Abstract
In 2021/2022, galls formed by a nematode, Anguina woodi, were found on the stems, leaves and leaf sheaths of dune grass, Ehrharta villosa var. villosa on Milnerton Beach, Blouberg Beach and Langebaan, Western Cape Province, South Africa. These galls were spongy in texture, deep purple to blackish in colour and non-pedunculate. They were found in clusters, but also as single entities. Larger, harder galls varying from beige to dark brown in colour, apparently caused by insects, were also found on the stems of dune grass at Blouberg Beach and Langebaan Nature Reserve. Some nematode galls were found immediately next to or on top of the insect galls. Those found on top of insect galls seemed to be harder and drier than those found on stems not infected by insect galls. The co-infection of insects and anguinid nematodes has not been reported from the current study areas and was thus included in the present study. Both molecular and morphological studies were conducted on the nematodes and wasps leading to the identification of a host specific, gall-forming nematode from all three localities. The wasps were identified morphologically and molecularly to the family Eurytomidae (Hymenoptera). No insect galls were found on dune grass from Milnerton Beach. The paper includes speculations on a probable association between nematodes and insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2021/2022, anguinid galls were found on dune grass, Ehrharta villosa Schult. at Milnerton Beach, Blouberg Beach and Langebaan, Western Cape Province, South Africa. In basic morphology the nematode galls seemed conspecific with galls formed by Anguina woodi Swart et al. 2004 from Milnerton Beach on dune grass E. villosa Schult. var. villosa (Swart et al. 2004). According to Subbotin et al. (2004), preliminary molecular data suggest that anguinid nematodes are generally associated with host plants from the same or related systematic groups in the Poaceae and the Asteraceae. However, they suggested that the combined analysis of the ITS region of different genes would enhance our understanding of gall-forming nematode phylogeny and their coevolution with host plants.
Further examination of the dune grass stems showed larger, hard, dark brown galls, which on opening them contained insect larvae and showed small exit holes of wasps. Nematode galls sometimes situated on top of, or next to the insects’ galls, were always brown to dark brown in colour, and appeared harder and drier than those found on grass stems and leaves. This study was therefore expanded to include an examination and counts of the occupants of the nematode galls that were in close proximity to the insect galls. In South Africa, the gall midge Mitodiplosis graminis Kieffer induces stem galls on E. villosa var. villosa (Kolesik and Wood 2019). They also reported that the large, empty larval chambers left by the midge were occupied by A. woodi, a host-specific, gall-forming nematode. Kolesik and Wood (2019) argued that it seemed unlikely that this nematode was involved in a mutualistic relationship with the gall midge, as is found between the nematodes of the genus Fergusobia and the gall flies of the genus Fergusonina in Australasia. Another report from South Africa described the association between the nematode Schistonchus africanus Vovlas, Troccoli, Van Noord & Van den Berg, 1998, a strangler fig tree (Ficus thonningii Blume) and its pollinator wasp, Elisabethiella stuckenbergi Grandi (Vovlas et al. 1998).
The present paper presents the results of the identification of the Anguina nematodes found in three localities in South Africa, as well as the counts of the nematodes within the galls at the different localities. The insects inside the insect galls were also identified and possible associations between the nematodes and insects were explored.
Materials and methods
Study sites
The study areas selected for this study were Milnerton Beach, Langebaan, (West Coast National Park) and Blouberg Beach (Kelpbaai) in the Western Cape Province and will be referred to as Milnerton, Langebaan and Blouberg in the rest of this study. These localities were chosen, as known and unknown Anguina species have been reported from them (Swart et al. 2004; Marais 2022; A. Wood, personal communication). A research permit to sample organisms associated with E. villosa in the West Coast and Agulhas National Parks was obtained by A. Wood (Permit No. CRC/2022–2023/005—2022/V1).
Grass samples with galls were collected by A. Wood from Milnerton Beach (S 33° 50´41.6 E´ 18° 29 15.9’) (Fig. 1 a-c), Langebaan (S 33° 07ʹ 33.9″ E 18° 03ʹ 37.7″) (Fig. 2b and c), and from Blouberg Beach (S 33° 45ʹ 17.9″ E 18° 26ʹ 31.8″) (Fig. 2a). Twenty galls were randomly chosen from each locality and the nematodes within them were sorted into females, males, juveniles and eggs, which were counted per gall. Insect galls were found on grass from Langebaan and Blouberg only.
