenberg 1981). It is an intracellular protozoan parasite that induces a spectrum of disease symptoms and is highly pathogenic to cattle. The disease occurs over a wide geographic area ranging from Southern Europe and extending to Southern Russia, the Middle East, Central Asia, China, India, Northern Africa and Sudan, Eritrea, and Mauritania (McCosker 1979; Dolan 1989; Minjauw and McLeod 2000).
Several methods for the diagnosis of T. annulata infection are available which are however not well suited for direct testing in the field. Primarily, these include the routine clinical diagnosis for theileriosis and the microscopic detection of parasites from sampled blood smears. Although the microscopic examination of blood smears is uncomplicated and is of value for the detection of acute cases, it has limited value for chronic cases because of the low number of parasitemia in those animals, and additionally, it is difficult to differentiate between piroplasm species according to morphology (Hooshmand-Rad 1974; Friedhoff 1997).
Research in molecular biology has delivered precise tools for the detection of parasite DNA, and a number of polymerase chain reaction (PCR)-based assays with high sensitivity and specificity for the diagnosis of T. annulata in the bovine host have been developed, including PCR (d'Oliveira et al. 1995; Shayan et al. 1998; Kirvar et al. 2000; Habibi et al. 2007) and reverse line blotting to detect and differentiate all known Theileria and Babesia species on the basis of differences in their 18S subunit rRNA gene sequences (Gubbels et al. 1999; Schnittger et al. 2004). These techniques require equipped laboratories and are expensive and impractical for field diagnosis. Recently, a loop-mediated isothermal amplification assay was developed and evaluated for diagnosis of tropical theileriosis, which operated at high specificity, efficiency, and rapidity (Salih et al. 2008) but which has not been validated in the field yet.
Serological tests are ideally suited for epidemiological studies. The indirect fluorescence antibody test (IFAT) has successfully been used to detect antibodies against T. annulata infection in cattle (Burridge and Kimber 1973) and has been reported to be more sensitive than examination of blood smears (Dhar and Gautam 1977; Darghouth et al. 1996). However, it has the major drawback of cross-reactivity between different Theileria species (Burridge et al. 1974). In recent years, efforts have been made to develop and validate an indirect enzyme-linked immunosorbent assay (iELISA) based on recombinantly expressed antigens, such as the Tams1 (Gubbels et al. 2000) and the T. annulata surface protein (TaSP) antigen (Bakheit et al. 2004). The immunodominant antigen TaSP (Schnittger et al. 2002) has been especially proven to be highly suitable for detection of T. annulata-specific antibodies (Bakheit et al. 2004; Seitzer et al. 2007, 2008), and it has been validated in the field (Salih et al. 2005) and used for epidemiological studies (Salih et al. 2007a, b; Mohammad Al-Saeed et al. 2010). Furthermore, a competitive ELISA (cELISA) based on the TaSP antigen was recently established and validated (Renneker et al. 2008, 2009) to increase specificity of the detection of circulating antibodies against TaSP.
ELISAs are the method of choice for epidemiological studies and large-scale investigations; however, the applications are time consuming and labor intensive and also require professional personnel, special laboratory materials, and equipment. Hence, a convenient, rapid, and sensitive diagnostic test, such as an immunochromatographic test, that does not require instrumentation or specially trained personnel would be extremely valuable for the use in both clinical and field applications for the diagnosis of tropical theileriosis. Given the high suitability of the TaSP antigen for serodiagnosis of T. annulata infection, the following study was performed to establish an immunochromatographic strip test (lateral flow device, LFD) on the basis of this protein for use as a rapid point-of-care assay.
