Abstract
The diversity of insects can be explained by their ability to fill various ecological niches, which includes the foraging from diverse sources. The cuticle-based feeding structures interact with the food and show adaptations in shape, material composition and mechanical properties to it. In this study, we focus on the mouthparts of a very prominent ambush predator, the antlion larvae of Euroleon nostras. By nanoindentation, we tested the hardness and the Young’s modulus of the mouthparts, which are significantly harder and stiffer than other insect cuticle structures. To gain insight into the origins of the high values, we studied the degree of tanning using confocal laser scanning microscopy. Additionally, we determined the content of transition and alkaline earth metals by energy dispersive X-ray spectroscopy. We found that the proportions of Zn, Mn, Fe, Cu, Ca, Mg, and Si correlate with the mechanical property values. We also conducted experiments on the breaking stress, the puncturing and biomechanical behaviour of the jaws, which highlighted their extraordinary strength. These findings are not only valuable for biologists, but also for material scientists, as they contribute to our understanding of the origins of mechanical property heterogeneities in insect cuticle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects represent the most species-rich and diverse animal group (e.g. Chapman 2009). Since they are abundant in nearly all habitats, they play one of the most important roles in each ecosystem and provide essential ecological functions (for review see Krenn 2019a). The diversity of taxa can be explained, among other factors, by the strong variety of lifestyles, which includes the foraging on different food sources. Since mouthparts can be regarded as the interfaces between the organism and its food, studies on them can contribute to the understanding of insect ecology, behaviour and evolution (for review see Krenn 2019b).
The morphologies of insect mouthparts were intensively investigated, giving important insights into their functional principles and the associated ecology of the animal (for review see Krenn 2019a, 2019b). With regard to the mechanical properties (e.g. hardness and Young’s modulus), which, together with shape, determine functionality, there are, however, large gaps in knowledge. Only few studies are devoted to the mechanical properties and their origins (i.e. the degree of tanning or the inorganic content) of mouthparts (see Hopkins and Kramer 1992; Vincent and Wegst 2004; Andersen 2012; Politi et al. 2019).
In this context, it was previously detected, that the degree of sclerotization and the distribution of proteins, e.g. resilin, determine the mechanical properties. It was also unravelled, that inorganic compounds affect the biomechanical behaviour of structures by providing more hardness and stiffness to insect cuticle (Hillerton and Vincent 1982; Schofield et al. 2002; Kundanati and Gundiah 2014; see reviews by Liu et al. 2017 and Politi et al. 2019). This includes transition metals (Cu, Fe, Mn, Zn), which ions participate in the formation of complex molecules by binding strongly to the biopolymers and increase the cross-linking density (Waite et al. 2004; Pontin et al. 2007; Broomell et al. 2008; Politi et al. 2012; Degtyar et al. 2014; Liu et al. 2017). Additionally, there are indications for the involvement of biomineralization in insect cuticle as alkaline earth metals (Ca, Mg) were previously detected (Vincent 2002; Lichtenegger et al. 2003; Quicke et al. 2004; Polidori and Wurdack 2019).
To provide more insight into the composition, mechanical properties and their relationship in insect cuticle, we here study the mouthparts of the antlion larvae of Euroleon nostras (Geoffroy, 1785) (Neuroptera, Myrmeleontidae). The antlions are represented by about 2000 species and are prominent due to the predatory lifestyle of their larvae, which in some species build pits to trap small arthropods or other prey. Prey is squeezed between their enormous sickle-like jaws, consisting of elongated mandibles and maxillae, then pierced. Afterwards, they inject venom and enzymes and finally suck out the liquefied contents by fluid uptake trough a food canal, which is formed by both the mandible and maxilla (van Zyl and van der Linde 2000; Zimmermann et al. 2019; Acevedo Ramos et al. 2020; Lehnert et al. 2022a, b). With regard to the biomechanics of this structure, it was recently discovered, by applying confocal laser scanning microscopy (CLSM), that the mandible tip is heavily sclerotized to pierce the prey item (Lehnert et al. 2022a, b). Afterwards the mandible and maxilla perform antiparallel movement during feeding, which exposes the hydrophilic food canal and enables fluid uptake, similar to a sucking pump (Lehnert et al. 2022a, b).
We here add onto existing knowledge about antlion larvae mouthpart biomechanics by examining the mechanical properties hardness H and Young’s modulus E by nanoindentation. To determine the factors influencing these properties, elemental analyses by energy dispersive X-ray spectroscopy (EDX, EDS) were performed on the same sites, to identify inorganic content of the cuticle. Additionally, CLSM was applied to study the autofluorescence, which can provide information on the distribution of proteins and the degree of tanning. In general, we found, that the mechanical property values, which are high in comparison to those of other insects, directly relate to the autofluorescence signal, but also correlate with the content of Zn, Mn, Fe, Cu, Ca, Mg, and Si. To gain insight into the functional consequences of the mechanical property heterogeneities, we performed experiment on the breaking and puncturing behaviours of the jaws. These data set is new for antlions and shall provide deeper insight into the biomechanics of insect mouthparts’ cuticle.
Materials and methods
Specimens and documentation
Individuals were collected in Pevestorf, Germany, directly under the eaves of the Elbauenstation (coordinates: 53.057829, 11.454801) in in the course of the annual undergraduate excursion. They were collected in 07/28/2022 alive, killed by a short deposit in the freezer, fixed and stored in 70% EtOH. Specimens were identified by employing the relevant literature (Badano and Pantaleoni 2014).
First, every specimen was cleaned by a short ultrasonic bath and documented by light microscopy, employing a Keyence Digital Microscope VHX-7000 (KEYENCE, Neu-Isenburg, Germany) equipped with automatic stacking software (see Fig. 1).
Scanning electron microscopy (SEM)
For documentation of the mouthpart morphology, the heads of two specimens were dried at room temperature and mounted on SEM sample holders by double-sided adhesive carbon tape. They were then sputter coated with platinum (5 nm thick layer) and visualized with the Zeiss LEO 1525 (One Zeiss Drive, Thornwood, USA) with 5 kV (see Fig. 2). Magnification and working distance were always adjusted to receive high quality images. Terminology was adapted from the literature (Beutel et al. 2010; Devetak et al. 2010; Zimmermann et al. 2019; Acevedo Ramos et al. 2020; Lehnert et al. 2022a, b).
