Abstract
From a Clonorchis sinensis adult worm cDNA library, we isolated a cDNA clone encoding a novel lactate dehydrogenase (LDH) gene which encoded a putative protein with a predicted molecular weight of 35.6 kDa. The optimum pH and temperature for the enzyme were 7.5 and 50°C in the pyruvate reduction while 11 and 80°C in the lactate oxidation reaction, respectively. CsLDH showed no substrate inhibition by high lactate and NAD+ concentration, and the optimal pyruvate and optimal NADH concentrations were 10 and 0.5 mmol/l, respectively. The relative activities of these 2-oxocarboxylic acids were pyruvic acid>2-ketobutyrate>oxalacetic acid>α-ketoglutaric acid>phenylpyruvate. The cofactor 3-acetylpyridine adenine dinucleotide was much more effective than NAD+. The cofactor analogs in which the nicotinamide ring is replaced by 3-pyridinealdehyde were lower activity cofactors, while the nicotinamide ring is replaced by nicotinic acid or thionicotinamide which is not a cofactor to CsLDH. The succinic acid and malic acid are not substrates of CsLDH. Cu2+, Fe2+, and Zn2+ greatly inhibited the CsLDH activity both in the direction of pyruvate reduction and in the direction of lactate oxidation. The inhibition of CsLDH by gossypol may make gossypol a potential therapy drug or a lead compound for C. sinensis. Accordingly, the CsLDH may be a novel potential drug target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human clonorchiasis is endemic in southern China, Japan, Korea, and Vietnam. It has been reported that millions of people are infected by Clonorchis sinensis in these areas via the consuming of raw or undercooked freshwater fish (Crompton 1999). The adult fluke habitats in bile ducts and provokes epithelial hyperplasia and periductal fibrosis, resulting in abdominal discomfort, liver enlargement and obstructive jaundice, cholangitis, cholelithiasis, and the development of cholangiocarcinoma (Chung and Lee 1976; Lee et al. 1994). Despite its clinical importance, many aspects of biology and biochemistry of C. sinensis have not yet been defined. Search of new therapeutic drugs, vaccine candidates, and diagnostic antigens require the discovery of new potential targets and investigation of their biochemical characteristics among the C. sinensis proteins essential for the survival.
Lactate dehydrogenase (LDH, EC 1.1.1.27) is a glycolytic enzyme which catalyzes the interconversion of pyruvate and lactate in the presence of the nicotinamide adenine dinucleotide coenzyme. The enzyme is widely distributed among bacteria, plants, and animals. In vertebrates, LDH is a tetramer whose subunits are coded by three independent loci, LDH-A, LDH-B, LDH-C. Each of LDH isoenzymes exhibits distinct tissue expression and kinetic, physicochemical, and immunochemical properties (Mulkiewicz et al. 2000). LDH-A is found predominantly in white skeletal muscle, where it functions in the reduction of pyruvate to lactate under anaerobic conditions; LDH-B predominates in more aerobic tissues, such as heart and brain, and is kinetically suited to the oxidation of lactate to pyruvate (Stock and Whitt 1992). Another isoform LDH-C predominates in testis (Baumgart et al. 1996). While in invertebrate, the LDHs catalyzing the pyruvate and lactate interconversion are not well characterized. Two kinds of lactate dehydrogenase have been found in invertebrate tissues. l-LDH reduces pyruvate to l-lactate, whereas the same reaction when carried out by a different dehydrogenase (LDH, EC 1.1.1.28) yields only d-lactate. All species contain either a d(−) or l(+) lactic-acid-specific LDH (Mulkiewicz et al. 2001).
Schistosomes, trematodes dwelling in blood vessels, are anaerobic parasites that utilize exogenous glucose for energy generation through glycolytic pathway. These parasites can ingest as much glucose as 26% of their dry body weight per hour. Adult C. sinensis is also an anaerobic trematode and also takes up as much external glucose as schistosome does. C. sinensis transports the external glucose in muscular tissues and breaks it down through glycolysis producing lactate as the major end-product to supply energy and intermediate products needed for its physiological metabolism. Glycolytic enzymes are recognized as crucial molecules for trematode survival and have been targeted for vaccine development (Hong et al. 2000).
To our knowledge, no studies on lactate dehydrogenase of C. sinensis have been reported, though it is a very important enzyme. The focus of this study was on the identification of a novel C. sinensis LDH cDNA and on the molecular characterization of its gene product. The enzyme activity, pH and temperature optimum, kinetic parameters, substrate specificity, metal cations, and inhibitor of the purified recombinant C. sinensis LDH were studied.
Materials and methods
Chemicals
Lactate, pyruvic acid, malic acid, 3-acetylpyridine adenine dinucleotide (APAD), thionicotinamide adenine dinucleotide, nicotinic acid adenine dinucleotide, 3-pyridinealdehyde adenine dinucleotide, sodium phenylpyruvate, 2-ketobutyrate, oxalacetic acid, and gossypol were purchased from Sigma (St. Louis, MO, USA). Nicotinamide adenine dinucleotide and its reduced form (NADH), α-ketoglutaric acid, and succinic acid were purchased from Sangon (Shanghai, China). All other reagents were local products of analytical grade.