Materials and methods
Preparation of recombinant TaSP
Recombinant expression and purification of the predicted extracellular domain of TaSP (recombinant TaSP (rTaSP), aa 25–156) was performed as described before (Schnittger et al. 2002). This first round of purified recombinant protein was applied to repurification using the Äkta prime high system (Amersham Bioscience, Uppsala, Sweden) using 5 ml HiTrap columns packed with Ni Sepharose 6 Fast Flow (GE Healthcare Europe GmbH, Freiburg, Germany). The Ni chromatography was carried out according to the manufacturer's instructions using the following chromatography program: (1) washing of the column with 5–10-column-volume (cv) H2O with a flow rate of 5 ml/min, (2) equilibration with 10-cv application buffer (20 mM Tris–HCl, pH 8.0, 8 M urea, 0.5 M NaCl, 5 mM imidazole), (3) application of the sample to the column with a flow rate of 1 ml/min, (4) washing with 20-cv washing buffer (20 mM Tris–HCl, pH 8.0, 8 M urea, 0.5 M NaCl, 5 mM imidazole) at a flow rate if 3 ml/min until the absorbance at A 280 is constant, (5) collection of 1-ml fractions after application of 1-cv elution buffer (20 mM Tris–HCl, pH 8.0, 8 M urea, 0.5 M NaCl, 1 M imidazole) with a linear gradient of 500 mM to 1 M imidazole until the protein peak appears at a flow rate of 1 ml/min. Collected fractions were analyzed for the presence of protein in Western blot using antihistidine antibody (RGS-His™ mouse antihistidine antibody, Qiagen, Hilden, Germany), T. annulata-positive serum samples, and anti-TaSP antiserum (Schnittger et al. 2002). A protein band of approximately 41 kDa was observed (Fig. 1). Purity was assessed by Coomassie gel staining, and protein concentration was determined using the Bio-Rad Micro-DC Assay kit (Bio-Rad, Munich, Germany). Fractions containing the protein were pooled, dialyzed against phosphate-buffered saline (PBS, 137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl), and adjusted to a concentration of 0.2 mg/ml. This repurified protein was used in all experiments with Western blot and LFD.
Purification of recombinant TaSP protein. a Recombinantly expressed and purified rTaSP was subjected to repurification using column chromatography as described in the “Methods” section. Collected fractions (lanes 1–22) were analyzed by silver staining (top panel) and Western blot using anti-TaSP antiserum (bottom panel). Fractions 11 to 13 were pooled for further use. b The pooled fractions were analyzed in silver staining (lane 1) and were tested for reactivity with anti-TaSP antiserum (lane 2) and two sera from T. annulata-infected cattle (lanes 3 and 4). Sera from uninfected cattle were tested in lanes 5 and 6. Lane 7: anti-bovine secondary antibody control. Lane 8: anti-rabbit secondary antibody control
Sera from experimentally infected animals and field sera
Serum samples from animals experimentally infected with T. annulata were previously generated at the Free University Berlin, Germany (Ahmed et al. 1989) and the University of Utrecht (kindly provided by Frans Jongejan). Serum from animals experimentally infected with Babesia bovis, Babesia bigemina, or Anaplasma marginale was kindly provided by Jiansan Wu, Qingdao, China. Serum samples from animals experimentally infected with Trypanosoma brucei or Theileria parva were kindly provided by Dirk Geysen, Antwerp, Belgium. Serum containing antibodies against Theileria mutans was from Svanova Biotech, Uppsala, Sweden. Ninety field serum samples were collected at random from cattle in Sudan (Bakheit et al. 2004) in areas where tropical theileriosis is known to be endemic. Sera from Theileria lestoquardi-infected animals were from Sudan (Bakheit et al. 2006). Results obtained with the T. annulata LFD (Ta-LFD) were compared to previously generated data using IFAT, indirect ELISA, or cELISA (Bakheit et al. 2004; Renneker et al. 2008). The specificity and sensitivity were calculated using the following formulas: specificity (Sp = (# of samples negative in both tests/total number of negative samples in the reference test) × 100), sensitivity (Se = (# of samples positive in both tests/total number of positive samples in the reference test) × 100). The Kappa test (Cohen's kappa measure of association) was used to calculate the degree of agreement between the Ta-LFD and compared tests using Intercooled Stata 6.0 (StataCorp LP, College Station, TX, USA) and SPSS 11.5.0 for Windows (SPSS Inc., Chicago, IL, USA) (Thomas et al. 1988). Interpretation of kappa can be rated as follows: kappa less than 0.0, “poor” agreement; between 0.0 and 0.20, “slight” agreement; between 0.21 and 0.40, “fair” agreement; between 0.41 and 0.60, “moderate” agreement; between 0.61 and 0.80, “substantial” agreement; and between 0.81 and 1.00, “almost perfect” agreement (Landis and Koch 1977). Hypothesis tests of whether kappa is significantly greater than 0 are based on standard errors derived from approximations to its variance (Bishop et al. 1975).
