Abstract
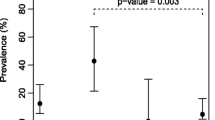
Zebra mussels (Dreissena polymorpha) from throughout the Shannon River drainage area in Ireland were tested for the anthropozoonotic waterborne parasites Cryptosporidium parvum, Giardia lamblia, Encephalitozoon intestinalis, E. hellem, and Enterocytozoon bieneusi, by the multiplexed combined direct immunofluorescent antibody and fluorescent in situ hybridization method, and PCR. Parasite transmission stages were found at 75% of sites, with the highest mean concentration of 16, nine, and eight C. parvum oocysts, G. lamblia cysts, and Encephalitozoon intestinalis spores/mussel, respectively. On average eight Enterocytozoon bieneusi spores/mussel were recovered at any selected site. Approximately 80% of all parasites were viable and thus capable of initiating human infection. The Shannon River is polluted with serious emerging human waterborne pathogens including C. parvum, against which no therapy exists. Zebra mussels can recover and concentrate environmentally derived pathogens and can be used for the sanitary assessment of water quality.


Similar content being viewed by others
References
Avery SW, Undeen AH (1987) The isolation of microsporidia and other pathogens from concentrated ditch water. J Am Mosq Control Assoc 3:54–58
Blackburn BL, Soave R (1997) Prophylaxis and chemotherapy: human and animal. In: Fayer R (ed) Cryptosporidium and cryptosporidiosis. CRC, Boca Raton, pp 111-128
Bornay-Llinares FJ, DaSilva AJ, Moura H, Schwartz DA, Visvesvara GS, Pieniazek NJ, Cruz-Lopez A, Hernandez-Jauregui P, Guerrero J, Enriquez FJ (1998) Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J Infect Dis 178:820–826
Breitenmoser AC, Mathis A, Burgi E, Weber R, Deplazes P (1999) High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology 118:447–453
Brieger G, Hunter RD (1993) Uptake and depuration of PCB 77, PCB 169, and hexachlorobenzene by zebra mussels (Dreissena polymorpha). Ecotoxicol Environ Safety 26:153—165
Bryan RT, Schwartz DA (1999) Epidemiology of microsporidiosis. In: Wittner M, Weiss LM (eds) The microsporidia and microsporidiosis. ASM, Washington, DC, pp 502–516
Buckholt MA, Lee JH, Tzipori S (2002) Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol 68:2595–2599
Chalmers RM, Sturdee AP, Mellors P, Nicholson V, Lawlor F, Kenny F, Timpson P (1997) Cryptosporidium parvum in environmental samples in the Sligo area, Republic of Ireland: a preliminary report. Lett Appl Microbiol 25:380-384
Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens MA, Trepo C (1999) Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J Infect Dis 180:2003–2008
DaSilva AJ, Schwartz DA, Visvesvara GS, Moura H, Slemenda SB, Pieniazek NJ (1996) Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (Microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol 34:986–987
DaSilva AJ, Slemenda SB, Visvesvara GS, Schwartz DA, Wilcox CM, Wallace S, Pieniazek NJ (1997) Detection of Septata intestinalis (Microsporidia) Cali et al. 1993 using polymerase chain reaction primers targeting the small subunit ribosomal RNA coding region. Mol Diagn 2:47–52
DaSilva AJ, Bornay-Llinares FJ, Moura LNS, Slemenda SB, Tuttle JL Pieniazek (1999) Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn 4:57–64
De Groote MA, Visvesvara GS, Wilson ML (1995) Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in patient with AIDS: successful therapy with albendazole. J Infect Dis 171:1375–1378
De Lafontaine Y, Gange F, Blaise C, Costan G, Gangon, Chan HM (1999) Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St. Lawrence River (Canada). Aquat Toxicol 50:51–57