C. sinensis adult worm
C. sinensis adult worms were obtained from the bile ducts of infected cats in Guangdong province, China. The total RNA was extracted with Trizol (Yang et al. 2005).
cDNA library construction
A cDNA library was constructed in the modified pBluescript II SK(+) vector with C. sinensis mRNA extracted. The modified pBluescipt II SK(+) vector was constructed by inserting a DNA fragment with SfiIA (5′-GGCCATTATGGCC-3′) and SfiIB (5′-GGCCGCCTCGGCC-3′) recognition sites into the EcoRI and NotI sites of pBluescriptII SK(+) (Stratagene). Double-strand cDNA was synthesized using a SMART cDNA Library Construction Kit (Clontech) following the manufacturer’s instruction. After SfiI digestion and cDNA size fraction, cDNAs longer than 500 bp were ligated into SfiIA and SfiIB sites of the modified pBluescript II SK(+) vector. The constructs were then transformed into Escherichia coli DH5α cells. Individual clones were cultured overnight in Luria–Bertani (LB) broth with 100 μg/ml ampicillin, and plasmids were isolated using a QIAwell plasmid purification system (QIAGEN).
Sequencing of the cDNA insert
The cDNA inserts were sequenced on an ABI PRISM 377 DNA sequencer (PerkinElmer) using the BigDye Terminator Cycle Sequencing Kit and BigDye Primer Cycle Sequencing Kit (PerkinElmer). A-21M13 primer, M13Rev primer, and synthetic internal walking primers were designed according to the obtained cDNA sequence fragments. Each part of the insert was sequenced at least three times bidirectionally. Subsequent editing and assembly of all the sequences from one clone was performed using Acembly (Sanger Center).
Bioinformatics analysis of C. sinensis LDH gene
DNA and the deduced protein sequences were analyzed using BLAST2.0 by BLAST-N, BLAST-P, and BLAST-X algorithms at NCBI Web server (http://www.ncbi.nlm.nih.gov/BLAST). The open reading frame (ORF) was predicted by ORF finder program (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Protein analysis was done with Proteomics and sequence analysis tools, SWISS-MODEL Repository (http://www.expasy.ch). Other sequence analyses were performed using Genedoc and Generunner software.
Construction of a recombinant C. sinensis LDH clone for protein expression
The polymerase chain reaction (PCR) was employed to amplify the ORF for the CsLDH from the plasmid DNA with the sense primer: 5′-GTCCCATGGAAATGTCATCAATATTGAAACCCG -3′ with the Met initiation codon (boldfaced), with a NcoI site (underlined), and the antisense primer: 5′-GGCCTCGAGCCATTTGATGCCCTGGATAACTTTC-3′, with a XhoI site (underlined). Amplification conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min for 30 cycles using Pfu DNA polymerase (Sangon), following the manufacturer’s instructions. The amplified fragment was double digested with NcoI and XhoI endonucleases and subcloned into the corresponding restriction sites of the prokaryotic expression vector, pET-28b (Novagen). The recombinant plasmid was transformed into E. coli strain BL21. The transformants were selected on LB agar plates containing 50 μg/ml kanamycin. The clones containing recombinant plasmid were further selected by DNA sequencing using primers designed from positions on the vector upstream or downstream of the insert.
Expression and purification of recombinant C. sinensis LDH
The transformed E. coli cells were grown in LB medium containing 50 μg/ml kanamycin at 37°C. A clone which had the ORF of CsLDH in frame to the pET-28b 6× His-tagged protein was selected for an overproduction of recombinant CsLDH. Overnight culture of the plasmid (1/50 volume of the culture medium) was inoculated into the LB culture medium containing 50 μg/ml kanamycin and cultured to reach an OD600 about 0.9. Isopropyl-β-d-1-thiogalactopyranoside (IPTG) was added to the culture to a final concentration of 1 mmol/l, and then cultured for another 3 h. All of the subsequent protein purification procedures were carried out at 0–4°C. The bacterial cells were pelleted by spinning the culture medium and resuspended in a start buffer (10 mmol/l imidazole, 137 mmol/l NaCl, 10 mmol/l Na2HPO4, 2 mmol/l KH2PO4, 2.7 mmol/l KCl, pH 7.4) with 2 mmol/l 2-mercaptoethano and 0.5 mmol/l phenylmethylsulfonyl fluoride. The cells were lysed and homogenized in an ice bath with a sonicator at 15% power for 10 min. The homogenate was centrifuged at 10,000×g, 4°C for 10 min, the supernatant was stored for a further purification of the recombinant CsLDH, and the insoluble material of the cell lysate was removed by the centrifugation.