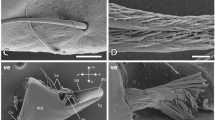
SEM images of the jaws. A, C, E Dorsal view on mandible (A) with magnification of the proximal region (C) and the mandibular tooth I and II (E). B, D, F Ventral view on mandible and maxilla (B) with magnifications of the proximal region (D) and the tip (F). G Dorsal, inner cuticle of the maxilla with serrated teeth. H During breaking stress experiments, the very tip of the maxilla bends when loaded. When the force is however very high, the maxilla is pushed out of the mandible grooves and structural failure occurs. Abbreviations: As attachment site with needle; Md mandible; Mx maxilla; Sto serrated tooth; Ti tip; To I mandibular tooth 1; To II mandibular tooth 2. Scale bars: A, B, 600 µm; C–E, 200 µm; F, 30 µm; G, 20 µm; H, 100 µm
Confocal laser scanning microscopy (CLSM)
To visualize the cuticle autofluorescence, three additional individuals were used. They were arranged on object glass slides and surrounded by a ring of modelling clay. Each ring was filled with glycerine (greater than or equal to 99.5%, free of water; Carl Roth GmbH and Co. KG, Karlsruhe, Germany) and covered with a glass cover slip. Mouthparts were visualized employing a Zeiss LSM 700 confocal laser scanning microscope (Carl Zeiss Microscopy GmbH, Jena, Germany), following the protocol of Michels and Gorb (2012). Four stable solid-state lasers with wavelengths of 405 nm, 488 nm, 555 nm, and 639 nm were used. Bandpass or longpass emission filters, transmitting light of wavelengths 420–480 nm, greater than or equal to 490 nm, greater than or equal to 560 nm, or greater than or equal to 640 nm were applied.
Afterwards, for multi-colour images, the autofluorescence produced by the laser of 405 nm was assigned a blue colour, of 488 nm a green, of 555 nm and 639 nm a red one (50 % for each) (following Michels and Gorb 2012). Finally, images were superimposed with maximum intensity projection, using the software Zeiss Efficient Navigation (Zen) (Carl Zeiss MicroImaging GmbH, Jena, Germany).
The whole head of one specimen was scanned in dorsal view (see Fig. 3). Additionally, the heads of two specimens were dissected, the mouthparts carefully extracted, the mandible and maxilla separated and individually scanned (two mandibles and maxillae were visualized from dorsal and two from ventral side).
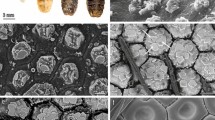
CLSM images. A, B. Whole head in dorsal view from one specimen with magnification (B). Anatomical orientations are aligned with the left mandible. C–H. Mandibles (D, H) and maxillae (C, E, F, G), of another specimen, are scanned individually. D. Mandible in dorsal view. The teeth are numbered; I: 1, II: 2, III: 3. E, F. Maxilla in dorsal view (E) with magnification of the tip (F). C, G. Maxilla in ventral view (G) with magnification of the tip (C). H. Mandible in ventral view. Scale bars: A, 800 µm; B, 400 µm; C, 200 µm, D, 800 µm; E, 800 µm; F, 800 µm; G, H, 800 µm
Nanoindentation
To test the mechanical properties of the exocuticle under wet, native conditions, nanoindentation was performed on six additional individuals. The experiments were performed with rewetted cuticle structures, since water is known to be the major modulator of mechanical properties, as its presence tends to reduce the Young’s modulus (E; elastic modulus) and Hardness (H) values (Klocke and Schmitz 2011). Mandibles and maxillae were first carefully separated, dried at room temperature, glued to small glass object slides (three mandibles and maxillae were attached at their dorsal side, three at their ventral side), which were then mounted on the nanoindentation sample holders. Then, samples were placed in a small plastic box, which was filled with wet paper towels. The box was closed and sealed for two days to rewet the samples by high air humidity.
A nanoindenter SA2 (MTS Nano Instruments, Oak Ridge, Tennessee, USA) equipped with a Berkovich indenter tip and a dynamic contact module (DCM) head was employed. H and E were determined from force-distance curves by applying the continuous stiffness mode with the same settings (e.g., a Poisson’s ratio of 0.3) as in our previous studies on chitinous radular teeth (Krings et al. 2019; Gorb and Krings 2021). All tests were performed under normal room conditions and each indent and corresponding curve were both manually controlled. E and H were determined at penetration depths of 600–2000 nm to ensure that the exocuticle was tested. For each site indented, we received ~ 100 values obtained at different indentation depths, which were averaged to receive one H and one E mean value per indent. We tested overall 1668 localities (on the mandibles: 510 tested localities were on the dorsal and 510 on the ventral side of the structure; on the maxillae: 324 on the dorsal and 324 on the ventral side) (see Fig. 4 for localities). On the ventral sides of the maxillae, we did not test the grooves as these structures are too steep, but measured between the grooves and next to them.
Results from nanoindentation (hardness H and Young’s modulus E, both given in GPa). The tested localities are designated in the light microscopy images (dorsal view). Based on six specimens, i.e. 12 mandibles and 12 maxillae; six from each structure were tested from ventral (red boxplots) and six from dorsal (black boxplots). For values, see Supplementary Table 2. A Results for the mandible. B Results for the maxilla. C Mandible in dorsal view with localities. D Maxilla in dorsal view with localities. Abbreviations: B basis of mandibular tooth; S stylus of mandibular tooth; T tip of mandibular tooth. Scale bar: 350 µm
Elemental dispersive X-ray spectroscopy (EDX, EDS)
By EDX, we tested the same localities of the exocuticle previously tested by nanoindentation (overall 1668 sites). After indentation, samples were carefully loosened from the glass slides, their orientation altered at 90°, and arranged on object glass slides by double-sided adhesive carbon tape. Then, each mouthpart was surrounded by a small metallic ring. This was then filled with epoxy resin (Reckli Epoxy WST; RECKLI GmbH, Herne, Germany) that the structures were completely covered, following our previous protocol (Krings et al. 2022a, 2022b). After polymerization, lasting for three days at room temperature, glass object slides and adhesive tape were removed. Samples were polished with sandpapers of different roughness until sections of the target localities were on display. Then they were smoothened with aluminium oxide polishing powder suspension of 0.3 μm grainsize (PRESI GmbH, Hagen, Germany) on a polishing machine (Minitech 233/333; PRESI GmbH, Hagen, Germany) to receive a plain smooth surface. Samples were first cleaned in an ultrasonic bath, lasting five minutes, then mounted on SEM sample holders, and coated with platinum (5 nm layer). Elemental composition was determined with the SEM Zeiss LEO 1525 equipped with an Octane Silicon Drift Detector (SDD) (micro analyses system TEAM; EDAX Inc., New Jersey, USA). For each sample, the same settings were used (i.e. an acceleration voltage of 20 kV, working distance 15 mm, lens opening 60 µm, measuring time for each point measurement 30 s, resolution 137.6 eV). Before analysis of each sample, the detector was calibrated using copper; thus, our results are semi-quantitative. Small areas were analysed to gain information. Some elements were not discussed as they are either the elemental basis of chitin and proteins (H, C, N, O), the coating (Pt), or the polishing powder (Al, O); the other elements were here summed to “all elements, Ae”.