Deplazes P, Mathis A, Muller C, Weber R (1996) Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J Eukaryot Microbiol 43:93S
Deplazes P, Mathis A, Weber R (2000) Epidemiology and zoonotic aspects of microsporidia of mammals and birds. In: Petry F (ed) Cryptosporidiosis and microsporidiosis. Karger, New York, pp 236–260
Dorsch MR, Veal DA (2001) Oligonucleotide probes for specific detection of Giardia lamblia cysts by fluorescent in situ hybridization. J Appl Microbiol 90:836–842
Dowd SE, Gerba CP, Pepper IL (1998) Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma cornea in water. Appl Environ Microbiol 64:3332–3335
Fournier S, Liguory O, Santillana-Hayat M, Guillot E, Sarfati C, Dumoutier N, Molina J, Derouin F (2000) Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol Med Microbiol 29:95–100
Frischer ME, Nierzwicki-Bauer SA, Resto M, Toro A, Toranzos GA (1999) Zebra mussels as possible biomonitors/filters of the protozoan pathogen Cryptosporidium. Dreissena Natl Aquat Nuisance Species Clearinghouse 10:1–4
Glaberman S, Moore JE, Lowery CJ, Chalmers RM, Sulaiman I, Elwin K, Rooney PJ, Millar BC, Dooley JS, Lal AA, Xiao L (2002) Three drinking-water-associated cryptosporidiosis outbreaks, Northern Ireland. Emerg Infect Dis 8:631–633
Graczyk TK (2003a) Human protozoan parasites in molluscan shellfish: epidemiology and detection. In: Villalba A, Requere B, Romalde JL, Beiras R (eds) Molluscan shellfish safety. Conselleria de Pesca e Asuntos Maritimos, Hunta de Galicia, pp 397–405
Graczyk TK (2003b) Human protozoan parasites in molluscan shellfish. J Parasitol 89:S57-S61
Graczyk TK, Fayer R, Cranfield MR (1997) Zoonotic potential of Cryptosporidium parvum: implications for waterborne cryptosporidiosis. Parasitol Today 13:348-351
Graczyk TK, Fayer R, Lewis EJ, Trout JM, Farley CA (1999) Cryptosporidium oocysts in Bent mussels (Ischadium recurvum) in the Chesapeake Bay. Parasitol Res 85:518-521
Graczyk TK, Marcogliese DJ, De Lafontaine Y, DaSilva AJ, Mhangami-Ruwende B, Pieniazek NJ (2001) Cryptosporidium parvum oocysts in zebra mussels (Dreissena polymorpha): evidence from the St. Lawrence River. Parasitol Res 87:231–234
Graczyk TK, Bosco-Nizeyi J, DaSilva AJ, Moura INS, Pieniazek NJ, Cranfield MR, Lindquist HD (2002) A single genotype of Encephalitozoon intestinalis infects free-ranging gorillas and people sharing their habitats in Uganda. Parasitol Res 88:926–931
Graczyk TK, Grimes BH, Knight R, DaSilva AJ, Pieniazek NJ, Veal DA (2003a) Detection of Cryptosporidium parvum and Giardia lamblia carried by synanthropic flies by combined fluorescent in situ hybridization and monoclonal antibody. Am J Trop Med Hyg 68:228–232
Graczyk TK, Conn DB, Marcogliese DJ, Graczyk H, De Lafontaine Y (2003b) Accumulation of human waterborne parasites by zebra mussels (Dreissena polymorpha) and Asian freshwater clams (Corbicula fluminea). Parasitol Res 89:107–112
Hester FD, Linquist HDA, Bobst AM, Schaffer FW (2000) Fluorescent in situ detection of Encephalitozoon hellem spores with a 6-carboxyfluorescein-labeled ribosomal RNA-targeted oligonucleotide probe. J Eukaryot Microbiol 47:299–308
Horgan MJ, Mills EL (1999) Clearance rate and filtering activity of zebra mussels (Dreissena polymorpha): implications for freshwater lakes. Can J Fish Aquat Sci 54:249–255
Jenkins MC, Trout J, Abrahamsen MS, Higgins J, Fayer R (2000) Estimating viability of Cryptosporidium parvum oocysts using reverse transcriptase-polymerase chain reaction (RT-PCR) directed at mRNA encoding amyloglucosidase. J Microbiol Methods 34:97–106
Jenkins M, Trout JM, Higgins J, Dorsch M, Veal D, Fayer R (2003) Comparison of tests for viable and infectious Cryptosporidium parvum oocysts. Parasitol Res 89:1–5
