Abstract
A new population of Pseudacrobeles (Pseudacrobeles) macrocystis and Poikilolaimus oxycercus is described from South Africa. Poikilolaimus oxycercus was collected from soil covered by a natural grass in South Africa, which is morphologically similar to the original description. The South African population of P. oxycercus is characterised by having a small size (807–818 µm in males and 703–779 µm in males), female tail cupola-shaped (24–35 µm), and spicule length (27–35 µm). The South African population of P. (P.) macrocystis, collected from natural grass, is characterised by having a small size (611–786 µm), a lateral field with three incisures, lip region with lips bearing seta-like processes and blunt conoid labial probolae, primary and secondary axils smooth, nerve ring and excretory pore at the posterior part of the pharyngeal corpus, spermatheca well developed, postvulval uterine sac poorly developed, female tail conoid-elongate, male tail conoid with thin acute mucro and spicules small (31–36 µm). Measurements and line illustrations of the species are given. In addition, LM, SEM photographs and the phylogenetic position of P. (P.) macrocyctis are provided. The 18S and 28S rDNA analyses show that P. macrocystis is closely related to other species of the genus Pseudacrobeles having lips with seta-like processes. This is the first report of P. (P.) macrocystis from South Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematodes of the order Rhabditida are an important group in the phylum Nematoda and can occupy different ecological niches (Shokoohi and Abolafia 2019). This group has a significant environmental role (mineralization of soil, pathogenicity on insects, the transmission of bacterial pathogens of vegetables, feed on bacteria, etc.). The taxonomy of these nematodes was revised by different authors (Andrássy 2005; Blaxter et al. 1998; De Ley and Blaxter 2002; Sudhaus and Fitch 2001; Shokoohi et al. 2007; 2008; Shokoohi and Abolafia 2019). This order contains numerous species (Andrássy 2005).
One of these species is Poikilolaimus oxycercus (de Man 1895) Sudhaus 1980, described previously from South Africa as Cuticularia mathesoni by van der Linde (1938) and, more recently, reported by Borgonie et al. (2015).
Furthermore, Pseudacrobeles (Pseudacrobeles) macrocystis De Ley and Siddiqi 1991 was described by De Ley and Siddiqi (1991) from Malaysia, providing SEM study. Later, this species was redescribed by De Ley et al. (1993a) from Brazil, Cameroon, and Tanzania, providing a new SEM study of the lip region and male posterior end. Then, Shokoohi and Abolafia (2012) described this species from Iran. Although this species was not found previously in South Africa, other species of this genus, Pseudacrobeles elongatus (de Man, 1884) Abolafia and Peña-Santiago 2005 (as Cephalobus elongatus de Man, 1884), was described in South Africa by van der Linde (1938).
Several years ago, we started a research project on the identification and biodiversity of free-living and plant-parasitic nematodes in South Africa. The present paper deals with two known species belonging to the families Poikilolaimidae Dougherty 1955 and Cephalobidae Filipjev 1934 (taxonomic classification according to Shokoohi and Abolafia, 2019) collected in natural areas from South Africa. Therefore, the study aimed to (i) study Poikilolaimus and Pseudacrobeles using morphological and morphometrical analyses and (ii) study Pseudacrobeles using molecular analysis.
Materials and methods
Nematode extraction and processing
Nematodes were collected from wild grass soil samples and extracted from them using the Baermann (1917) funnel technique. Extracted individuals were fixed with a hot 4% formaldehyde solution and transferred to anhydrous glycerin utilising the method of De Grisse (1969). Fixed specimens were mounted on permanent glass slides (see methodology provided by Abolafia (2022).