The single peak of P overlaps with one of the peaks in the complex spectrum of Pt. Because of this, the software could not discriminate between these two elements and P content could not be reliably determined. Therefore, P and Pt were discussed together (P + Pt). Even though sputter-coating caused artefacts, we followed this protocol, because the sputtering allowed us to determine, if the measurement was successful (e.g., if a very high or no Pt content was determined, we excluded this measurement). We however measured 20 areas of pure epoxy to receive values on the Pt content (mean ± SD; 0.15 ± 0.02 atomic %) to further estimate the proportions of P. The testing of the epoxy additionally revealed that none of the discussed elements (Mn, Zn, Ca, Si, etc.) was present there and that there are no artefacts caused by sample preparation.
Experiments on the biomechanical behaviour of the jaw
To receive data on the mechanical behaviour of the structure and the stress it could resist to, ten specimens were dissected and the intact jaws glued to a needle at their proximal ventral region (at As, see Fig. 2B). Each needle was firmly mounted onto the force transducer FORT-1000 (World Precision Instruments, Sarasota, USA), which was connected to the amplifier (Biopac System Inc., Goleta, USA) and computer-based data acquisition and processing system (Acq Knowledge™, Biopac Systems, Inc., version: 3.7.0.0; World Precision Instruments, Sarasota, USA). The force transducer was attached to a remote-controlled micromanipulator (DC 3001R; World Precision Instruments Inc., Sarasota, USA), which allowed precise movements. During the whole procedure, forces were recorded. For our analyses, we used the maximal forces, calculated from the obtained force–time diagrams (see Krings et al. 2021a, 2021b).
Before experiments, jaws were rewetted by drippling distilled water on the sample. Large drops were then absorbed with filter paper. All experiments were performed under binocular microscope (Fig. 5).
Schematic illustration of the experiments on the biomechanical behaviour of the mouthparts. A Illustration of the experiment on an object glass slide (‘breaking’ experiments, i.e., leading to structural failure). B Illustration of ‘puncturing’ experiments with prey. C Illustration of ‘bending experiment. For this setup, each jaw was turned to 30° to allow contact between the tip of maxilla and object slide. While the whole structure was then moved, the maxilla tip interacted with the sandpaper and its very tip bent. Abbreviations: As attachment site; Md mandible; Mx maxilla
We performed (a) breaking experiments to measure the resistance of the jaw to structural failure. For this, the jaw tips of two specimens were pressed onto an object glass slide, which was covered with sandpaper to prevent the jaw from sliding, until the structures broke (Fig. 5A).
Additionally, we punctured (b) two common prey items (the isopod Porcellio scaber and the ant Formica rufa, which were tested in fresh condition) of the antlion to receive data on the force needed to pierce the prey cuticle with the jaw. For this, six woodlouses and four ants were attached, laying on their ventral side, to object glass slides with double-sided adhesive tape and pierced with the jaws of five specimens either between the sclerites (with three jaws), into their sclerites (with three jaws) or into the abdomen of the ants (with four jaws) (Fig. 5B). The ants and isopods were collected alive in September 2022. The woodlouses were part of student course with focus on the animals’ behaviour. Ca. 100 individuals were collected alive for the class (the criteria for selection: male, adult and not freshly moulded). Afterwards we were able to select individuals of similar size. All ants and isopods were then killed by quickly changing the temperature (i.e., placing them in the freezer for 10 min, then keeping them at room temperature for 10 min, and placing them again in the freezer for 10 min).
We also tested the biomechanical behaviour of the jaw (c) and measured the force, needed to bend the tip of the maxilla to slightly enlarge the opening of the food canal. The jaws of three specimens were carefully pressed onto an object glass slide, which was covered with sand paper, and moved forwards (see Fig. 5C) until the very tip of each maxilla bent (into the opposite direction) and enlarged the opening of the food canal. For this experiment, we had to change the angle between object slide and jaw at ~ 30°, so that the tip of the maxilla could interact with the object slide without the mandible tip being in the way (Fig. 5C).
Statistical analysis
All statistical analyses on elemental proportions were performed with JMP Pro, Version 14 (SAS Institute Inc., Cary, USA). Mean values and standard deviations were calculated and Shapiro–Wilk-W-tests for testing of normality was conducted. As the data was normally distributed, an ANOVA, followed by pairwise comparison with Tukey–Kramer method, was carried out. With this software, relationships between parameters were visualized and correlation coefficients calculated.
Results
Autofluorescence
The ventral and dorsal sides of the mandible tip and of the mandibular teeth, the region of insertion with the head capsule and the ventral side of the maxilla tip are of a strong red colour (have a high autofluorescence from the lasers of 555 nm and 639 nm wavelength) (Fig. 3). In the mandible, the central region is blue to green (has a strong autofluorescence signal from the lasers of 405 nm and 488 nm wavelength), whereas the outer and the inner edges are red (Fig. 3D). Ventrally, each mandible interacted with the maxilla through two grooves, forming the food canal (Fig. 3H). These grooves were red in contrast to the surrounding regions showing a blue to green colour. The ventral cuticle of the maxilla exhibited gradients in the autofluorescence; the tip was of a strong red colour, which decreased towards proximal (Fig. 3C and G). In the dorsal cuticle, however, we observed a different pattern; here, the proximal region and the tip were of a strong green colour (has a strong autofluorescence signal from the laser of 488 nm wavelength) (Fig. 3E and F).
Elemental analysis
H (hydrogen), C (carbon), N (nitrogen), O (oxygen), Pt (platinum), Al (aluminium), Ca (calcium), Cl (chlorine), Cu (copper), F (fluorine), Fe (iron), K (potassium), Mn (manganese), Mg (magnesium), Na (sodium), P (phosphorus), S (sulphur), Si (silicon), and Zn (zinc) were detected and their proportions measured.
The discussed elements were abundant with the following proportions (sorted from highest to lowest mean proportions): mean > 1 atomic %: F (values range from 0.16 to 1.70%); mean: < 1 atomic %: P + Pt (1.33–0.26 atomic %), Zn (0.04–3.38%), Mn (0.04–3.38%), Si (0.00–2.33%), Ca (0.02–3.47%), Cu (0.04–2.36%), Mg (0.00–0.53%), Fe (0.04–1.82%), K (0.02–1.73%), S (0.00–0.79%), Cl (0.00–0.56%), and finally Na (0.00–0.61%) (see Table 1 and Supplementary Table 1).