Components of the Ta-LFD
Adsorption of rTaSP and anti-TaSP antibody to the membrane
rTaSP was used as the solid-phase antigen in the test line by jetting it linearly onto the membrane (Hi-flow membrane no. R90N93558-Hf1350, Millipore, USA) at a concentration of 2 mg/ml using Bio-Dot airbrush equipment (Bio-Dot Ltd., West Sussex, UK). Fifty microliters of the TaSP solution was added per 30 cm of the membrane. The control line comprised rabbit anti-TaSP antibody, prepared by immunization of a rabbit with the recombinant TaSP (Schnittger et al. 2002) and subsequent purification of specific IgG antibodies by affinity chromatography (HYDRA®-gelmatrix, Charles River Laboratories, Kisslegg, Germany), which were applied to the control line at a concentration of 2 mg/ml parallel to the rTaSP test line (test band). The membranes were then dried at 37°C for 45 min and stored in sealed foil sachets until use.
Conjugation of rTaSP to gold microparticles
Purified rTaSP labeled with colloidal gold was used as the mobile phase by coupling to 40-nm colloidal gold particles using a proprietary method and storing at 4°C until use.
Adsorption of gold/rTaSP conjugate to filters
The gold/Ab conjugate was sprayed onto the conjugate filter (Whatman, UK) using Bio-Dot airbrush equipment (Bio-Dot Ltd., West Sussex, UK) at a volume of 1-μl/mm filter. The filters were dried at room temperature for 45 min and then stored in sealed foil sachets until required.
Assembly of the strip test device
Sequentially, the membrane, absorbent pad, conjugate pad, and sample pad were assembled on an adhesive card and cut into 0.8-cm-wide strips with a Bio-Dot cutter (Bio-Dot Ltd., West Sussex, UK). The membrane strips were assembled into a device as described previously (Brüning et al. 1999; Ferris et al. 2009).
Results
Optimum antigen concentration and sample buffer
The system for the lateral flow device was optimized by determining the physical and chemical conditions for maximum sensitivity of detecting a positive sample while assuring a negative signal for negative serum. These studies showed that the optimum concentration of rTaSP for binding to microspheres and the nitrocellulose was 0.2 μg/μl and 2 mg/ml, respectively. In addition, different sample volumes were tested, and 180 μl was chosen as the optimal sample volume to solubilize the conjugate and to facilitate capillary flow.
Ta-LFD test operation
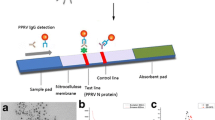
During the assay performance, 20 μl of bovine sera mixed with 160 μl of chase buffer was applied to the sample pad. This resulted in rehydration of the air-dried rTaSP-conjugated and dyed gold particles and their migration by capillary action along the nitrocellulose membrane, representing the reaction matrix. If anti-TaSP antibodies were present in the sample, then the antibody–rTaSP–conjugate complex was captured by the immobilized rTaSP on the membrane at the “T” (test) line and resulted in their accumulation, which could be visualized as a red line to signify a positive result. Excess (or unbound) rTaSP-labeled gold particles continued to migrate along the device until being captured by the immobilized rabbit anti-TaSP antibody and the formation of a red “C” (control) line to validate the test. The rest of the liquid was entrapped in the wick or absorbent pad. The results could be judged within 8–10 min and were recorded as shown in Fig. 2.
Test strip and assembled Ta-LFD setup of the test strip (1–3), showing the nitrocellulose strip with the control line (C) and test line (T) and the lower and upper affixed sample, conjugate, and absorbent pads. The assembled LFD device (4–6) correspondingly shows a sample window, test window, and control window. Application of serum from an uninfected animal (1, 4) results in only the control line becoming visible; application of serum from an infected animal (2, 5) or anti-TaSP antiserum as control (3, 6) results in also the test line becoming visible
Sensitivity and specificity of the Ta-LFD
The sensitivity and specificity of the Ta-LFD were determined by detecting anti-TaSP-specific antibodies in field sera using the optimized conditions and comparing the results with data obtained by investigation of the serum samples using IFAT, iELISA, and cELISA (Bakheit et al. 2004; Renneker et al. 2008). In comparison to IFAT as the reference test, the sensitivity and specificity of the Ta-LFD were 96.34% (79/82) and 87.5% (7/8), respectively (Table 1). Seventy-nine samples were positive and seven were negative in both tests, whereas one was positive using the Ta-LFD but negative using IFAT, and three were negative using Ta-LFD but positive using IFAT. The relative agreement was calculated to be 95.5% (kappa = 0.75; substantial agreement; p < 0.0000). Using the iELISA as the reference method, 78 samples were positive and nine negative in both tests. Two samples were positive using the Ta-LFD and negative using iELISA, and only one sample was negative in the Ta-LFD test but positive in the iELISA, leading to a calculated sensitivity and specificity of the Ta-LFD of 98.7% (78/79) and 81.1% (9/11), respectively. The relative agreement was 96.6% (kappa = 0.83; almost perfect agreement; p < 0.0000). Lastly, results using the Ta-LFD test were compared to results obtained with competitive ELISA as the reference test. Here, 69 samples were positive and 10 were negative in both tests, whereas 11 samples were positive using the Ta-LFD and negative using cELISA, resulting in a sensitivity and specificity of 100% (68/68) and 47.6% (10/21), respectively, with an agreement of 86.6% (kappa = 0.56; moderate agreement; p < 0.0000).