Nematode studies
Morphological identification
A total of 20 galls were randomly chosen from Milnerton and Langebaan, and 20 females and 20 males were selected from each locality. Working under a Nikon dissecting microscope, fitted with a light source from above, a scalpel was used to cut open the galls. Two layers were covering each nematode gall, the first being harder and the inner layer softer and lighter in colour. After removing the second layer, the nematodes were visible or were spontaneously released from the galls (Fig. 10b). Nematodes were killed and fixed in FAA (6 ml formalin (40%), 20 ml ethanol (95%), 1 ml acetic acid and 40 ml distilled water) at a temperature of 60–70 °C. After 2 days, the nematodes were transferred to the dehydrating mixture of 1.25 ml glycerol and 98.75 ml distilled water to start their processing into pure glycerol. The Syracuse dish with the nematode suspension was partly covered and kept for 6 weeks at room temperature to allow the dehydration mixture to slowly evaporate, leaving the nematodes in pure glycerol. The Syracuse dish was then stored in a desiccator, with CaCl2 as the desiccant, until the specimens could be mounted (Hooper 1970; Kleynhans et al. 1996; Marais et al. 2017). The nematodes were mounted on permanent glass microscope slides and measurements and drawings were done by means of a Nikon Labophot-2 Research Microscope equipped with a drawing tube at the Nematology Unit at Agricultural Research Council-Plant Health and Protection (ARC-PHP), Roodeplaat East Campus, Pretoria. Measurements of curved structures were made along the median line. The Blouberg anguinids were identified by molecular means only. As they were molecularly conspecific with the anguinids from Milnerton and Langebaan, a morphological identification was deemed unnecessary.
The following morphometrical terms were used in the study:
L: total body length in millimetres (mm).
a: body length/body width at vulva.
b: body length/distance from anterior end to end of pharynx lobes.
c: body length/tail length (anus or cloaca to tail terminus).
cʹ: tail length/body width at anus or cloaca.
V: distance from anterior end to vulva/body length × 100 (%).
DGO: length from the posterior end of stylet knobs to dorsal oesophageal gland opening (µm).
PUS: length of the post-uterine sac from the vulva to the posterior end of the sac (µm).
Molecular identification
For DNA extraction, nematodes were collected from galls and put into 1.5 µl micro centrifuge tubes containing 8 µl of distilled water. Three micro centrifuge tubes were used and labelled as follows: M for Milnerton, L for Langebaan and B for Blouberg. Tubes M1 and L1 contained one nematode, tubes M2 and L2 had two nematodes and tubes M3 and L3 had 10 nematodes. Ten microlitres (10 μl) nematode extraction buffer was added containing the following ingredients: 10 mM Tris, pH 8.2; 2.5 mM MgCl2; 50 mM KCl2; 0.45% Tween 20; 0.05% gelatine and 60 μg/ml Proteinase-K (Thomas et al. 1997) was added and frozen at − 70 °C for 15 min. The extract was put in a water bath at 60 °C for 60 min and then the Proteinase-K was denatured by heating at 95 °C for 15 min (Ma et al. 2011). The DNA amplification was performed using conventional PCR (Powers et al. 2001) in the Thermal Cycler (Bio-Rad T100 machine,) according to the cycling parameters described in Table 1 (See supplementary information). PCR was performed in 0.2 ml microcentrifuge tubes with the reaction mixture containing 10× PCR buffer for DNA polymerase and 12.5 μl OneTaq™ (Master Mix), 1 μl of the forward TW81: 5ʹ-GTTTCCGTAGGTGAACCTGC-3ʹ and reverse AB28: 5′-ATATGCTTAAGTTCAGCGGGT-3ʹ (Howlett et al. 1992) primers, 2 μl of DNA was added and 8.5 μl of a double-distilled water to obtain a final volume of 25 μl. The parameter composition had a concentration of 1.5 uM and 10 µl of DNA added. The parameters in Table 1 were used to run PCR using TW81 and AB28 primers with a total volume of 25 μl for samples M1, M2, M3, L1, L2 and L3, each sample with 1 μl of DNA. The third PCR was run with parameters on Table 1 with total volume of 25 μl, but with 2 μl DNA added. The PCR products were analysed using agarose gel electrophoresis to assess the quantity and quality of the DNA (Ami et al. 2019). The PCR products were sent for Sanger sequencing at inqaba biotec™ in Pretoria (South Africa) with the same primers used in the amplification of DNA during PCR. New sequences were submitted to GenBank under accession numbers: SUB12685428 B1-TW-AB OQ342843; SUB12685428 L1-TW-AB OQ342844; SUB12685428 M1-TW-AB OQ342845.
Phylogenetic analyses were conducted in MEGA11. Sequences were aligned using MUSCLE algorithm. Initial tree for the heuristic search was obtained automatically by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then selecting the topology with superior log-likelihood value.
Insect studies
Morphological identification
Emergence boxes was used to collect insects that emerged from the grass galls as described by Uys and Urban (2006). The galled plant material was placed in emergence boxes with the vial facing the light. The flying insects were attracted to the light, they accumulated in the vials and were collected with tweezers or a brush. The collected insects were preserved in 70% alcohol to prevent fungal growth before putting them into gelatine capsules to be stored for future use, which were held on a standard insect pin. A small wad of cotton wool was placed inside the capsule to prevent the specimens from being shaken. The cotton wool was smoothened and compacted before inserting it to prevent the insects becoming entangled in the fibres. Morphological identification of insects was accomplished by using characters in the following taxonomic keys: one emerged fly was tentatively identified to the family Chloropidae Rondani 1856, and the wasp, Eurytomidae Walker 1832, according to Goulet and Huber (1993). Because the Eurytomidae specimens could not be identified to species level by using morphological keys, it was decided to identify them by molecular approach. As only one fly was available for the study, further identification of this specimen was abandoned.