Mechanical properties
The Young’s modulus (E) describes the stiffness of a solid material and the relationship between tensile stress and axial strain. It correlates with the ability of the material to transmit force, relates to the puncturing behaviour and resistance to failure (for review on puncture mechanics, see Anderson 2018). The hardness (H) is the measure of the resistance to local plastic deformation induced by indentation or abrasion.
In the antlion jaws, H values ranged from 0.17 to 1.98 GPa (mean ± SD: 0.80 ± 0.26 GPa) and E from 3.47 to 20.88 GPa (8.28 ± 2.88 GPa) (see Fig. 4, Table 1, and Supplementary Tables 1 and 2). Most localities differed significantly in E and H values (see Supplementary Table 3 for p-values). In the mandible, the dorsal areas were harder and stiffer than the ventral ones. This was in contrast to the maxilla, where the ventral regions were harder and stiffer than the dorsal cuticle.
In the mandible, the hardest and stiffest regions were at the surfaces interacting with the prey (see Fig. 4 and Supplementary Tables 1 and 2): the most distal region of the tip was the hardest and stiffest region, with E and H values decreasing towards the posterior parts. In the mandible teeth, which were the second hardest and stiffest items, the same pattern, with decreasing values along the stylus towards the tooth basis, were observed. For the posterior parts of the mandible, we detected that the inner edge was the hardest and stiffest region, followed by the outer edge, and finally the central region as the softest and most flexible area. Here, similar to the tip and the teeth, E and H values decreased towards proximal region. However, the most proximal region, where the mandibles interacted with the head capsule, was slightly harder and stiffer.
In the maxilla, the medial edge was moderately harder and stiffer than the lateral edge. In general, E and H values increased from proximal to distal—except for the region interacting with the head capsule, which was slightly harder and stiffer. For the tip, E and H values first remained somewhat constant and decreased significantly towards distal region.
Experiments on the biomechanical behaviour
Forces of 700.00 ± 195.92 mN (mean ± SD) were needed to break the jaws (see Table 1 and Fig. 6). During this structural failure, the mandible tip was bent and the mandible, as consequence of applying further load, broke loose from the maxilla (Fig. 2H). During puncturing experiments, the highest forces were needed to penetrate the stiff cuticle of Porcellio scaber (300.77 ± 24.17 mN), followed by puncturing softer cuticle between the sclerites (97.33 ± 20.53 mN) and finally puncturing the abdomen of the ant Formica rufa (87.25 ± 9.87 mN). For bending of the maxilla tip, a force, similar to puncturing between the sclerites and puncturing of the ant abdomen was needed (86.10 ± 16.14 mN).
Results from experiments on the biomechanical behaviour; force, given in mN, for breaking (failure) (see Fig. 2H for result), puncturing (between and into sclerites of Porcellio scaber and into the abdomen of Formica rufa) and bending experiments (bending of the maxilla tip). Abbreviations: As attachment site; Md mandible; Mx maxilla
Relationship between parameters
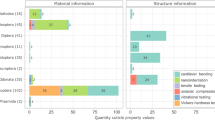
Most parameter correlated positively (moderate to very high) (see Supplementary Table 4 for correlation coefficients and Supplementary Figs. 1–15 for the relationship between the individual elements and the mechanical properties). Na and Cl showed mostly very low positive correlation coefficients to the other parameters (r = 0.07–0.20). Only F correlated negatively to each parameter (very low to low; r = – 0.50–0.11). H and E showed a very high positive correlation (r = 0.96); both mechanical properties correlated very highly with Mg, Si, S (r = 0.83–0.87) and highly with P + Pt, Ca, Mn, Fe, Cu, Zn, and with the total elemental content Ae (r = 0.63–0.80).
Discussion
Functionality of antlion larva jaws
Due to the strong hardness and stiffness of the antlion mandible tips, the puncturing of prey exocuticle with relatively low applied force, is facilitated. Additionally, the high values of the mechanical properties probably reduce structural failure; this is supported by the breaking stress experiments, where very high forces were needed to break the jaws. The softer and flexible regions of the jaws and the mandibular teeth potentially serve as a shock absorber, reducing the risk of structural failure (see also Lehnert et al. 2022a, b). In the mandible, the dorsal cuticle and, in the maxilla, the ventral cuticle are harder and stiffer; this probably reduces wear caused by the interaction between the surrounding sand during trap building and ambush behaviour. The tip of the maxilla is interestingly found to be softer and more flexible. The biomechanical consequences of this could be overserved during the biomechanical experiments: when the maxilla tip interacted with the object glass slide, it bent towards exterior, slightly enlarging the entrance of the food canal. Due to these observations, we propose that the hard and stiff mandible tips pierce the prey, and afterwards, with little force involved, the tips of the maxillae bend. Afterwards, fluid uptake and thus feeding is then facilitated by antiparallel movements of mandible together with maxilla, as detected by Lehnert et al. (2022a, b). The cuticle on the ventral side of the mandible and the dorsal side of the maxilla can probably adjust to the ingested fluids and particles, as they are softer and more flexible.
The insect cuticle as composite material
The arthropod cuticle is a chitinous composite material of various components with different material properties, including stiff and hard or soft and flexible regions. The mechanical properties show a huge range from KPa to GPa (see review by Vincent and Wegst 2004; Stamm et al. 2021), depending on the region tested and the water content of the cuticle, as water is an important modulator for mechanical properties (Klocke and Schmitz 2011; Krings et al. 2021a, 2021b).
Structures that require wear resistance, such as mouthparts, joints or claws (see e.g. Schofield et al. 2002, 2021; Barbakadze et al. 2006; Cribb et al. 2008a; Zhang et al. 2019; Tadayon et al. 2020; Kundanati et al. 2021), are significantly harder and stiffer than e.g. legs, eyes, elytra or wings (e.g. Smith et al. 2000; Lomakin et al. 2011; Dirks and Taylor 2012; Chen et al. 2013; Hayot et al. 2013; Oh et al. 2017; Li et al. 2022), even though studies cannot be directly comparable due to different techniques or sample preparation (please see review by Stamm et al. 2021).