Serum from a cattle experimentally infected with T. annulata was collected at 2, 3, 4, and 5 weeks postinfection and tested by indirect ELISA (Bakheit et al. 2004), competitive ELISA (Renneker et al. 2008), and Ta-LFD. Comparable results were obtained with the three tests, indicating that the Ta-LFD can detect infection 3 weeks postinfection (Table 2).
Testing for possible cross-reactivity of the Ta-LFD was performed using sera from animals experimentally infected with B. bovis, B. bigemina, T. brucei, A. marginale, T. mutans, and T. parva as well as serum from an animal testing positive in an ELISA for detection of infection with T. lestoquardi (Bakheit et al. 2006). No cross-reactivity was observed in comparison to the T. annulata-positive and T. annulata-negative controls (Fig. 3).
Testing for cross-reactivity using the Ta-LFD serum from animals infected with T. brucei, A. marginale, B. bigemina, B. bovis, T. lestoquardi, and T. parva was done in the Ta-LFD. Serum from an uninfected animal was used as the negative control and serum from an animal infected with T. annulata as the positive control. C control line, T test line
Discussion
Lateral flow immunoassays are well established as a valuable tool in medical, veterinary, food, agricultural, environmental, and industrial diagnostics. Immunochromatographic tests have also been developed for the diagnosis of many protozoan diseases, such as babesiosis (Huang et al. 2004b; Kim et al. 2007, 2008), malaria (Mills et al. 1999), cryptosporidiosis (Chan et al. 2000), leishmaniasis (Reithinger et al. 2002), coccidiosis (Liao et al. 2005), toxoplasmosis (Huang et al. 2004a), and trypanosomosis (Houghton et al. 2009). Regarding the serodiagnosis of infection with T. annulata, there have been several reports in the last years, including IFAT (Burridge and Kimber 1973; Dhar and Gautam 1977; Darghouth et al. 1996), indirect ELISA (Gubbels et al. 2000; Bakheit et al. 2004), and competitive ELISA (Renneker et al. 2008). Besides having cross-reactivity problems for instance using IFAT (Burridge et al. 1974), these methods involve complex procedures that require expensive laboratory materials, equipment, and trained personnel. Thus, although the ELISA assays are specific and highly suitable for large-scale epidemiological studies carried out in the laboratory, these methods are unsuited for rapid diagnosis for use by the veterinarian in the field. In the present study, we thus developed a new rapid immunochromatographic strip assay for the detection of infection with T. annulata (Ta-LFD) based on the same protein (TaSP) which was used in the previously established ELISA assays (Bakheit et al. 2004; Renneker et al. 2008). The application of this Ta-LFD is simpler and more convenient, provides results that can very rapidly be judged by the naked eye compared to the standard serodiagnostic tests, and can be applied by veterinarians in the field especially in countries where laboratories are less well equipped.
Several critical aspects in the establishment of an LFD need to be considered, such as the quality of antigen and/or antibody, pretreatment of the sample, and conjugate pad or membrane properties (Zhang et al. 2006). In the establishment of the Ta-LFD, the quality of TaSP used was crucial for the sensitivity and specificity of the assay, indicating that purification and consistency of supply are important to minimize nonspecific binding effects. In addition, to maximize the adsorption of proteins, these should be applied to the membrane in buffers that are preferably free of salt, surfactants, and sugars (Mansfield 2005; Millipore Technical Publication “Rapid Lateral Flow Test Strips: Considerations for Product Development” available at http://www.millipore.com/techpublications/tech1/tb500en00). Therefore, TaSP was repurified, and respective fractions were pooled and dialyzed against PBS to ensure that it exhibits sufficient sensitivity, specificity, purity, and stability to execute the performance requirements of the completed product.