Molecular identification
For molecular identification, five adult wasps were collected from Blouberg, four adults and two larvae from Langebaan. The collected insects were immediately placed in absolute alcohol (95% ethyl alcohol) to preserve DNA for molecular identification. The collected adults and larvae from Langebaan were placed in PCR tubes and labelled 2-folmer-HCO and 3-folmer-HCO, respectively. Adult wasps from Blouberg were placed in a PCR tube and labelled Ib2. DNA extraction was done by using the Qaigen Kit (DNeasy Plant Maxi) following the manufacturer’s protocol.
Amplification of the CO I region using primer sequence specific to HCO (Folmer FW)5ʹ TAA ACT TCA GGG TGACCA AAA AAT CA 3ʹ (Folmer 1994) LCO (Folmer Rev) and5' GGT CAA CAA ATC ATA AAGATA TTG G 3ʹ insects (Folmer et al. 1994). Each PCR tube contained 2 µl of 10–20 ng total DNA, 2 µl of 10 µM of the forward primer, 2 µl of 10 µM of the reverse primer, 12.5 µl of the Taq MasterMix from Qaigen and nuclease-free water was added to a total volume to 25 µl. The electrophoresis gel consisted of 0.6 g agarose, 50 ml 1× TBE buffer and 1 µl ethidium bromide. One (1 µl) loading dye + 5 µl PCR product were loaded in the gel and ran for 30 min at 100 V. The amplification parameters used for PCR are shown in Table 2 (See Supplementary information). The PCR products were sent to Inqaba Biotechnology lab for Sanger sequencing. The sequence data was edited using BioEdit program. The consensus sequences were blasted and compared for similarity to other nucleotide sequences on the National Center for Biotechnology Information (NCBI) database. New sequences are submitted in GenBank under accession number: SUB12685453 3-FOLMER OQ351110 for Langebaan and SUB12685453 B2-LCO-HCO OQ351111 for Blouberg.
Phylogenetic analyses were conducted in MEGA11. Sequences were aligned using MUSCLE algorithm. Initial tree for the heuristic search were obtained automatically by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then selecting the topology with superior log likelihood value.
Results
Morphological characterization of galls formed by Anguina woodi Swart Subbotin, Tiedt & Riley, 2004
The nematode galls measured 0.5–1 mm in diameter for all the localities. Most nematode galls were single and separated from each, but some were clustered together (Fig. 5a and c). The nematode galls on the grass stems were softer in texture than those situated on insect galls and were deep purple to blackish in colour, non-pedunculate but slightly elevated above the surface of the grass stems and leaf sheaths (Fig. 5a–c). Galls on dry, brown stems and leaves were dark brown and somewhat elevated, but never pedunculated (Fig. 5f). The nematodes inside them were still alive. Insect galls were found on grass from Langebaan and Blouberg only. The nematode galls situated on top of the insect galls were always clustered, brown to dark brown in colour, matching the colour of the insect galls (Fig. 5d). They were also harder and drier and some of these dried-up galls contained no nematodes but were filled with particles that looked like debris under the microscope. No nematode galls were found on the insect galls from Blouberg (Table 3).
Measurements of Anguina woodi from Milnerton and Langebaan
See Table 4.
Morphological description of Anguina woodi from Milnerton and Langebaan
Female (Milnerton)
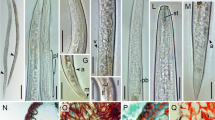
Female body obese, curved into a circular shape, fusiform and considerably broader than the body of the male. Lip region flattened anteriorly and offset from body. Anterior part of neck region, just posterior of lip region displays prominent annules or folds of cuticle with a short stylet and well-defined rounded knobs. Median bulb oval to roundish in shape with distinct valves. Basal bulb large, trapezoid. Excretory pore was located at the same level as posterior end of basal bulb. Lateral lines ambiguous/unclear. Vulva prominent transversely with lips protruding slightly from the body contour. Tail tapering gradually to a minutely pointed or slightly rounded terminus.
Female (Langebaan)
The description of females from Langebaan was the same as that from Milnerton. However, the following small morphological differences were noticed between females not associated with insect galls and those associated with insect galls: lower c-value [39 ± 7.1 (28–54) vs 34 ± 5.6 (23–46)], lower cˈ-value [1.9 ± 0.9 (0.6–3.1) vs 1.6 ± 0.2 (1.3–2)] and shorter tail [(60.2 ± 11 (42–79) vs 68.9 ± 7.4 (52–84)].
Male (Milnerton)
Morphology of males was the same as females except for the following characters: the body was slenderer than that of females, slightly curved to almost straight, spicules ventrally curved, gubernaculum slightly ventrally curved. Tail was long, narrowing very gradually to an acute tip with bursa not reaching tail tip.
Male (Langebaan)
The description of males from Langebaan was the same as that from Milnerton. However, the bursa length differed between males not associated with insect galls and those associated with insect galls [106 ± 16.5 (71.5–132) µm vs 92.2 ± 19.27 (62–120) µm], the bursa of males not associated with insect galls being longer.
Diagnosis and relationships
The nematodes from Milnerton and those from Langebaan are sufficiently close to each other morphologically and morphometrically to consider them conspecific.