In insect mandibles, mechanical property values can range from ~ 6 to ~ 11 GPa for E and from 0.4 to 1.2 GPa for H in termites (Cribb et al. 2008a), from ~ 7 to ~ 11 GPa for E and from ~ 0.7 to ~ 1.0 GPa for H in dragonfly nymphs (Kundanati et al. 2021), or from ~ 7 to ~ 11 GPa for E and ~ 0.3 to ~ 1.0 GPa for H in beetle larvae (Kundanati et al. 2020). The values of E and H of the antlion jaws exceed the previous measured mouthparts and are comparable to limpet (Gastropoda, Mollusca) or some polyplacophoran (Mollusca) radular teeth (Ukmar-Godec et al. 2017; Krings et al. 2022a).
The mechanical properties of the cuticle (see review by Politi et al. 2019) can have their origin in the degree of sclerotization by quinone reactions (Hopkins and Kramer 1992; Andersen 2010), the microstructure of the chitin, the mineral content and the abundance of proteins or transition metals and halogens (e.g. Hillerton and Vincent 1982; Schofield et al. 2002; for review on mechanical property gradients and their various origins, see Liu et al. 2017).
The degree of tanning and its influence on the mechanical parameters were well investigated in various arthropod cuticle structures. Autofluorescence signals, that were received after laser excitation via CLSM according to the protocol of Michels and Gorb (2012), are commonly studied to identify cuticle regions with certain dominating material compositions.
The following material properties were previously related to the following autofluorescence signals:
(a) sclerotized, stiff cuticle to the signal received after excitation with lasers of 555 nm and 639 nm wavelength (red colour was assigned to the signal in the CLSM images).
(b) weakly sclerotized chitin to the signal received from the laser of 488 nm wavelength (green colour was assigned to the signal in the CLSM images). These regions are flexible and relatively tough.
(c) Regions containing high proportions of resilin, an elastic and flexible protein (see Andersen and Weis-Fogh 1964; for review see Michels et al. 2016), or several other proteins (e.g. Garcia-Castineiras et al. 1978; Giurginca et al. 2015) emit a strong autofluorescence, when excited with the laser of 405 nm wavelength (blue colour was assigned to the signal in the CLSM images).
Regions containing resilin and weakly sclerotized chitin the assigned colours overlay and can appear brown, yellow, or pink.
This protocol (Michels and Gorb 2012) was applied to various chitinous structures, such as insect endocuticle (Wang et al. 2019), wings (Ma et al. 2022), foot attachment devices (Peisker et al. 2013), thorax structures (Casey et al. 2022), antennae (Saltin et al. 2019), genitals (Matsumura et al. 2021a), and also mouthparts (Büsse and Gorb 2018; Weihmann and Wipfler 2019; Matsumura et al. 2021b; Lehnert et al. 2021; Sun et al. 2021; Wei et al. 2022).
A relationship between the autofluorescence signals and the material properties (see Eshghi et al. 2018) was previously cross-validated for leg attachment devices in lady bird beetles employing AFM-nanoindentation (Peisker et al. 2013) and for chitinous radular teeth (Krings et al. 2022b). The same relationship is also detected here, as we determined that blue to green areas are softer and more flexible (e.g. the central region of the mandible) than regions of red colour (e.g. the tips of the mandible teeth or of the mandible).
In contrast to other arthropod lineages, specifically crustaceans, the insect cuticle lacks higher proportions of inorganic contents. However, diverse transition metals (Cu, Fe, Mn, Zn), with colocalized halogens (Cl), and alkaline earth metals (Ca, Mg) were found to be abundant in insect structures, which are prone to structural failure or wear as ovipositors (e.g. Quicke et al. 2004; Polidori et al. 2013; Lehnert et al. 2019), claws (Zhang et al. 2019), or mouthparts (Hillerton and Vincent 1982; Hillerton et al. 1984; Quicke et al. 1998; Fawke et al. 1997; Schofield et al. 2002, 2021; Morgan et al. 2003; Cribb et al. 2008a, 2008b; Jorge et al. 2017; Polidori et al. 2020; Laiolo et al. 2021; Lehnert et al. 2022a, b).
Here, Cu, Fe, Mn and/or Zn ions probably serve as cross-links (Schofield 2001, 2005; Lichtenegger et al. 2003; Birkedal et al. 2006; Degtyar et al. 2014; Jorge et al. 2017; Schofield et al. 2021), whereas Ca and Mg could potentially be present in crystalline form and be part of biomineralization (Vincent 2002; Lichtenegger et al. 2003; Quicke et al. 2004; Polidori and Wurdack 2019).
In general, transition and alkaline earth metals occur, sometimes also with P, Si, Si, Cl, K or Na, in same insect cuticle region (Gilby and McKellar 1976; Darlington et al. 1983; Leschen and Cutler 1994; Rasch et al. 2003; Quicke et al. 2004; Cribb et al. 2005; Garcia-Guinea et al. 2011; Stewart et al. 2011; Vega et al. 2017; Baio et al. 2019; Lehnert et al. 2019, 2022a, b; Polidori and Wurdack 2019; Zhang et al. 2019).
Most of the above elements, especially Zn, Mn, Ca and Mg, were previously found to be directly related to an increase in hardness and wear resistance in insects (Hillerton et al. 1982; Hillerton and Vincent 1982; Edwards et al. 1993; Schofield et al. 2002, 2021; Cribb et al. 2008a; Andersen 2010; Vega et al. 2017; Zhang et al. 2019; Kundanati et al. 2020; Johnston et al. 2022).
For the antlion Euroleon nostras (see Fig. 7 for the relationship between the elements that correlated strongly to very strongly with the mechanical properties; see Supplementary Table 4 for correlation coefficients), we detected that Cu, Fe, Mn, and Zn content correlated strongly with the mechanical properties (r = 0.63–0.75), which suggest that the cuticle of antlion jaws is enhanced by cross-linking with these transition metals. Additionally, the E and H values correlate strongly to very strongly with Ca (r = 0.70–0.71) and Mg content (r = 0.77–0.86), which indicates that they are involved in the harden- and stiffening of the jaws, similar to the exoskeletons of ants (Li et al. 2020). If these elements act as cross-links or are present in crystalline form in the cuticle awaits further investigation, as by EDX the bonding condition cannot be determined.
Availability of data and materials
The datasets used are available from the corresponding author on reasonable request.