For evaluation of the Ta-LFD, the results of testing field sera from a tropical theileriosis endemic region in Sudan using the Ta-LFD assay were compared to those obtained by indirect and competitive TaSP ELISA. These comparisons showed that the Ta-LFD had a significant correlation with all other tests (IFAT, iELISA, cELISA), the highest agreement being between the Ta-LFD and iELISA (96.7%), followed by IFAT (95.5%) and cELISA (86.6%), indicating that the use of the Ta-LFD would be reliable for use in the field. The comparison also showed that the Ta-LFD had a high sensitivity compared to the respective reference tests, ranging from 100% to 98.7% and 96.34% compared to the cELISA, iELISA, and IFAT, respectively. Thus, only a minor number of samples detected as positive in the reference tests were tested negative with Ta-LFD. The specificity of the Ta-LFD compared to the reference tests was also high, ranging from 87.5% for the IFAT to 81.8% for the iELISA and 47.6% for the cELISA. The latter value is due to the fact that 11 samples testing negative in the cELISA were positive using the Ta-LFD, a discrepancy which was also observed when comparing the cELISA to the iELISA (Renneker et al. 2008). This is perhaps attributable to the fact that detection of T. annulata infection using the cELISA relies on the inhibition of binding of a monoclonal antibody to an epitope of the TaSP protein by antibodies in the test serum, which may not be present in sufficient amounts in selected samples, giving false-negative results.
In conclusion, the developed Ta-LFD for serological detection of infection with T. annulata required no sophisticated equipment to be carried out and delivered results which are easy-to-read and interpretable by the naked eye after 10 min of application of the sample. The newly established test is sensitive and specific and may be regarded as a suitable diagnostic tool for the detection of theileriosis in cattle under field conditions. Considering also that the completely assembled Ta-LFD is stable when stored without refrigeration, this rapid test should be easily applicable by veterinarians as a point-of-care diagnostic assay.
References
Ahmed JS, Rothert M, Steuber S, Schein E (1989) In vitro proliferative and cytotoxic responses of PBL from Theileria annulata-immune cattle. Zentralbl Veterinärmed B 36:584–592
Bakheit MA, Schnittger L, Salih DA, Boguslawski K, Beyer D, Fadl M, Ahmed JS (2004) Application of the recombinant Theileria annulata surface protein in an indirect ELISA for the diagnosis of tropical theileriosis. Parasitol Res 92:299–302
Bakheit MA, Seitzer U, Ahmed JS (2006) A new recombinant protein-based ELISA for the diagnosis of malignant theileriosis of sheep and goats. Parasitol Res 98:145–149
Bishop YMM, Feinberg SE, Holland PW (1975) Discrete multivariate analysis. MIT Press, Cambridge
Brüning A, Bellamy K, Talbot D, Anderson J (1999) A rapid chromatographic strip test for the pen-side diagnosis of rinderpest virus. J Virol Methods 81:143–154
Burridge MJ, Kimber CD (1973) Duration of serological response to the indirect fluorescent antibody test of cattle recovered from Theileria parva infection. Res Vet Sci 14:270–271
Burridge MJ, Brown CG, Kimber CD (1974) Theileria annulata: cross-reactions between a cell culture schizont antigen and antigens of East African species in the indirect fluorescent antibody test. Exp Parasitol 35:374–380
Chan R, Chen J, York MK, Setijono N, Kaplan RL, Graham F, Tanowitz HB (2000) Evaluation of a combination rapid immunoassay for detection of Giardia and cryptosporidium antigens. J Clin Microbiol 38:393–394
Darghouth ME, Bouattour A, Ben Miled L, Sassi L (1996) Diagnosis of Theileria annulata infection of cattle in Tunisia: comparison of serology and blood smears. Vet Res 27:613–621
Dhar S, Gautam OP (1977) Indirect fluorescent antibody test for serodiagnosis in cattle infected with Theileria annulata. Indian J Anim Sci 47:720–723