Morphometrically, the mean body length of the females from Milnerton and Langebaan is longer than that of the females in the original description of A. woodi (2.3 − 2.5 mm vs 2.1 mm) and more obese (a = 11.7–12.3 vs 17.4). The slightly more obese body can be attributed to the flattening of the female body during mounting on glass slides. Other slight differences are the shorter mean length of the pharynx (b = 15.2–17.2 vs 11), longer stylet (12.1–12.3 µm vs 11.2 µm), broader head width (10.3–10.8 µm vs 9.6 µm) and longer PUS (91–101 µm vs 86 µm). Morphometrically, the mean body length of the males from Milnerton and Langebaan are also slightly longer than those of the original description of A. woodi (1.8 -1.9 mm vs 1.6 mm) and slenderer (a = 14.7–15.4 vs 21.7). Additional differences are the longer pharynx (b = 11.6–12.8 vs 8.9), slightly longer mean stylet length (11.6–11.9 µm vs 11.4 µm), longer mean tail length (74–76.9 µm vs 69 µm), percentage of the bursa covering the tail (67.8 – 72.7% vs 59.3%) and the shorter mean gubernaculum length (9.1–9.4 µm vs 12.3 µm). The morphology of the galls is the same: like A. woodi, the species from Milnerton and Langebaan induces galls on stems and leaves of Ehrharta villosa var. villosa, the mature galls also vary in colour from purplish to dark brown and forms roundish elevations, which are not pedunculated.
Morphologically and morphometrically, the anguinid specimens of Milnerton and Langebaan are also close to A. australis Steiner 1940, mainly in the strongly ventrally curled obese females, the deep cuticular folds in the anterior part of the neck region in both sexes and the smaller and slenderer males. The females differ from those of A. australis mainly in the broader average head region (10.3–10.8 µm vs 7.4 µm), slightly longer stylet (11–13 µm vs 9.9–11.1 µm) and longer average PUS (91–101 µm vs 75 µm). The males differ from those of A. australis mainly in the longer body (1.5–2.2 mm vs 1.1–1.55 mm) and the percentage of the bursa covering the tail (67.8–72.7% vs 75.8%).
In conclusion, the anguinids from Milnerton and Langebaan are morphologically close enough to A. woodi to consider them as belonging to the same species (Figs. 5, 6, 7, 8).
Nematode and insect galls. a Ehrharta villosa grass showing nematode galls from Milnerton. b Dissected nematode gall with nematodes from Blouberg. c Nematode galls on Ehrharta villosa grass from Langebaan. d Nematode gall (arrow 1) as well as an insect gall (arrow 2) on Ehrharta villosa grass from Langebaan. e Insect gall showing emerging aperture (arrow). f Ehrharta villosa grass with nematodes and insects galls
Counts of Anguina woodi specimens from galls of the different localities, not associated with/or associated with insect galls (Table 3)
Milnerton (Fig. 6)
Each gall contained females, males, juveniles, and eggs (Fig. 6). The clustered galls contained more adult nematodes than the single galls. A single gall contained a maximum of three adult nematodes, but four galls contained one female and one male per gall. The clustered galls had more adult nematodes ranging from four to ten per gall, with more females than males (1–7 females vs 1–4 males). Juveniles and eggs were present in high numbers in both single and clustered galls, ranging from 5 to 35 juveniles per gall and eggs ranging from 5 to 31 eggs per gall. Gall number 12 had the highest number of females at seven per gall and galls number 5, 6, 7 and 8 contained the lowest count at one female per gall. Gall number 4 had the highest number of males at four per gall and the lowest count were in gall numbers 5, 6, 7, 8, 9, 15, 16, 17 and 18 with one male per gall. Gall number 9 had the highest number of juveniles at 35 per gall with gall number 2 having the lowest juvenile count at four juveniles per gall. The highest number of eggs were in gall number 1 with 30 eggs per gall and the lowest in gall number 9 with five per gall (Fig. 6). The total number of females, males, juveniles, and eggs in the 20 galls was 73, 34, 305, and 325, respectively.
Langebaan: galls not associated with insect galls (Fig. 7)
Nematode counts on 20 grass galls from Langebaan not associated with galls induced by insects were the following: the highest female nematode count was in gall number 8 with four females per gall, and the lowest count were in gall numbers 2, 3, 4, 12, 7 and 9 with one female per gall (Fig. 7). The highest male count was in gall number 1 with 4 males/gall count, and the lowest count was gall number 19 with no males. Only one gall, number 1, contained five eggs. Four galls contained juveniles, with gall numbers 17 and 18 containing the highest number of seven and five juveniles each. Gall number 10 and 13 contained 4 and 13 juveniles, respectively. The total number of females, males, juveniles, and eggs in the 20 galls was 98, and the average numbers were 4.9. The average nematode calculation from the total number of 20 galls was 1.9 for males, 2.05 for females, 0.95 for juveniles and 0.25 for the egg count. The total count for males was 38, 41 for females, 19 for juveniles and 5 for eggs (Fig. 7).