References
Acevedo Ramos F, Monserrat VJ, Contreras-Ramos A, Pérez-González S (2020) Comparative study of sensilla and other tegumentary structures of Myrmeleontidae larvae (Insecta, Neuroptera). J Morphol 281(10):1191–1209
Andersen SO (2010) Insect cuticular sclerotization: a review. Insect Biochem Mol Biol 40:166–178
Andersen SO (2012) 6 - Cuticular sclerotization and tanning. In: Gilbert LI (ed) Insect molecular biology and biochemistry. Academic Press, pp 167–192
Andersen SO, Weis-Fogh T (1964) Resilin. A rubber-like protein in arthropod cuticle. Adv Insect Physiol 2:1–65
Anderson PSL (2018) Making a point: shared mechanics underlying the diversity of biological puncture. J Exp Biol. 221:jeb187294
Badano D, Pantaleoni RA (2014) The larvae of European Myrmeleontidae (Neuroptera). Zootaxa 3762(1):001–071
Baio JE, Jaye C, Sullivan E, Rasmussen MH, Fischer DA, Gorb SN, Weidner T (2019) NEXAFS imaging to characterize the physio-chemical composition of cuticle from African flower scarab Eudicella gralli. Nat Commun 10(1):4758
Barbakadze N, Enders S, Gorb SN, Arzt E (2006) Local mechanical properties of the head articulation cuticle in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J Exp Biol 209(4):722–730
Beutel RG, Friedrich F, Aspöck U (2010) The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zool J Linn Soc 158:533–562
Birkedal H, Khan RK, Slack N, Broomell C, Lichtenegger HC, Zok F, Stucky GD, Waite JH (2006) Halogenated veneers: protein cross-linking and halogenation in the jaws of Nereis, a marine polychaete worm. ChemBioChem 7(9):1392–1399
Broomell CC, Chase SF, Laue T, Waite JH (2008) Cutting edge structural protein from the jaws of Nereis virens. Biomacromol 9:1669–1677
Büsse S, Gorb SN (2018) Material composition of the mouthpart cuticle in a damselfly larva (Insecta: Odonata) and its biomechanical significance. R Soc Open Sci 5:172117
Casey C, Yager C, Jankauski M, Heveran CM (2022) The flying insect thoracic cuticle is heterogenous in structure and in thickness-dependent modulus gradation. Acta Biomater 138:422–429
Chapman A (2009) Numbers of living species in Australia and the world. Department of the Environment, Water, Heritage and the Arts, Canberra, Australia
Chen YH, Skote M, Zhao Y, Huang WM (2013) Stiffness evaluation of the leading edge of the dragonfly wing via laser vibrometer. Materials Lett 97:166–168
Cribb BW, Rasch R, Barry J, Palmer CM (2005) Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae). Arthropod Struct Dev 34(1):41–48
Cribb BW, Stewart A, Huang H, Truss R, Noller B, Rasch R, Zalucki MP (2008a) Insect mandibles-comparative mechanical properties and links with metal incorporation. Sci Nat 95:17–23
Cribb BW, Stewart A, Huang H, Truss R, Noller B, Rasch R, Zalucki MP (2008b) Unique zinc mass in mandibles separates drywood termites from other groups of termites. Sci Nat 95:433–441
Darlington MV, Meyer HJ, Graf G, Freeman TP (1983) The calcified puparium of the face fly, Musca autumnalis (Diptera: Muscidae). J Insect Physiol 29:157–162
Degtyar E, Harrington MJ, Politi Y, Fratzl P (2014) The mechanical role of metal ions in biogenic protein-based materials. Angew Chem 53:12026–12044
Devetak D, Lipovšek S, Pabst MA (2010) Larval morphology of the antlion Neuroleon microstenus (McLachlan, 1898) (Neuroptera, Myrmeleontidae), with notes on larval biology. Zootaxa 2428:55–63
Dirks JH, Taylor D (2012) Veins improve fracture toughness of insect wings. PLoS ONE 7(8):e43411
Edwards AJ, Fawke JD, McClements JG, Smith SA, Wyeth P (1993) Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting ant Atta sexdens rubropilosa. Cell Biol Int 17:697–698
Eshghi S, Jafarpour M, Darvizeh A, Gorb SN, Rajabi H (2018) A simple, high-resolution, non-destructive method for determining the spatial gradient of the elastic modulus of insect cuticle. J R Soc Interface 15:20180312
Fawke JD, McClements JG, Wyeth P (1997) Cuticular metals: quantification and mapping by complementary techniques. Cell Biol Int 21:675–678
Garcia-Castineiras S, Dillon J, Spector A (1978) Non-tryptophanfluorescence associated with human lens protein; apparent complexity and isolation of bityrosine and anthranilic acid. Exp Eye Res 26:461–476
Garcia-Guinea JC, Jorge AC, Tormo L, Furio M, Crespo-Feo E, Correcher V, Prado-Herrero P, Soria AC, Sanz J, Nieves-Aldrey JL (2011) Ossification vesicles with calcium phosphate in the eyes of the insect Copium teucrii (Hemiptera: Tingidae). Sci World J 11:186–198
Gilby AR, McKellar JW (1976) The calcified puparium of a fly. J Insect Physiol 22(11):1465–1468
Giurginca A, Šustr V, Tajovský K, Giurginca M, Matei I (2015) Spectroscopic parameters of the cuticle and ethanol extracts of the fluorescent cave isopod Mesoniscus graniger (Isopoda, Oniscidea). ZooKeys 515:111–125
Gorb SN, Krings W (2021) Mechanical property gradients of taenioglossan radular teeth are associated with specific function and ecological niche in Paludomidae (Gastropoda: Mollusca). Acta Biomater 134(15):513–530
Hayot CM, Enders S, Zera A, Turner JA (2013) Nanoindentation to quantify the effect of insect dimorphism on the mechanical properties of insect rubberlike cuticle. J Mater Res 28(18):2650–2659
Hillerton JE, Vincent JFV (1982) The specific location of zinc in insect mandibles. J Exp Biol 101:333–336
Hillerton JE, Reynolds SE, Vincent JFV (1982) On the indentation hardness of insect cuticle. J Exp Biol 96:45–52
Hillerton JE, Robertson B, Vincent JFV (1984) The presence of zinc or manganese as the predominant metal in the mandibles of adult stored-product beetles. J Stored Prod Res 20:133–137
Hopkins TL, Kramer KJ (1992) Insect cuticle sclerotization. Annu Rev Entomol 37(1):273–302
Johnston R, Said M, Labonte D, Russell J, Sackett E, Board R (2022) Correlative structure-property characterisation of the leafcutter ant (Atta cepholotes) mandible. Microsc Microanal 28(S1):1342–1346