The soluble fraction of the recombinant 6× His-tagged CsLDH was purified by Ni–NTA metal-affinity chromatography (QIAGEN) under native conditions. The supernatant of the disrupted cells was bound to 3 ml Ni–NTA spin column. After the column had been washed with 20 ml start buffer (10 mmol/l imidazole, 137 mmol/l NaCl, 10 mmol/l Na2HPO4, 2 mmol/l KH2PO4, 2.7 mmol/l KCl, pH 7.4) and 20 ml wash buffer (50 mmol/l imidazole, 0.3 mol/l NaCl, 50 mmol/l NaH2PO4, pH 8.0), respectively, 6× His-tagged CsLDH was eluted with 10 ml elution buffer (100 mmol/l imidazole, 0.3 mol/l NaCl, 50 mmol/l NaH2PO4, pH 8.0). The eluted fraction containing 6× His-tagged CsLDH was concentrated by ultrafiltration through a microsep 30-kDa Filtron membrane with 20 mmol/l Tris (pH 7.5). Protein concentration was determined by the method of Bradford using bovine serum albumin (BSA) as a standard (Bradford 1976). The integrity of the 6× His-tagged CsLDH was checked by sodium dodecyl sulfide-polyacrylamide gel electrophoresis (SDS-PAGE).
Assay of enzymatic activity
The assay of CsLDH activity was conducted using a modification of the procedure of Dando et al. (2001). CsLDH activity for the reduction of pyruvate to lactate was determined in 100 mmol/l Tris, pH 7.5, with 10 mmol/l pyruvate and 0.5 mmol/l NADH. For the oxidation of lactate to pyruvate, LDH activity was measured in 100 mmol/l Tris, pH 9.0, with 100 mmol/l lactate and 1 mmol/l NAD+, 37°C, by following changes in optical density at 340 nm, ɛ=6.22 mmol−1 cm−1. The reaction was started by the addition of CsLDH enzyme and incubated at 37°C. The enzyme reactions were measured with a U-3000 spectrophotometer (Hatachi, Japan) for 1 min after starting the reaction. Each datum represented the mean value of three samples. One unit of activity was defined as 1 μmol of product (NAD+) formed for the reduction of pyruvate to lactate and 1 μmol of product (NADH) formed for the oxidation of lactate to pyruvate per minute per milligram of protein.
Effect of pH and temperature on enzyme activity
The enzyme activity was measured in the pH range for the reduction of pyruvate to lactate from 6.0 to 11.5 and for the oxidation of lactate to pyruvate from 7.0 to 13.0. Buffers used were 100 mmol/l Tris–HCl (pH 6.0–10.0) and 100 mmol/l glycine–sodium hydroxide (pH 11.0–13.0). To test the effect of temperature on enzyme activity, reactions were performed at various temperatures (10–100°C). The reaction temperatures were adjusted in a temperature-controlled water circulation bath.
Enzyme kinetics assay of C. sinensis LDH
For analysis of enzyme kinetics of CsLDH, varying concentrations (0.25, 0.5, 0.8, 1.0, 5.0, 10.0 mmol/l) of pyruvate was used while the NADH was kept constant at 0.5 mmol/l; varying concentrations (1.0, 1.25, 2.0, 2.5, 5.0, 10.0, 100 mmol/l) of lactate was used while the NAD+ was kept constant at 1 mmol/l; varying concentrations (0.05, 0.1, 0.2, 0.25, 0.5 mmol/l) of NADH was used while the pyruvate was kept constant at 10 mmol/l; varying concentrations (0.1, 0.125, 0.2, 0.5, 1.0, 2.0 mmol/l) of NAD+ was used while the lactate was kept constant at 100 mmol/l. The results were analyzed by double reciprocal Lineweaver–Burk plot by using Origin 6.0 and SPSS10.0. Kinetic parameters (Km, Vmax) were computed from these plots.
Substrate inhibition study of C. sinensis LDH
The assays for substrate inhibition were initiated by the addition of enzyme to reaction mixtures containing excessive substrates whose concentrations were varied from 5, 10, 15, 20, 25, to 30 mmol/l for pyruvic acid and from 0.25, 0.5, 0.75, 1, 1.25, 1.5, to 2 mmol/l for NADH.
Substrate specificity study of C. sinensis LDH
Various combinations—2-ketobutyrate, phenylpyruvate, α-ketoglutaric acid, and oxalacetic acid for pyruvic acid; succinic acid and malic acid for lactate; APAD, thionicotinamide AD, nicotinic acid AD, and 3-pyridinealdehyde AD for NAD+—were tested for their ability to act as substrates of the CsLDH. The test conditions of standard reaction were as described, except for using the corresponding substrate concentration. Results with the tested combinations are compared with the standard reaction.
The effects of cations study of C. sinensis LDH
To determine the effects of cations, the samples were incubated in the presence of 2 mmol/l each of divalent metal ions (Zn2+, Mg2+, Cu2+, Fe2+, Ba2+, Ni2+, Ca2+, and Mn2+).