Langebaan: galls associated with insect galls (Fig. 9)
The 20 nematode galls from Langebaan associated with insect’s galls contained only females and males. The highest female count per gall was found in gall number 16 with seven females. The lowest female count was in five galls, gall numbers 3, 7, 9, 11 and 20, with one female nematode per gall. The highest number of males were found in gall numbers 8 and 13 with seven males per gall. The lowest male count was one male per gall from gall numbers 2, 3, 6, 9, 10, 12, 14, 15, 18 and 20 (Fig. 8). The total count from the 20 galls was 56 females and 42 males, with an average of 2.8 females and 2.1 males (see Table 3).
Blouberg: galls not associated with insect galls
Nematodes that are not associated with insects from Blouberg had only one gall (gall number 2) with nematode eggs. This gall contained one female, two males and seven eggs. Two galls (numbers 3 and 5) contained stage 4 juveniles. Galls number 1,7,14 and 15 contained one female and one male nematode per gall. Galls number 9 and 20 contained two female and two male nematodes per gall each. Galls number 4, 16 and 18 contained only one female nematode per gall. Galls number 6, 11, 12 and 17 had two female and one male nematode per gall. Galls 8 and 13 contained three females and one male nematode per gall and finally gall number 10 contained one female and two male nematodes. The total count of females was 32 with an average of 1.6 per gall nematodes and the total count of males was 22 with average of 1.1 per gall (see Table 3). The total count of juveniles was 13 with an average of 0.65 and the total egg count was seven with an average of 0.35.
Blouberg: galls associated with insect galls (Fig. 8)
The Blouberg nematode count from 20 galls were 7 galls with one female and one male in each gall (gall numbers 1,3, 5,13,15,16 and 18), and 7 galls (numbers 2, 3, 4,11,17,19 and 20) with two females and one male per gall. Two galls (numbers 9 and 12) contained three females and two males per gall. Galls number 10 and 14 had the highest female count with a total of four nematodes per gall. Gall number 10 had the highest male count with a total of three males in the gall. The twenty galls had a total number of 34 female nematodes, with an average of 2.05(see Table 3), and a total number of 24 male nematodes with an average of 1.9 (Fig. 9). There were no juveniles and eggs found in these galls.
Molecular identification and phylogeny of Anguina woodi Swart et al. 2004
The findings showed that the amplification of the ITS and 18S rRNA genes of these populations yielded single fragments of 764 nucleotide base pairs. Furthermore, the result showed that the percentage of base pairs similarity was 100% for the sequenced samples as compared to that of the reference specimen of A. woodi, (AY307122).
The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree had the highest log likelihood of (− 11,074.77) and with analysis involving 32 nucleotide sequences (Fig. 10).
Maximum likelihood tree of DNA sequence evolution, Ln likelihood = (− 11,074.77)) obtained from analyses of internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence for 32 nematode species and populations of Anguina woodi. Position of Milnerton, Langebaan and Bloubergstrand species are shown by bold letters
The fly was tentatively identified as belonging to the family Chloropidae and the wasp to family Eurytomidae Walker 1832
Description of galls
Langebaan, Western Cape
Insect galls were light to dark brown in colour, about 5 mm in width and 10 mm in length), oval in shape and rough on the outside (Fig. 5d, e and f) The galls were very hard to cut through and the inside was cream in colour (Fig. 11d). The hardness of the galls extended to the grass stalk surface. The galls were traversed by tunnels that ended with a cavity containing the larval stage of the wasp (Fig. 5d and e). On close inspection of the insect gall, the aperture of another, small tunnel was observed (Fig. 5d). This aperture was probably made by a wasp (Hymenoptera: Eurytomidae), which was caught in the insect trap.
Wasps and fly from the Langebaan samples that emerged from the insect trap emerging box (LM). a, b and c indicate wasps that emerged from the samples taken in 2020 and 2021, respectively; d Dissected insect gall with wasp larvae (arrow 1), tunnel (arrow 2) and emerging aperture of wasp (arrow 3); e and f show fly that emerged from the samples taken in 2021
Blouberg, Western Cape
Insect galls from Blouberg Beach were beige to light brown in colour. The galls were oval, 5 mm wide and 10 mm long and smooth on the outside. They were traversed by tunnels that ended with a cavity containing the larval stage of an insect, probably a gall-forming fly of the family Chloropidae. Wasp exit apertures were visible on the surface of some galls (Fig. 5d and e).
Morphological identification of the wasp from the family Eurytomidae
Two wasps and one fly from the Langebaan 2020 samples emerged after 14 days in the insect trap emerging box. The wasps and fly measured 1 mm and 2 mm in length, respectively. The insects were photographed by using a Nikon compound microscope (Fig. 11a, b, c, e and f). The wasps were taken to the Entomology Unit, Biosystematics, ARC-PHP for morphological identification where they were identified to the family Eurytomidae and probable genus Bruchophagus Ashmead 1888. Seven wasps from the January 2022 samples from Blouberg Beach emerged after 30 days. Out of the seven wasps, five were used for molecular identification and the remaining two were preserved in vials for further morphological identification. As a single fly was obtained from the Langebaan samples and nothing from the Blouberg samples, the authors were able to make a preliminary morphological identification of this specimen only. The single fly from Langebaan was identified to the family Chloropidae (Fig. 11 e and f). Future studies on more specimens will be needed to confirm this identification.