Light microscopy (LM)
Observations were made using a Leitz Laborlux S (Leitz, Wetzlar, Germany), a Nikon Eclipse 80i (Nikon, Tokyo, Japan), and a Zeiss (Axio Lab, Germany) microscope. Measurements (see methodology provided by Abolafia (2023) were taken with the Leitz and Zeiss (Axio Lab) microscopes, which have a drawing tube (camera lucida) attached to it, and the Demanian indices (de Man 1881) and other ratios were calculated. Drawings were made using a camera attached to a Zeiss microscope (Axio Lab, Germany), whereas LM pictures were taken with the Leitz microscope and the Nikon microscope, which is equipped with differential interference contrast (DIC) optics and a Nikon Digital Sight DS-U1 camera. Micrographs were combined using Adobe® Photoshop® CS. The terminology used for the morphology of stoma, spicules, and gubernaculum follows the proposals by De Ley et al. (1995) and Abolafia and Peña-Santiago (2017).
Scanning electron microscopy (SEM)
Specimens preserved in glycerine were selected for observation under SEM, according to Abolafia (2015). They were hydrated in distilled water, dehydrated in a graded ethanol-acetone series, critical point dried, coated with gold, and observed with a Zeiss Merlin microscope (5 kV) (Zeiss, Oberkochen, Germany).
DNA extraction, PCR, and sequencing
DNA was extracted from fresh nematodes following Holovachov et al. (2009). The specimens were picked using a fine-tipped needle and transferred together to a 1.5 ml capacity microtube containing 25 μl of double distilled water. The presence of the specimens in the tube was verified using a Zeiss microscope at the Aquaculture Research Unit. The nematodes were crushed with a sterile needle, after which 20 μl of 5% Chelex-100 (Sigma, USA) solution and 5 μl proteinase K (20 mg ml–1) were added to the nematode substrate. The homogenate was incubated at 56 °C for 2 h and then at 95 °C for 10 min (Shokoohi 2022). The supernatant was extracted from the tube and stored at – 20 °C. Partial 18S rDNA sequences were amplified using polymerase chain reactions (PCR) with the forward primer SSU_F_04 (5'-GCTTGTCTCAAAGATTAAGCC-3') and reverse primer SSU_R_26 (5'-CATTCTTGGCAAATGCTTTCG-3') (Blaxter et al. 1998). In addition, the 28S D2A (5'-ACAAGTACCGTGAGGGAAAGTTG-3') and D3B (5′-TCGGAAGGAACCAGCTACTA-3') primers were used for the D2-D3 segment amplification (De Ley et al. 1999). The PCR reaction was done using 8 μl of nematode DNA extract, 8 μl of nuclease-free ddH2O, 12.5 μl ready to use Master Mix including Taq polymerase (Promega, USA), 1 μl of each of the two primers (10 pmol μl−1) to a final volume of 30.5 μl. The amplification was done using an Eppendorf Mastercycler gradient thermal cycler (Eppendorf, Hamburg, Germany) with the following conditions: initial denaturation at 94 °C for 3 min, followed by 37 cycles entailing denaturation at 94 °C for 45 s, annealing at 57 °C for 45 s, and extension at 72 °C for 1 min. The final extension was achieved at 72 °C for 6 min. (Koohkan et al. 2015 and Shokoohi et al. 2023) for 18S rDNA and 28S rDNA, respectively). Following DNA amplification, 5 μl of PCR product was loaded in a 1.5% agarose gel in TBE buffer (40 mM Tris, 40 mM boric acid, and 1 mM EDTA) for evaluation of the DNA bands. The bands were stained with SafeView (abm, Canada) and visualised and photographed on a UV transilluminator. The DNA product was stored at – 20 °C until sequencing commenced. PCR products were sent to a commercial sequencing company (Inqaba Biotechnical Industries (Pty) Ltd, Pretoria, South Africa) for purification and sequencing. The obtained sequences were deposited in NCBI GenBank under accession numbers MW298529 (18S rDNA) and MW301158 (28S rDNA) for P. (P.) macrocystis.