Gossypol inhibition effect on the C. sinensis LDH
The inhibition of gossypol for CsLDH was assayed in the reaction by adding various amounts of Gossipol (0.01, 0.1, 0.25, 0.5, 0.75, 1 mmol/l) to the standard reaction mixture.
Results
Identification of the Figures LDH cDNA
The CsLDH cDNA was identified from an adult C. sinensis cDNA library by large-scale sequencing of the expressed sequence tag (EST). The 1,230-bp cDNA spanned an ORF from 83 to 1,069 and encoded a putative protein of 328 amino acids with a predicted molecular mass of 35.6 kDa. It is a full-length cDNA encoding a putative protein because an inphase stop codon (TAG) were present 5′ to the ATG start codon (Fig. 1). Significantly, the putative protein contained a single lactate dehydrogenase catalytic domain (amino acid 20 Lys–324 Gln), which is conserved in known lactate dehydrogenase. In a search against the GenBank database, the protein identified here was found to be 76% identity to Schistosoma japonicum LDH, 29% identity to Plasmodium falciparum LDH, 37% identity to Bacillus stearothermophilus LDH, 24% identity to Trichomonas vaginais LDH; 30% identity to Toxoplasma gondii LDH, 58% identity to Homo sapiens LDH-A, 56% identity to H. sapiens LDH-B, and 56% identity to H. sapiens LDH-C. Therefore, we concluded that the cDNA we cloned encoded a new member of lactate dehydrogenase. The gene was then named CsLDH. Residues in the crystal structure of P. falciparum LDH were replaced with the CsLDH sequence according to the alignment in Fig. 2. The CsLDH consists of two domains: the N-terminal dinucleotide cofactor binding domain (residues 1–156) and the C-terminal catalytic domain (residues 157–325). The dinucleotide binding domain has the typical Rossmann fold, comprising a twisted parallel six-stranded β-sheet (S1–S6) and flanking α-helices (H1–H4). The catalytic domain is similar to those of other LDHs. It consists of four long helices (H5–H8) and six strands (S7–S12) (Lee et al. 2001; Fig. 2).
Nucleotide and deduced amino acid sequences of the Clonorchis sinensis LDH gene. The nucleotide sequence of the 1,230-bp cDNA is shown on the top line, and its predicted amino acid sequence was shown below in a single-letter code. Numbers on the left refer to the first nucleotide in each corresponding line. An upstream inphase stop codon (TAG) at 5′ to the start codon is shaded in gray. Asterisk indicates the stop codon. The putative poly (A)-addition signal is set in bold italic. The sequence data reported here have been deposited in the GenBank (GenBank accession no. AY666121). The substrate specificity loop of CsLDH is underlined
Alignment of the deduced amino acid sequences of the LDH genes of C. sinensis and other organisms. Accession numbers (GenBank) for the other sequences are as follows: Plasmodium falciparum LDH, Bacillus stearothermophilus LDH, Trichomonas vaginais LDH, Toxoplasma gondii LDH, Schistosoma japonicum LDH, Homo sapiens LDH-A, H. sapiens LDH-B, H. sapiens LDH-C, whose GenBank accession numbers are AF323520, M19396, AF060233, U35118, AY225190, BC067223, BC071860, and NM_002301, respectively. Numbers on the right refer to the last amino acid in each corresponding line. Identity is indicated by a black box, and similarity is indicated by a gray box. Secondary structure elements are indicated above the sequence. Â-strands, α-helices, and 310-helices are labeled sequentially as S1–S12, H1–H8, G1–G5, respectively. L1 and L2 are the active-site and active-control loops, respectively (Lee et al. 2001). Underneath the alignment, asterisks mean that the residues in that column are nicotinamide binding site, pound signs indicate the pyruvate binding site according to the B. stearothermophilus LDH crystal model (Sessions et al. 1997), and plus symbols indicate the pyruvate binding sites according to the Trichomonas vaginalis LDH crystal model (Wu 1989)
Purification of C. sinensis LDH
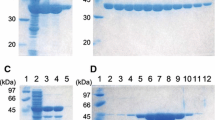
CsLDH samples from the different purification steps were analyzed by electrophoresis on 10% SDS-PAGE. One major protein band corresponding to the expected 35.6-kDa 6× His-tagged CsLDH was detected in the elution fraction (Fig. 3). In total, 0.57 mg protein was obtained from 500 ml cell culture, and the concentration was 1.14 mg/ml. In a representative purification procedure, the final preparation represented 5.33-fold enrichment and had a specific activity of about 415.8 U/mg (Table 1).