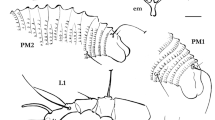
Morphologically the authors identified the wasp to family level (Eurytomidae Walker 1832) by using the taxonomic key by Goulet and Huber (1993). The following characters were used for identification: pronotum in dorsal view with large rectangular to quadrate collar at least half as long as mesoscutum (ms). Posterior margin of collar often incurved, but sides subparallel and anterior margin usually abruptly shoulder-like lateral to concavely narrowed neck. Propodeum often widely depressed medially or with median longitudinal channel. Body rarely with metallic lustre, usually coarsely sculptured or with distinct mesh-like or granular sculpture. Head without occipital carina (though gena often ridged). Scutellum without frenum. Ovipositor sheath extending at most slightly beyond apex of last metasomal tergum.
Molecular identification and phylogeny of the wasp Eurytomidae sp.
After DNA extraction, parameters shown in Table 1 (See supplementary information) were used for PCR and the cytochrome oxidase subunit I (CO1) gene partial cds mitochondrial was targeted for amplification. The gel electrophoresis showed visible bands for all three samples. In the negative control no band was observed, while the positive control got from previous DNA extracted from citrus insects showed a visible band. The sequence results showed 90.71% similarity to Eurytomidae for Langebaan species and 88.70% for Blouberg species. Evolutionary analysis was done by the maximum likelihood method and Tamura–Nei model using MEGA 11. The tree shows a set of possible nucleotides (states) at each ancestral node based on their inferred likelihood at site 1. For each node, only the most probable state is shown. Initial tree for the heuristic search was obtained automatically by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura–Nei model, and then selecting the topology with superior log likelihood value. The rates among sites were treated as being uniform among sites (Uniform rates option). This analysis involved 28 nucleotide sequences and here was a total of 1630 positions in the final dataset. According to the tree constructed for this study, the species of Eurytomidae from Langebaan and Blouberg show more relatedness to other Eurytoma species as compared to Bruchophagus species (Fig. 12).
Discussion
Anguina woodi was collected from three locations in the Western Cape: Milnerton, Langebaan and Blouberg. These findings were also compared with those of the originally described species, A. woodi, from Milnerton. Like A. woodi, the species from Milnerton, Langebaan and Blouberg incited the same type of galls on stems and leaves of Ehrharta villosa var. villosa. The nematodes from the present study were also compared to the close relative of A. woodi, A. australis, a native of Western Australia, which also parasitizes a grass (Ehrharta longiflora Smith) (Riley et al. 2001). However, the combination of slight differences in morphology and morphometry, different hosts and huge geographical separation made conspecifity of the anguinids of the present study with A. australis, unlikely. Another interesting observation was the female:male sex ratio in the Milnerton nematode galls of 3.65:1.7 per gall (present study). This indicates that, according to its sex ratio, A. woodi from Milnerton is nearer to A. australis. For instance, Riley and Swart (2004) reported that, in their study, the sex ratio of A. woodi females to males was close to 1:1, while A. australis has more females than males per gall (proportion of females per gall was 57.3%). Riley and Swart (2004) speculated that A. australis might occur in South Africa but has not been found yet. Further molecular studies using more samples on the Western Cape anguinids need to be done to explore this possibility.
The molecular study of the nematodes from Milnerton, Langebaan and Blouberg showed an exact match between the sequences of the ITS and 18S rRNA genes of the original Anguina woodi description (reference sequence of A. woodi from NCBI (AY307122)) and that of Anguina species from the three localities. The phylogenic tree also showed that the nematodes from the three localities have the same genetic structure. The slight differences seen might be attributed to different localities, each with a slightly different climate. The same argument was put forth by Ami et al. (2019). According to these authors, the molecular identification of wheat seed gall nematode Anguina tritici parasitizing durum and bread wheat cultivars which were collected from two cities, Erbil and Duhok, from the Kurdistan Region and Iraq, respectively, emphasized that both nematode isolates are of the same genetic structure and confirmed their belonging to the same nematode race. While the pathogenicity of the wheat race varies by different cultivars of wheat as well as the infection behaviour and severity may change in different regions and under different environmental circumstances (Ami et al. 2019).
The nematode galls found during the present study, are morphologically very near those described from Milnerton Beach by Swart et al. (2004). However, some galls from the present study appears to be slightly larger (0.5–1 mm in diameter in the present study vs. 0.5 X 0.4 mm on green stems and 0.8 X 0.6 mm on dry, brown stems in the 2004 study). At Langebaan and Blouberg, the anguinid galls were found alone or in close proximity with galls formed by, presumably, the following insects: a gall-forming fly and a wasp. The morphometrics and life cycle of the inhabitants of the anguinid galls situated close to, or on insect galls, seem to be influenced by the presence of the insects, because only nematodes associated with insect galls seemed to be affected. In Langebaan, the females not associated with insect galls, had on average a longer tail than those associated with these galls. Likewise, males not associated with insect galls had, on average, a longer bursa than those associated with insect galls. Of great importance is the fact that no juvenile stages or eggs were found in the nematode galls associated with insect galls. This suggests a negative association where the life cycle of the nematode is not completed when their galls are associated with the insect galls. Different authors suggested that the nematodes are the first to colonize the grass where they start to form galls (Riley et al. 2001; Riley and Swart 2004). Kolesik and Wood (2019) also argued that the insects might be attracted to these sites where they enter the plant tissues, causing their own galls. According to Lotfalizadeh et al. (2007), the majority of the eurytomid wasp’s larvae are endophytic: as seed eaters, gall formers, or as parasitoids of phytophagous insects and are, primary or secondary parasitoids, attacking eggs, larvae or pupae of various arthropod groups.