Jorge A, Polidori C, Garcia-Guinea J, Nieves-Aldrey JL (2017) Spectral cathodoluminescence analysis of hymenopteran mandibles with different levels of zinc enrichment in their teeth. Arthropod Struct Dev 46:39–48
Klocke D, Schmitz H (2011) Water as a major modulator of the mechanical properties of insect cuticle. Acta Biomater 7(7):2935–2942
Krenn HW (2019) Introduction: ecological importance of insect feeding. In: Krenn H (ed) Insect mouthparts. Zoological monographs, vol 5. Springer, Cham, pp 1–7
Krenn HW (2019) Form and function of insect mouthparts. In: Krenn H (ed) Insect mouthparts. Zoological monographs, vol 5. Springer, Cham, pp 9–46
Krings W, Kovalev A, Glaubrecht M, Gorb SN (2019) Differences in the Young modulus and hardness reflect different functions of teeth within the taenioglossan radula of gastropods. Zoology 137:125713
Krings W, Kovalev A, Gorb SN (2021a) Influence of water content on mechanical behaviour of gastropod taenioglossan radulae. Proc Royal Soc B 288:20203173
Krings W, Kovalev A, Gorb SN (2021b) Collective effect of damage prevention in taenioglossan radular teeth is related to the ecological niche in Paludomidae (Gastropoda: Cerithioidea). Acta Biomater 135:458–472
Krings W, Brütt J-O, Gorb SN (2022a) Ontogeny of the elemental composition and the biomechanics of radular teeth in the chiton Lepidochitona cinerea. Front Zool 19:19
Krings W, Brütt J-O, Gorb SN (2022b) Material gradients in gastropod radulae and their biomechanical significance: a combined approach on the paludomid Lavigeria grandis. Sci Nat 109:52
Kundanati L, Gundiah N (2014) Biomechanics of substrate boring by fig wasps. J Exp Biol 217:1946–1954
Kundanati L, Chahare NR, Jaddivada S, Karkisaval AG, Sridhar R, Pugno NM, Gundiah N (2020) Cutting mechanics of wood by beetle larval mandibles. J Mech Behav Biomed Mater 112:104027
Kundanati L, Das P, Pugno NM (2021) Prey capturing dynamics and nanomechanically graded cutting apparatus of dragonfly nymph. Materials 14(3):559
Laiolo P, Pato J, Illera JC, Obeso JR (2021) Selection for functional performance in the evolution of cuticle hardening mechanisms in insects. Evolution 75:1132–1142
Lehnert MS, Reiter KE, Smith GA, Kritsky G (2019) An augmented wood-penetrating structure: cicada ovipositors enhanced with metals and other inorganic elements. Sci Rep 9:19731
Lehnert MS, Johnson DD, Wu J, Sun Y, Fonseca RJ, Michels J, Shell JS, Reiter KE (2021) Physical adaptations of butterfly proboscises enable feeding from narrow floral tubes. Funct Ecol 35:1925–1937
Lehnert MS, Lanba A, Reiter KE, Fonseca RJ, Minninger J, Hall B, Huff W (2022) Mouthpart adaptations of antlion larvae facilitate prey handling and fluid feeding in sandy habitats. J. Exp. Biol. 225(19):jeb244220
Lehnert MS, Tarver LA, Feng J (2022b) Material properties and morphology of prestomal teeth in relation to the feeding habits of Diptera (Brachycera). Insects 13:207
Leschen RAB, Cutler B (1994) Cuticular calcium in beetles (Coleoptera: Tenebrionidae: Phrenapetinae). Ann Entomol Soc Am 87:918–921
Li H, Sun C-Y, Fang Y, Carlson CM, Xu H, Ješovnik A, Sosa-Calvo J, Zarnowski R, Bechtel HA, Fournelle JH, Andes DR, Schultz TR, Gilbert PUPA, Currie CR (2020) Biomineral armor in leaf-cutter ants. Nat Comm 11:5792
Li C, Rajabi H, Gorb SN (2022) Conflicting requirements for transparency and mechanical stability in the compound eyes of desert locusts. Adv Mater Interfaces 9:2200766
Lichtenegger HC, Schöberl T, Ruokalainen JT, Cross JO, Heald SM, Birkedal H, Waite JH, Stucky GD (2003) Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proc Natl Acad Sci 100:9144–9149
Liu Z, Meyers MA, Zhang Z, Ritchie RO (2017) Functional gradients and heterogeneities in biological materials: design principles, functions, and bioinspired applications. Prog Mater Sci 88:467–498
Lomakin J, Huber PA, Eichler C, Arakane Y, Kramer KJ, Beeman RW, Kanost MR, Gehrke SH (2011) Mechanical properties of the beetle elytron, a biological composite material. Biomacromol 12(2):321–335
Ma Y, Ren H, Ning J, Gorb SN (2022) The combination of structure and material distribution ensures functionality of the honeybee wing-coupling mechanism. Soft Matter 18:956–963
Matsumura Y, Jafarpour M, Reut M, Moattar BS, Darvizeh A, Gorb SN, Rajabi H (2021a) Excavation mechanics of the elongated female rostrum of the acorn weevil Curculio glandium (Coleoptera; Curculionidae). Appl Phys A 127:348
Matsumura Y, Kovalev A, Gorb SN (2021b) Mechanical properties of a female reproductive tract of a beetle and implications for penile penetration. Proc R Soc B 288:20211125
Michels J, Gorb SN (2012) Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J Microsc 245(1):1–16
Michels J, Appel E, Gorb SN (2016) Functional diversity of resilin in Arthropoda. Beilstein J Nanotechnol 7:1241–1259
Morgan TD, Baker P, Kramer KJ, Basibuyuk HH, Quicke DLJ (2003) Metals in mandibles of stored products insects: do zinc and manganese enhance the ability of larvae to infest seeds? J Stored Prod Res 39:65–75
Oh JK, Behmer ST, Marquess R, Yegin C, Scholar EA, Akbulut M (2017) Structural, tribological, and mechanical properties of the hind leg joint of a jumping insect: using katydids to inform bioinspired lubrication systems. Acta Biomater 62:284–292
Peisker H, Michels J, Gorb SN (2013) Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat Commun 4:1661
Polidori C, Wurdack M (2019) Mg-enriched ovipositors as a possible adaptation to hard-skinned fruit oviposition in Drosophila suzukii and D. subpulchrella. Arthropod-Plant Interact 13:551–560
Polidori C, Jorge A, Nieves-Aldrey JL (2013) Breaking up the wall: metal enrichment in ovipositors, but not in mandibles, covaries with substrate hardness in gall-wasps and their associates. PLoS ONE 8:1–7
Polidori C, Jorge A, Keller A, Ornosa C, Tormos J, Asís JD, Nieves-Aldrey JL (2020) Strong phylogenetic constraint on transition metal incorporation in the mandibles of the hyper-diverse Hymenoptera (Insecta). Org Divers Evol 20:511–526