Expression and purification of CsLDH. The samples were analyzed by SDS-PAGE stained with Coomassie brilliant blue R-250. Lane 1, protein molecular marker; lane 2, BL21 cell containing pET28b without isopropyl-β-d-1-thiogalactopyranoside (IPTG) induction; lane 3, BL21 cell containing pET28b with IPTG induction; lane 4, BL21 cell containing pET28b-CsLDH without IPTG induction; lane 5, BL21 cell containing pET28b-CsLDH with IPTG induction; lane 6, insoluble fraction of BL21 cell containing pET28b-CsLDH with IPTG induction; lane 7, soluble fraction of BL21 cell containing pET28b-CsLDH with IPTG induction; lane 8, the elution fraction (CsLDH) of purified 6× His-CsLDH
Effect of pH on CsLDH enzyme activity
Figure 4a shows pH profiles for pyruvate reduction and lactate oxidation. In the pyruvate reduction, reaction CsLDH had pH optima at pH 7.5 and the enzyme was inactive below pH6.0. In the lactate oxidation reaction CsLDH had broad pH optima between 9.0 and 13.0. For the pyruvate reduction reaction, CsLDH activity sharply decreased below 7.0, and for the lactate oxidation reaction, CsLDH activity sharply decreased below 9.0.
a pH activity curves for CsLDH in the direction of pyruvate reduction (▪) and lactate oxidation (▾). The CsLDH activity was measured as described in “Materials and methods” at 37°C. b Effect of temperature on CsLDH enzyme activity. CsLDH activity was measured in a standard reaction as described in “Materials and methods” in the direction of pyruvate reduction (▪) at pH 7.5 and in the direction of lactate oxidation (▾) at pH 9.5. c Substrate inhibition of CsLDH by pyruvate. The reactions were measured in a standard as described in “Materials and methods” except the various concentrations of pyruvate, and the reactions were measured at pH 7.5, 37°C. d Substrate inhibition of CsLDH by NADH. The reactions were measured in a standard as described in “Materials and methods” except the various concentrations of NADH, and the reactions were measured at pH 7.5, 37°C. e Effects of divalent metal ions on CsLDH activity. The reaction mixtures were incubated in the presence of 2 mmol/l each of the indicated divalent metal ions. The left black column was in the direction of pyruvate reduction at pH 7.5, 37°C, and the right white column was in the direction of lactate oxidation at pH 9.5, 37°C. f Gossypol inhibition effect on the CsLDH. CsLDH activity was measured in a standard reaction as described in “Materials and methods” except for adding various concentrations of gossypol
Effect of temperature on CsLDH enzyme activity
In the direction of pyruvate reduction, the temperature optimum was at 50°C, and the temperature curve was a standard overturned bell shape. In the direction of lactate oxidation, the temperature optimum was at 80°C. From 10 to 100°C, the CsLDH activity had a slight change except that there is a pinnacle around 80°C (Fig. 4b).
Determination of catalytic parameters, Km, Vmax, of CsLDH
Michaelis constant values for pyruvate, NADH, lactate, and NAD+ and Vmax values for pyruvate and lactate were calculated and were listed in Table 2. The Km of pyruvae was about one sixth of the Km of lactate, and the Km of NADH was four times to the Km of NAD+. The Vmax of pyruvate was largely more than the Vmax of lactate.
Substrate inhibition study of C. sinensis LDH
The activity of the CsLDH was inhibited by high concentration of pyruvate and reduced to 6% of maximal activity at 25 mmol/l pyruvate (Fig. 4c). Also, the activity of the CsLDH was inhibited by high concentration of NADH and reduced to 4% of maximal activity (1.25 mmol/l NADH; Fig. 4d). The optimal pyruvate concentration was 10 mmol/l, and the optimal NADH concentration was 0.5 mmol/l. CsLDH showed no substrate inhibition by high lactate and NAD+ concentration (data not shown).
Substrate specificity
Substrate specificity of CsLDH was determined at pH 7.5 of 2-oxocarboxylic acid. Pyruvic acid, 2-ketobutyrate, oxalacetic acid, α-ketoglutaric acid, and phenylpyruvate were substrates of CsLDH. The relative activities of these 2-oxocarboxylic acids were pyruvic acid>2-ketobutyrate>oxalacetic acid>α-ketoglutaric acid>phenylpyruvate. The reverse reaction at pH 9.5 was studied with lactate as substrate, while the succinic acid and malic acid are not substrates of CsLDH. The cofactor specificities of CsLDH were studied. The APAD was much more effective than NAD+. The cofactor analogs in which the nicotinamide ring is replaced by 3-pyridinealdehyde were lower activity cofactors, while the nicotinamide ring is replaced by nicotinic acid or thionicotinamide which is not a cofactor to CsLDH (Table 3).
The effects of cations study of C. sinensis LDH
In the direction of pyruvate reduction, 2 mmol/l Mg2+, Ba2+, and Ca2+ enhanced the CsLDH activity, whereas Mn2+ and Ni2+ only caused a very small enhancement of the activity; in the direction of lactate oxidation, 2 mmol/l Ni2+ inhibited the CsLDH activity, while Mg2+, Ba2+ Mn2+, and Ca2+ showed a little effect on the activity. Cu2+, Fe2+, and Zn2+ greatly inhibited the CsLDH activity both in the direction of pyruvate reduction and in the direction of lactate oxidation (Fig. 4e).