Kilani RT, Sekla L (1987) Purification of Cryptosporidium oocysts and sporozoites by cesium chloride percoll gradient. Am J Trop Med Hyg 36:505-508
Kotler DP, Orenstein JM (1999) Clinical syndromes associated with microsporidiosis. In: Wittner M, Weiss LM (eds) The microsporidia and microsporidiosis. ASM Press, Washington, DC, pp 258–292
Kucerova-Pospisilova Z, Carr D, Leitch G, Scanlon M, Visvesvara GS (1999) Environmental resistance of Encephalitozoon spores. J Eukaryot Microbiol 46:11S-13S
Lowery CJ, Nugent P, Moore JE, Millar BC, Xiru X, Dooley JS (2001a) PCR-IMS detection and molecular typing of Cryptosporidium parvum recovered from a recreational river source and an associated mussel (Mytilus edulis) bed in Northern Ireland. Epidemiol Infect 127:545–553
Lowery CJ, Millar BC, Moore JE, Xu J, Xiao L, Rooney PJ, Crothers L, Dooley JS (2001b) Molecular genotyping of human cryptosporidiosis in Northern Ireland: epidemiological aspects and review. Ir J Med Sci 170:246–250
Lowery CJ, Moore JE, Millar BC, McCorry KA, Xu J, Rooney PJ, Dooley JS (2001c) Occurrence and molecular genotyping of Cryptosporidium spp. in surface waters in Northern Ireland. J Appl Microbiol 91:774–779
McMahon RB (1991) Mollusca: bivalvia. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, pp 315–401
Minchin D, Lucy F, Sullivan M (2002a). Zebra mussel: impacts and spread. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe: distribution, impact and management. Kluwer, Dordrecht, pp 135–146
Minchin D, Lucy F, Sullivan M (2002b) Monitoring of zebra mussels in the Shannon-Boyle navigation, other navigable regions and principal Irish lakes, 2001 and 2002. Marine Institute, Dublin, Marine Environment and Health Series No. 5
Rinder H, Thomschke A, Sengjel B, Gothe R, Loscher T, Zahler M (2000) Close genetic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol 86:185–188
Rose JB, Lisle JT, LeChevallier M (1997) Waterborne cryptosporidiosis: incidence, outbreaks, and treatment strategies. In: Fayer R (ed) Cryptosporidium and cryptosporidiosis. CRC, Boca Raton, pp 93–110
Sharma S, Sachdeva P, Virdi JS (2003) Emerging water-borne pathogens. Appl Microbiol Biotechnol 5–6:424–428
Sparfel JM, Sarfati C, Liquory O, Caroff B, Dumoutier N, Gueglio B, Billaud E, Raffi F, Molina LM, Miegeville M, Derouin F (1997) Detection of microsporidia and identification of Enterocytozoon bieneusi in surface water by filtration followed by specific PCR. J Eukaryot Microbiol 44:78S
Vesey G, Ashbolt N, Fricker EJ, Deere D, William KL, Veal DA, Dorsch M (1998) The use of a ribosomal RNA targeted oligonucleotide probe for fluorescent labeling of viable Cryptosporidium parvum oocysts. J Appl Microbiol 85:429–440
Visvesvara GS, DaSilva AJ, Croppo GP, Pieniazek NJ, Ferguson D, Moura H, Wallace S, Slemenda SB, Tyrrel I, Moore DF, Meador J (1995) In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol 33:930–936
Wolfe MS (1992) Giardiasis. Clin Microbiol Rev 5:93–100
Acknowledgements
The study was supported by the U.S. Environmental Protection Agency, Washington, DC (grant no. R824995), The Center for A Livable Future, Baltimore, MD (grant no. H040-951-0180), the Polish Committee for Scientific Research (grant no. 604C02421), and a NATO Collaborative Linkage Grant, CLG 979765.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graczyk, T.K., Conn, D.B., Lucy, F. et al. Human waterborne parasites in zebra mussels (Dreissena polymorpha) from the Shannon River drainage area, Ireland. Parasitol Res 93, 385–391 (2004). https://doi.org/10.1007/s00436-004-1142-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1142-4