Dolan TT (1989) Theileriosis: a comprehensive review. Rev Sci Tech 8:11–36
d'Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F (1995) Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol 33:2665–2669
Ferris NP, Nordengrahn A, Hutchings GH, Reid SM, King DP, Ebert K, Paton DJ, Kristersson T, Brocchi E, Grazioli S, Merza M (2009) Development and laboratory validation of a lateral flow device for the detection of foot-and-mouth disease virus in clinical samples. J Virol Methods 155:10–17
Friedhoff KT (1997) Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 39:99–109
Gubbels JM, de Vos AP, van der Weide M, Viseras J, Schouls LM, de Vries E, Jongejan F (1999) Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J Clin Microbiol 37:1782–1789
Gubbels MJ, d'Oliveira C, Jongejan F (2000) Development of an indirect Tams1 enzyme-linked immunosorbent assay for diagnosis of Theileria annulata infection in cattle. Clin Diagn Lab Immunol 7:404–411
Habibi GR, Esmaeil-Nia K, Bozorgi S, Najjar E, Hashemi-Fesharki R, Bordbar N (2007) PCR-based detection of Theileria annulata infection and molecular characterization of Tams I T. annulata vaccine strain. Arch Razi Inst 62:83–89
Hooshmand-Rad P (1974) Blood protozoan diseases of ruminants. Bull Off Int Epizoot 81:779–792
Houghton RL, Stevens YY, Hjerrild K, Guderian J, Okamoto M, Kabir M, Reed SG, Leiby DA, Morrow WJ, Lorca M, Raychaudhuri S (2009) Lateral flow immunoassay for diagnosis of Trypanosoma cruzi infection with high correlation to the radioimmunoprecipitation assay. Clin Vaccine Immunol 16:515–520
Huang X, Xuan X, Hirata H, Yokoyama N, Xu L, Suzuki N, Igarashi I (2004a) Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J Clin Microbiol 42:351–353
Huang X, Xuan X, Xu L, Zhang S, Yokoyama N, Suzuki N, Igarashi I (2004b) Development of an immunochromatographic test with recombinant EMA-2 for the rapid detection of antibodies against Babesia equi in horses. J Clin Microbiol 42:359–361
Kim C, Alhassan A, Verdida RA, Yokoyama N, Xuan X, Fujisaki K, Kawazu S, Igarashi I (2007) Development of two immunochromatographic tests for the serodiagnosis of bovine babesiosis. Vet Parasitol 148:137–143
Kim CM, Blanco LB, Alhassan A, Iseki H, Yokoyama N, Xuan X, Igarashi I (2008) Development of a rapid immunochromatographic test for simultaneous serodiagnosis of bovine babesioses caused by Babesia bovis and Babesia bigemina. Am J Trop Med Hyg 78:117–121
Kirvar E, Ilhan T, Katzer F, Hooshmand-Rad P, Zweygarth E, Gerstenberg C, Phipps P, Brown CG (2000) Detection of Theileria annulata in cattle and vector ticks by PCR using the Tams1 gene sequences. Parasitology 120:245–254
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Liao M, Zhang S, Xuan X, Zhang G, Huang X, Igarashi I, Fujisaki K (2005) Development of rapid immunochromatographic test with recombinant NcSAG1 for detection of antibodies to Neospora caninum in cattle. Clin Diagn Lab Immunol 12:885–887
Mansfield MA (2005) The use of nitrocellulose membranes in lateral-flow assays. In: Wong RC, Tse HY (eds) Forensic science and medicine: drugs of abuse: body fluid testing. Humana, Totowa, pp 71–85
McCosker PJ (1979) Global aspects of the management and control of ticks of veterinary importance. In: Rodriguez JG (ed) Recent advances in acarology. II. Proceedings of the Vth International Congress of Acarology, East Lansing. Academic, New York, pp 45–53
Mills CD, Burgess DC, Taylor HJ, Kain KC (1999) Evaluation of a rapid and inexpensive dipstick assay for the diagnosis of Plasmodium falciparum malaria. Bull World Health Organ 77:553–559
Minjauw B, McLeod A (2000) Epidemiology and economics of tick-borne diseases: their effects on the livelihoods of the poor in East and Southern Africa and in India. Consultancy report to the Animal Health Programme (AHP) of the Department for International Development (DFID). Nairobi, Kenya, p 94