Gossypol inhibition effect on the C. sinensis LDH
In the direction of pyruvate reduction, gossypol had a lower inhibition effect on the CsLDH, while in the direction of lactate oxidation, gossypol had a strong inhibition effect on the CsLDH (>85% inhibition by 1.0 mmol/l gossypol; Fig. 4f).
Discussion
LDH is a tetrameric protein whose molecular mass of each unit is 35 kDa. The predicted molecular mass of the CsLDH is 35.6 kDa, which is identical to the most of lactate dehydrogenase from vertebrate and invertebrate organisms (Wyckoff et al. 1997; Turgut-Balik et al. 2004). In a search against the GenBank database, the identity of CsLDH to other organisms was from 24 to 76%. Though the identity varied from other organism LDHs, the CsLDH share the same catalytic residues that are conserved in all LDH, including arginine-165 (binds pyruvate), aspartate-162 and histidine-189 (that proton donor couple), and arginine-102 (polarizes the pyruvate carbonyl group; Turgut-Balik et al. 2004). A surface loop of 24 residues moves approx. 10 Å to close over the active site when substrate/cofactor is bound (Gerstein and Chothia 1991). This loop closure is the rate-limiting step in catalysis (Waldman et al. 1988; Clarke et al. 1989). To test whether CsLDH encodes a functional enzyme, we studied the enzymatic properties of the recombinant CsLDH expressed and purified from E. coli. The final preparation was a soluble CsLDH whose activity was about 416 U/mg. The expression and purification of CsLDH provided the possibility of estimating whether it has vaccine and diagnosis value or not, which also offered the backgrounds on what functions it has in the metabolism on the cell level of C. sinensis. Regularly, the kinetic of the purified recombinant C. sinensis was studied.
Among three types of LDH, the LDH-A isozyme is best suited for pyruvate reduction in anaerobic tissues (muscle), whereas the LDH-B isozyme is superior for lactate oxidation in aerobic tissue (heart; Mannen et al. 1996). Catalysis of CsLDH in the reverse reaction, the oxidation of lactate to pyruvate, was extremely low, exhibiting an approximate Vmax only 3.1% the rate of pyruvate reduction. Considering the CsLDH has much more activity in the direction of pyruvate reduction and the CsLDH sequence has higher identity to human LDH-A, the CsLDH maybe a new member of LDH-As. The classification of LDHs mainly depends on their location in the tissue. We are presently preparing antibodies against the CsLDH protein to study the tissue distribution and subcellular location of the CsLDH in detail.
The activity of the CsLDH was inhibited by high concentration of pyruvate and NADH. Substrate inhibition is a common property of LDH (Dunn et al. 1996). High concentrations of ketoacid substrate inhibit most nature hydroxyacid dehydrogenases due to the formation of an abortive enzyme–NAD+–ketoacid complex. An analysis of the mammalian LDHs showed that the amide of niconamide cofactor formed a water-bridged hydrogen bond to S163. The LDH of P. falciparum is not inhibited by its substrate and, uniquely, in this enzyme, the serine is replaced by a leucine. In the S163L mutant of human LDH-A4, pyruvate inhibition is indeed abolished and the enzyme retains high activity (Eszes et al. 1996). CsLDH showed no substrate inhibition by high lactate and NAD+ concentration. The biological significance of the lack of substrate inhibition of CsLDH by lactate and NAD+ and having substrate inhibition of CsLDH by pyruvate and NADH may be related to the metabolic adjustment. Considering the CsLDH has much more activity in the direction of pyruvate reduction than that in the direction of lactate oxidation, this can ensure that the cells have adequate lactate to be deposited. The lower lactate oxidation speed can ensure that the lactate will be used effectively. The steady level of lactate is important (Immke and McCleskey 2001) so the substrate inhibition effect can make the lactate level not higher.
The conserved residues involved in the binding of a substrate and the catalytic reaction have been identified in LDH. For example, an aspartate-162 and histidine-189 pair plays an essential role in the catalysis, and arginine-165 is important for substrate binding and orientation and recognition in the substrate-binding site (Taguchi and Ohta 1991). The substrate specificity of CsLDH was examined. The enzyme effectively catalyzed the pyruvic acid reduction. The relative activities of these 2-oxocarboxylic acids were pyruvic acid>2-ketobutyrate>oxalacetic acid>α-ketoglutaric acid>phenylpyruvate (Table 3). The longer and more complex (benzene ring) the carbon chain of the 2-oxocarboxylic acid substrate is, the lower the activity is. All these 2-oxocarboxylic acid substrates have similar 2-oxocarboxylic structure so they can be catalyzed by the CsLDH. The aliphatic chain and benzene ring may influence the combination and orientation of the substrate and enzyme active site. Most enzymes of lactate dehydrogenase/malate dehydrogenase family have high substrate specificity for either lactate or malate, although some less-specificity variants are also known; for example, in Mycoplama genitalium, the same protein is believed to have both lactate dehydrogenase and malate dehydrogenase activities (Wu et al. 1999). However, the malic and succinic acids are not substrates of CsLDH. This indicates that the CsLDH may be seen as a more stringent enzyme in the lactate oxidation reaction than in the pyruvate reduction reaction. The CsLDH was observed to function with several NAD analogs altered in either the purine or pyridine portion of the dinucleotide. 3-Pyridinealdehyde AD was 69% as effective as NAD, whereas the presence of nicotinic acid AD and thionicotinamide AD resulted had no function as coenzyme. The CsLDH has much more activity with APAD compared with NAD as cofactors. Since loop closure is rate determining for LDH, this suggests that loop closure for CsLDH is faster when APAD replaces NAD (Dando et al. 2001).