The establishment of the relationship between the nematodes and the insects will require another study with enough insects obtained from the study area. It will require, for instance, early detection of gall formation and monitoring the gall growth until maturity. Of interest, a few wasps were found dead or damaged in the insect galleries within the dissected galls. The cause of the wasps being trapped before emergence is currently unknown and further investigation is required. Another question that remains is the difference in surface texture between the insect galls from Langebaan and those from Blouberg, especially as the same grass, and possibly the same insects, are involved in the formation of the galls.
Conclusion
The present study presents evidence that the large, hard, brownish galls found on the stems of E. villosa in Langebaan and Blouberg, are inhabited and formed by a fly, possibly of the family Chloropidae. A wasp of the family Eurytomidae was also found within these galls, but whether they are parasites of the fly or contribute to the formation of the galls, must still be investigated.
The relationship between the gall-forming nematodes and the gall-forming insects still needs further investigation. One scenario might be that the nematodes invade the grass first, which start an infection site and gall formation. Secondary is the invasion by midges, flies and/or wasps, which might be attracted to the infection sites of the nematodes. In this scenario, the nematode galls get pushed to the periphery of the insect gall and thereby to a less favourable environment. This may explain the fact that fewer adult nematodes are found within the nematode galls situated directly next to, or on top of the insect galls. The question remains whether the relationship between insects and nematode is beneficial to the insects. With the knowledge that the nematode is an obligate plant parasite of the grass, we might conclude that the nematode is the first one to establish its galls and that the insects, as secondary invaders, incite much larger galls. This invasion distresses the xylem of the grass, thereby hardening the gall tissues, which is less favourable for gall formation by the nematode.
References
Ami SN, Taher IE, Hussen FSA (2019) First molecular identification of wheat seed gall nematode Anguina tritici races parasitized on wheat in Iraq. Acta Univ Sapientiae 11:5–15. https://doi.org/10.2478/ausae-2019-0001
Ashmead WH (1888) A revised generic table of the Eurytominae, with descriptions of new species. (Part I.). J Entomol Am 4:41–43
Brzeski M (1981) The genera of Anguinidae (Nematoda, Tylenchida). Rev De Nématol 4:23–24
de Man JG (1884) Die frei in der reinen Erde und im süssen Wasser lebenden Nematoden der Niederländischen Fauna. Eine Systematisch-Faunistische Monographie. E. J. Brill, Leiden.
Fawcett SGM (1938) A disease of the Australian grass Microlaena stipoides R.B. caused by a nematode Anguillulina microlaenae n. sp. J Helminthol 16:17–23
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Goulet H, Huber JT (1993) Hymenoptera of the world: An identification guide to families. Centre for Land and Biological Resources Research Ottawa, Ottowa
Hooper DJ (1970) Handling, fixing, staining and mounting nematodes. Technical bulletin, 5th edn. Ministry of Agriculture Fisheries and Food, pp 39–54
Howlett BJ, Brownlee AG, Guest DI, Adcock GJ, McFadden GI (1992) The 5S ribosomal RNA gene is linked to large and small subunit ribosomal RNA genes in the oomycetes, Phytophthora vignae, P. cinnamomi, P. megasperma f. sp. glycinea and Saprolegnia ferax. Curr Genet 22(6):455–461. https://doi.org/10.1007/BF00326410
Kieffer J-J (1914) Neue Gallmücken aus Süd-Afrika. Cent.bl. Bakteriol Parasitenkd Infekt Krankh 40:514–517
Kleynhans KPN, Van den Berg E, Swart A, Marais M, Buckley NH (1996) Plant nematodes in South Africa. Plant Protection Research Institute Handbook No. 8. ARC Plant Protection Research Institute, Pretoria
Kolesik P, Wood AR (2019) Redescription of Mitodiplosis graminis (Diptera: Cecidomyiidae), a gall midge inhibiting the flowering of pyp grass Ehrharta villosa (Poaceae) in South Africa. Zootaxa 1:173–179. https://doi.org/10.11646/zootaxa.4614.1.8
Lotfalizadeh H, Delvare G, Rasplus JY (2007) Phylogenetic analysis of Eurytominae based on morphological characters (Chalcidoidea: Eurytomidae). Zool J Linn Soc 151:441–510. https://doi.org/10.1111/j.1096-3642.2007.00308.x
Ma Y, Xie H, Wang J, Liu C (2011) Detection of second-stage juveniles of Anguina agrostis using TaqMan Real-time PCR. Russ J Nematol 19:151–158
Marais M (2022) Report N0447: South African plant parasitic nematode survey (SAPPNS). Agricultural Research Council Plant Health and Protection (ARC-PHP), Pretoria