Mohammad Al-Saeed AT, Omer LT, Abdo J, Habibi G, Salih DA, Seitzer U, Ahmed J (2010) Epidemiological studies on tropical theileriosis (Theileria annulata infection of cattle) in Kurdistan Region, Iraq. Parasitol Res 106:403–407
Reithinger R, Quinnell RJ, Alexander B, Davies CR (2002) Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol 40:2352–2356
Renneker S, Kullmann B, Gerber S, Dobschanski J, Bakheit MA, Geysen D, Shiels B, Tait A, Ahmed JS, Seitzer U (2008) Development of a competitive ELISA for detection of Theileria annulata infection. Transbound Emerg Dis 55:249–256
Renneker S, Abdo J, Ahmed JS, Seitzer U (2009) Field validation of a competitive ELISA for detection of Theileria annulata infection. Parasitol Res 106:47–53
Salih DE, Ahmed JS, Bakheit MA, Ali EB, El Hussein AM, Hassan SM, Shariff OE, Fadl M, Jongejan F (2005) Validation of the indirect TaSP enzyme-linked immunosorbent assay for diagnosis of Theileria annulata infection in cattle. Parasitol Res 97:302–308
Salih DA, El Hussein AM, Seitzer U, Ahmed JS (2007a) Epidemiological studies on tick-borne diseases of cattle in Central Equatoria State, Southern Sudan. Parasitol Res 101:1035–1044
Salih DA, Hassan SM, El Hussein AM (2007b) Comparisons among two serological tests and microscopic examination for the detection of Theileria annulata in cattle in northern Sudan. Prev Vet Med 81:323–326
Salih DA, Liu Z, Bakheit MA, Ali AM, El Hussein AM, Unger H, Viljoen G, Seitzer U, Ahmed JS (2008) Development and evaluation of a loop-mediated isothermal amplification method for diagnosis of tropical theileriosis. Transbound Emerg Dis 55:238–243
Schnittger L, Katzer F, Biermann R, Shayan P, Boguslawski K, McKellar S, Beyer D, Shiels BR, Ahmed JS (2002) Characterization of a polymorphic Theileria annulata surface protein (TaSP) closely related to PIM of Theileria parva: implications for use in diagnostic tests and subunit vaccines. Mol Biochem Parasitol 120:247–256
Schnittger L, Yin H, Qi B, Gubbels MJ, Beyer D, Niemann S, Jongejan F, Ahmed JS (2004) Simultaneous detection and differentiation of Theileria and Babesia parasites infecting small ruminants by reverse line blotting. Parasitol Res 92:189–196
Seitzer U, Bakheit MA, Salih DE, Ali A, Haller D, Yin H, Schnittger L, Ahmed J (2007) From molecule to diagnostic tool: Theileria annulata surface protein TaSP. Parasitol Res 101(Suppl 2):S217–S223
Seitzer U, Beyer D, Kullmann B, Bakheit MA, Ahmed JS (2008) Evaluation of Theileria annulata recombinant immunodominant proteins for the development of ELISA. Transbound Emerg Dis 55:244–248
Shayan P, Biermann R, Schein E, Gerdes J, Ahmed JS (1998) Detection and differentiation of Theileria annulata and Theileria parva using macroschizont-derived DNA probes. Ann N Y Acad Sci 849:88–95
Thomas EE, Puterman ML, Kawano E, Curran M (1988) Evaluation of seven immunoassays for detection of rotavirus in pediatric stool samples. J Clin Microbiol 26:1189–1193
Uilenberg G (1981) Theileria species of domestic livestock. In: Irvin AD, Cunningham MP, Young AS (eds) Advances in the control of theileriosis. Proc. Internat. Conf., 9–13 Feb. 1981, Nairobi. Martinus Nijhoff, The Hague, pp 4–37
Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM, Zhao D, Chai SJ, Guo JQ (2006) Development of an immunochromatographic lateral flow test strip for detection of beta-adrenergic agonist clenbuterol residues. J Immunol Methods 312:27–33
Acknowledgements
This study was supported in part by the EU-funded Coordinated Action “Integrated Consortium on Ticks and Tick-Borne Diseases” (ICTTD-3), project number 510561.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdo, J., Kristersson, T., Seitzer, U. et al. Development and laboratory evaluation of a lateral flow device (LFD) for the serodiagnosis of Theileria annulata infection. Parasitol Res 107, 1241–1248 (2010). https://doi.org/10.1007/s00436-010-1994-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1994-8