Temperature is the most relevant variable to be optimized for enzyme reactor operation (Illanes et al. 2001). Homologous proteins from organisms adapted to different temperatures have yielded important insights into the molecular basis of protein adaptation (Miyazaki et al. 2000). LDHs have adapted to vary temperature by changing flexibility in small areas of molecular that affect the mobility of adjacent active-site structures (Field and Somero 1998). In the direction of pyruvate reduction, the CsLDH has higher activity between 37 and 60°C. C. sinensis is a liver fluke that lives in the biliary passages of mammals. The broad temperature optimum of CsLDH may be relevant to the adaptation to the parasitical host under complex environment. In the direction of lactate oxidation between 30 and 60°C, the CsLDH activity has a slight change that makes the use of lactate on a stable level. In the pyruvate reduction reaction, CsLDH had pH optima at pH 7.5, and the enzyme was inactive below pH 6.0. In the lactate oxidation reaction, CsLDH had broad pH optima between 9.0 and 13.0. Considering the different behavior on reduction and oxidation reaction under different pH, the pH may be a factor of adjusting the CsLDH activity. The mechanism of different pH behavior is now under investigation by analysing its secondary structure. All the kinetic parameters such as temperature optimum, pH optimum, Km, Vmax, and cation effects, which were measured in the experiment, accumulated the necessary data for the research on the molecular catalytic mechanisms of CsLDH and the subsequent applying researches in the future. We have acquired the crystal of CsLDH. The crystallization of CsLDH with different substrates and structure determinations are currently underway.
Gossypol, a polyphenolic binaphthyl disequiterpene isolated from cotton seeds, has been studied extensively in China as a potential male antifertility agent (Wu 1989). Inhibition of CsLDH by gossypol is strictly competitive with cofactor binding. Molecular modeling studies predict that gossypol will be complex at the cofactor site, consistent with the kinetic result (Dando et al. 2001). Gossypol can be viewed as a prototype of inhibitors targeting to dinucleotide folds and compounds structurally related to gossypol, such as selective inhibitors of dehydrogenase (Yu et al. 2001). The inhibition of CsLDH by gossypol may make gossypol a potential therapy drug or a lead compound for C. sinensis through interfering the anaerobic glycolysis metabolic net. Accordingly, the CsLDH may be a novel potential drug target.
In conclusion, we have identified a novel C. sinensis LDH cDNA and the molecular characterization of its gene product. The recombinant CsLDH enzyme activity and kinetic were studied. Collectively, the results available to date suggest that the CsLDH could play a key role in the physiology of C. sinensis. Further studies will focus on the function of the CsLDH protein in vivo at cell level and the molecular catalysis mechanism.
References
Baumgart E, Fahimi HD, Stich A, Völkl A (1996) l-Lactate dehydrogenase A4- and A3B isoforms are bona fide peroxisomal enzymes in rat liver. J Biol Chem 271:3846–3855
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chung CS, Lee SK (1976) An epidemiological study of primary liver carcinomas in Busan area with special reference to clonorchiasis. Korean J Pathol 10:33–46
Clarke AR, Atkinson T, Holbrook JJ (1989) From analysis to synthesis: new ligand binding sites on the lactate dehydrogenase framework. Trends Biochem Sci 14:145–148
Crompton DW (1999) How much human helminthiasis is there in the world? J Parasitol 85:397–403
Dando C, Schroeder ER, Hunsaker LA, Deck LM, Royer RE, Zhou X, Parmley SF, Vander Jagt DL (2001) The kinetic properties and sensitivities to inhibitors of lactate dehydrogenases (LDH1 and LDH2) from Toxoplasma gondii: comparisons with pLDH from Plasmodium falciparum. Mol Biochem Parasitol 118:23–32
Dunn CR, Banfield MJ, Barker JJ, Higham CW, Moreton KM, Turgut–Balik D, Brady RL, Holbrook JJ (1996) The structure of lactate dehydrogenase from Plasmodium falciparum reveals a new target for anti-malarial design. Nat Struct Biol 3:912–915
Eszes CM, Sessions RB, Clarke AR, Moreton KM, Holbrook JJ (1996) Removal of substrate inhibition in a lactate dehydrogenase from human muscle by a single residue change. FEBS Lett 399:193–197
Fields PA, Somero GN (1998) Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc Natl Acad Sci U S A 95:11476–11481