Marais M, Swart A, Fourie H, Berry SD, Knoetze R, Malan AP (2017) Techniques and procedures. In: Fourie H, Spaull VW, Jones RK, Daneel MS, de Waele D (eds) Nematology in South Africa: a view from the 21st century. Springer, Cham
Powers TO, Szalanski AL, Mullin PG, Harris TS, Bertozzi T, Griesbach JA (2001) Identification of seed gall nematodes of agronomic and regulatory concern with PCR-RFLP of ITS1. J Nematol 33:191–194
Riley I, Swart A (2004) Aspects of reproduction and survival of Anguina woodi. Austral. Plant Pathol 5:5. https://doi.org/10.1071/AP04063
Riley IT, Schmitz A, de Silva P (2001) Anguina australis, a vector for Rathayibacter toxicus in Ehrharta longiflora Australas. Plant Pathol 30:171–175. https://doi.org/10.1071/AP01019
Rondani C (1856) Dipterologiae Italicae prodromus. Vol. II. genera Italica ordinis Dipterorum. A. Stocchi Parmae. Australas Plant Pathol 33:595–596. https://doi.org/10.1071/AP04063
Steiner G (1940) Opuscula miscellanea nematologica VIII. A new grass nematode, Anguina australis n. sp. Proc Helminthol Soc Wash 7:5–22
Subbotin SA, Krall EL, Riley IT, Chizhov VN, Staelens A, DeLoose M, Moens M (2004) Evolution of the gall-forming plant parasitic nematodes (Tylenchida: Anguinidae) and their relationships with hosts as inferred from Internal Transcribed Spacer sequences of nuclear ribosomal DNA. Mol Phylogenet Evol 30:226–235. https://doi.org/10.1016/S1055-7903(03)00188-X
Swart A, Subbotin SA, Tiedt LR, Riley IT (2004) Anguina woodi sp.n. (Tylenchida: Anguinidae) from dune grass, Ehrharta villosa, in South Africa. Nematology 6:129–144. https://doi.org/10.1163/156854104323073008
Thomas KW, Vida JT, Frisse LM, Mundo M, Baldwin JG (1997) DNA sequence from formalin-fixed nematodes: integrating molecular and morphological approaches to taxonomy. J Nematol 29:250–254
Uys VM, Urban RP (2006) How to collect and preserve insects and arachnids. Plant protection research institute handbook No. 7, 2nd edn. ARC-Plant Protection Research Institute, Pretoria
Vovlas N, Troccoli A, Van Noort S, Van den Berg E (1998) Schistonchus africanus n. sp. (Aphelenchida: Aphelenchoididae) associated with Ficus thonningii (Moraceae) and its pollinator wasp Elisabethiella stuckenbergi (Chalcidoidea: Agaonidae). J Nematol 30:404–410
Walker F (1832) Monographia Chalciditum. Entomol Mag 1:12–29
Acknowledgements
The authors thank Dr Alan Wood (ARC-PHP) for obtaining a research permit to sample organisms associated with Ehrharta villosa in the West Coast and Agulhas National Parks, for taking the grass samples for this study and for sending them to the Nematology Unit, Agricultural Research Council-Plant Health and Protection (ARC-PHP) for cold storage. He also shared his knowledge of plant pathology with Tinyiko Chauke and Antoinette Swart and took Tinyiko Chauke on a field trip to discover the intricacies between grass, nematodes and insects in different habitats. Thanks Alan, for sharing your experience and enthusiasm. The authors also thank the University of South Africa (UNISA) for financial support for Tinyiko Chauke’s study and the ARC-PHP for letting him use the facilities of the Nematology Laboratory for part of his studies. Thanks are also due to Beth Grobbelaar (ARC-PHP) for her help with the construction of an insect trap emerging box, as well as Dr Gerhard Prinsloo (ARC-PHP) for the preliminary morphological identification of the wasp to the family Eurytomidae. We also thank the reviewers of the manuscript for their very relevant recommendations.
Funding
Open access funding provided by University of South Africa.
Author information
Authors and Affiliations
Contributions
Contributions by authors. Mr. T.R. Chauke did the fundamental research from collecting data to writing. Dr. S.A. Subbotin for reviewing the manuscript.Contribution to molecular identification, and phylogenetics study of nematodes. Dr. Antoinette Swart for writing and reviewing the manuscript and guiding the research direction. Dr. P.D Malatji for administrating the MSc course and reviewing the manuscript. Ms. M.M. Mamabolo for her contribution to molecular identification of nematodes and insects and writing the manuscript. D.r Maseko for insect molecular and morphological identifications. Writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chauke, T.R., Malatji, D.P., Subbotin, S.A. et al. Gall-forming nematode, Anguina woodi (Nematoda: Anguinidae) and Chalcid wasp (Hymenoptera: Eurytomidae), on dune grass from the Western Cape, South Africa. Zoomorphology 143, 57–75 (2024). https://doi.org/10.1007/s00435-023-00631-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00631-6