Politi Y, Priewasser M, Pippel E, Zaslansky P, Hartmann J, Siegel S, Li C, Barth FG, Fratzl P (2012) A spider’s fang: how to design an injection needle using chitin-based composite material. Adv Funct Mater 22:2519–2528
Politi Y, Bar-On B, Fabritius HO (2019) Mechanics of arthropod cuticle-versatility by structural and compositional variation. In: Estrin Y, Bréchet Y, Dunlop J, Fratzl P (eds) Architectured materials in nature and engineering, vol 282. Springer, Cham, pp 287–327
Pontin MG, Moses DN, Waite JH, Zok FW (2007) A nonmineralized approach to abrasion-resistant biomaterials. Proc Natl Acad Sci USA 104:13559–13564
Quicke DLJ, Wyeth P, Fawke JD, Basibuyuk HH, Vincent JFV (1998) Manganese and zinc in the ovipositors and mandibles of hymenopterous insects. Zool J Linn Soc 124:387–396
Quicke DLJ, Palmer-Wilson J, Burrough A, Broad JR (2004) Discovery of calcium enrichment in cutting teeth of parasitic wasp ovipositors (Hymenoptera: Ichneumonoidea). Afr Entomol 12:259–264
Rasch R, Cribb B, Barry J, Palmer C (2003) Application of quantitative analytical electron microscopy to the mineral content of insect cuticle. Microsc Microanal 9(2):152–154
Saltin BD, Matsumura Y, Reid A, Windmill JF, Gorb SN, Jackson JC (2019) Material stiffness variation in mosquito antennae. J R Soc Interface 16(154):20190049
Schofield RMS (2001) Metals in cuticular structures. In: Brownell P, Polis G (eds) Scorpion biology and research. Oxford University Press, Oxford, pp 234–256
Schofield RMS (2005) Metal–halogen biomaterials. Am Entomol 51:45–47
Schofield RMS, Nesson MH, Richardson KA (2002) Tooth hardness increases with zinc content in mandibles of young adult leaf-cutter ants. Sci Nat 89:579–583
Schofield RMS, Bailey J, Coon JJ, Devaraj A, Garrett RW, Goggans MS, Hebner MG, Lee BS, Lee D, Lovern N, Ober-Singleton S, Saephan N, Seagal VR, Silver DM, Som HE, Twitchell J, Wang X, Zima JS, Nesson MH (2021) The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Sci Rep 11:17481
Smith CW, Herbert R, Wootton RJ, Evans KE (2000) The hind wing of the desert locust (Schistocerca gregaria Forskal). II. Mechanical properties and functioning of the membrane. J Exp Biol. 203(19):2933–2943
Stamm K, Saltin BD, Dirks JH (2021) Biomechanics of insect cuticle: an interdisciplinary experimental challenge. Appl Phys A 127:329
Stewart AD, Anand RR, Laird JS, Verrall M, Ryan CG, de Jonge MD, Paterson D, Howard DL (2011) Distribution of metals in the termite Tumulitermes tumuli (Froggatt): two types of Malpighian tubule concretion host Zn and Ca mutually exclusively. PLoS ONE 6(11):e27578
Sun Y, Zhang J, Tang X, Wu Z, Gorb SN, Wu J (2021) Specialized morphology and material properties make a honey bee tongue both extendible and structurally stable. Acta Biomater 136:412–419
Tadayon M, Younes-Metzler O, Shelef Y, Zaslansky P, Rechels A, Berner A, Zolotoyabko E, Barth FG, Fratzl P, Bar-On B, Politi Y (2020) Adaptations for wear resistance and damage resilience: micromechanics of spider cuticular “tools.” Adv Funct Mater 30:2000400
Ukmar-Godec T, Bertinetti L, Dunlop JWC, Godec A, Grabiger MA, Masic A, Nguyen H, Zlotnikov I, Zaslansky P, Faivre D (2017) Materials nanoarchitecturing via cation-mediated protein assembly: making limpet teeth without mineral. Adv Mater 29(27):1701171
van Zyl A, van der Linde TCK (2000) Anatomy and histology of the alimentary canals of the antlion larvae Furgella intermedia Markl and Palpares annulatus Stitz (Neuroptera: Myrmeleontidae), with reference to their feeding physiology. Afr Entomol 8:179–188
Vega FE, Bauchan G, Infante F, Davis S (2017) Mouthpart structure and elemental composition of the mandibles in the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). Ann Entomol Soc 110(4):381–389
Vincent JFV (2002) Arthropod cuticle – a natural composite shell system. Comp Part A 33:1311–1315
Vincent JFV, Wegst UGK (2004) Design and mechanical properties of insect cuticle. Arthropod Struct Dev 33(3):187–199
Waite JH, Lichtenegger HC, Stucky GD, Hansma P (2004) Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry 43:7653–7662
Wang L-Y, Jafarpour M, Lin C-P, Appel E, Gorb SN, Rajabi H (2019) Endocuticle sclerotisation increases the mechanical stability of cuticle. Soft Matter 15:8272
Wei J, Huo Z, Liang Y, Wu Z, Wu J, Gorb SN (2022) Hydrophilic and opened canals in honey bee tongue rods endow elastic structures with multiple functions. Acta Biomater 137:162–171
Weihmann T, Wipfler B (2019) The generalized feeding apparatus of cockroaches: a model for biting and chewing insects. In: Krenn H (ed) Insect mouthparts. Zoological monographs, vol 5. Springer, Cham, pp 203–262
Zhang Z, Zhang Y, Zhang J, Zhu Y (2019) Structure, mechanics and material properties of claw cuticle from mole cricket Gryllotalpa orientalis. PLoS ONE 14(9):e0222116
Zimmermann D, Randolf S, Aspöck U (2019) From chewing to sucking via phylogeny — from sucking to chewing via ontogeny: mouthparts of Neuroptera. In: Krenn H (ed) Insect mouthparts. Zoological monographs, vol 5. Springer, Cham, pp 361–385
Acknowledgements
We would like to thank Elke Woelken and Frank Friedrich from the Institute of Cell and Systems Biology of Animals, Universität Hamburg, for their support on the SEM and for discussion of the results. We are highly grateful for the helpful comments of the two anonymous reviewers.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was financed by the DFG grant KR 470833544.
Author information
Authors and Affiliations
Contributions
WK and SG together initiated and designed the study. WK wrote the first draft of the manuscript, which was discussed and corrected by SG. All authors have approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krings, W., Gorb, S.N. Mechanical properties of larval mouthparts of the antlion Euroleon nostras (Neuroptera: Myrmeleontidae) and their correlation with cuticular material composition. Zoomorphology 142, 423–438 (2023). https://doi.org/10.1007/s00435-023-00609-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00609-4