Gerstein M, Chothia C (1991) Analysis of protein loop closure. Two types of hinges produce one motion in lactate dehydrogenase. J Mol Biol 220:133–149
Hong SJ, Seong KY, Sohn WM, Song KY (2000) Molecular cloning and immunological characterization of phosphoglycerate kinase from Clonorchis sinensis. Mol Biochem Parasitol 108:207–216
Illanes A, Wilson L, Tomasello G (2001) Effect of modulation of enzyme inactivation on temperature optimization for reactor operation with chitin-immobilized lactase. J Mol Catal B Enzym 11:531–540
Immke DC, McCleskey EW (2001) Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4:869–870
Lee JH, Yang HM, Bak UB, Rim HJ (1994) Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two-step carcinogenesis. Korean J Parasitol 32:13–18
Lee BI, Chang C, Cho SJ, Eom SH, Kim KK, Yu YG, Suh SW (2001) Crystal structure of the MJ0490 gene product of the hyperthermophilic archaebacterium Methanococcus jannaschii, a novel member of the lactate/malate family of dehydrogenases. J Mol Biol 307:1351–1362
Mannen H, Tsoi SC, Pickford DB, Donald JA, Guillette LJ, Li SS (1996) Sequences of the lizard cDNAs encoding lactate dehydrogenase (LDH) isozymes A (muscle) and B (heart). Gene 171:303–304
Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH (2000) Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol 297:1015–1026
Mulkiewicz E, Zietara MS, Stachowiak K, Skorkowski EF (2000) Properties of lactate dehydrogenase from the isopod, Saduria entomon. Comp Biochem Physiol B Biochem Mol Biol 126:337–346
Mulkiewicz E, Zietara MS, Stromberg JO, Skorkowski EF (2001) Lactate dehydrogenase from the northern krill Meganyctiphanes norvegica: comparison with LDH from the Antarctic krill Euphausia superba. Comp Biochem Physiol B Biochem Mol Biol 128:233–245
Sessions RB, Dewar V, Clarke AR, Holbrook JJ (1997) A model of Plasmodium falciparum lactate dehydrogenase and its implications for the design of improved antimalarials and the enhanced detection of parasitaemia. Protein Eng 10:301–306
Stock DW, Whitt GS (1992) Evolutionary implications of the cDNA sequence of the single lactate dehydrogenase of a lamprey. Proc Natl Acad Sci U S A 89:1799–1803
Taguchi H, Ohta T (1991) d-Lactate dehydrogenase is a member of the d-isomer-specific 2-hydroxyacid dehydrogenase family, cloning, sequencing, and expression in Escherichia coli of the d-lactate dehydrogenase gene of Lactobacillus plantarum. J Biol Chem 266:12588–12594
Turgut-Balik D, Akbulut E, Shoemark DK, Celik V, Moreton KM, Sessions RB, Holbrook JJ, Brady RL (2004) Cloning, sequence and expression of the lactate dehydrogenase gene from the human malaria parasite, Plasmodium vivax. Biotechnol Lett 26:1051–1055
Waldman AD, Hart KW, Clarke AR, Wigley DB, Barstow DA, Atkinson T, Chia WN, Holbrook JJ (1988) The use of genetically engineered tryptophan to identify the movement of a domain of B. stearothermophilus lactate dehydrogenase with the process which limits the steady-state turnover of the enzyme. Biochem Biophys Res Commun 150:752–759
Wu D (1989) An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gyneacological disease. Drugs 38:333–341
Wu G, Fiser A, Ter Kuile B, Sali A, Muller M (1999) Convergent evolution of Trichomonas vaginalis lactate dehydrogenase from malate dehydrogenase. Proc Natl Acad Sci U S A 96:6285–6290
Wyckoff HA, Chow J, Whitehead TR, Cotta MA (1997) Cloning, sequence and expression of the l-(+) lactate dehydrogenase of Streptococcus bovis. Curr Microbiol 34:367–373
Yang G, Yu XB, Wu ZD, Xu J, Song LX, Zhang HM, Hu XC, Zheng NC, Guo LC, Xu J, Dai JF, Ji CN, Gu SH, Ying K (2005) Molecular cloning and characterization of a novel adenylate kinase 3 gene from Clonorchis sinensis. Parasitol Res 95:406–412
Yu Y, Deck JA, Hunsaker LA, Deck LM, Royer RE, Goldberg E, Vander Jagt DL (2001) Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem Pharmacol 62:81–89
Acknowledgements
This research was funded by grants from the Natural Science Foundation of Guangdong Province (team program), China, the key Science and Technique Foundation of Guangdong Province, China (No.2002B31005), and the key Science and Technique program of Guangzhou city, Guangdong Province, China (No. 200223-E4022). The experiments comply with the current laws of China in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, G., Jing, C., Zhu, P. et al. Molecular cloning and characterization of a novel lactate dehydrogenase gene from Clonorchis sinensis . Parasitol Res 99, 55–64 (2006). https://doi.org/10.1007/s00436-005-0125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0125-4