Phylogenetic analyses
The newly obtained 18S and 28S sequences were manually edited using Chromas 2.6.6 (Technelysium, Queensland, Australia) and aligned with other 18S or 28S rDNA sequences available in GenBank using ClustalW as implemented in MEGA7 (Kumar et al. 2016). Poorly aligned regions at either end were trimmed using MEGA7. The model of nucleotide substitution was statistically selected using jModelTest 2.1.10 (Darriba et al. 2012). Phylogenetic trees were generated with the Bayesian inference method using MrBayes 3.2.6 (Ronquist et al. 2012). Aphelenchus avenae (JQ348399) and Teratolobus sp. (KJ652552) were chosen as outgroups for 18S and 28S rDNA phylogenetic analysis. The General Time Reversible Plus Invariant sites plus Gamma distribution (GTR + I + G) model was selected with a random starting tree and run with the Markov Chain Monte Carlo (MCMC) analysis (Larget and Simon 1999) for 1 × 106 generations. The resulting trees were visualised and saved with FigTree 1.4.4 (Rambaut 2018).
Results
Poikilolaimus oxycercus (de Man 1895) Sudhaus 1980 (Fig. 1).
Material examined
Five females and five males in good condition.
Measurements
See Table 1.
Female
Body almost straight with slightly curved ventrad after fixation. Cuticle almost smooth, sock-like, 4.6–5.2 µm, finely annulated; annuli 1.2–1.3 µm wide. Lateral fields with one longitudinal line. Lip region is continuous with the neck, bearing small papillae. Amphids aperture is not visible. Stoma rhabditoid, with distinct cheilo-, gymno- and stegostom; cheilostom poorly cuticularised, gymnostom longer than cheilostom, with well-cuticularized walls; stegostom tubular at promesostegostom and with isomorphic and isotopic glottoid apparatus at metastegostom, bearing 1–2 denticles; telostegostom very short; gymnostom and promesostegostom fused, tubular; pharyngeal collar present surrounding the metatelosgostom. Pharynx rhabditoid; pharyngeal corpus, about 1.9–2.6 times isthmus length, with subcylindrical procorpus and slightly swollen metacorpus; isthmus robust, well distinguished from metacorpus; basal bulb spheroid, with prominent grinder with visible posterior haustrulum, this 16–20 µm long. Cardia conoid, surrounded by intestinal tissue. Nerve ring at isthmus level, at 66–68% of neck length. Secretory-excretory pore at isthmus level, at 79–80% of neck length. Deirids not visible. Intestine without distinct specialisation at anterior cardiac part. Reproductive system didelphic-amphidelphic; ovaries dorsally reflexed, lacking posterior flexures; oviducts short, tubular, not well differentiated from the ovary, differentiated posteriorly in a spheroid to oval-shaped spermatheca, with round sperm; uteri tubular, tubular with narrow lumen at the anterior part and swollen with wider lumen at the posterior part; vagina with thin walls, about one-third of the body diameter long; vulva with simple lips, located slightly postequatorial. Rectum 0.7–1.4 times anal body diameter. Tail slightly cupola shaped, with rounded anterior part and conoid posterior part. Phasmids located at base of the rounded part of tail.
Male
General morphology is similar to that of females. Body straight, slightly curved. Genital system monorchic, with testis ventrally reflexed. Tail cupola shaped with acute terminus. Bursa peloderan with eight genital papillae. Genital papillae comprise three pre-cloacal pairs and five post-cloacal pairs, in arrangement 1 + 2/1 + 1 + 3. Spicules free, ventrally curved, 1.3–1.5 times than the corresponding body diameter; manubrium rounded, hook-shaped, offset from calamus, calamus short, lamina ventrally curved with developed velum. Gubernaculum 29–38% of spicule length.
Remarks
Morphology and measurements of the South African population agree with those of the previous material examined (de Man 1895; van der Linde 1938; Sudhaus 1980; Andrássy 1983; Tahseen et al. 2009; Shokoohi et al. 2014; Kang et al. 2019); however, it differs in female tail length (24–35 vs 30–60 µm), and gubernaculum length (10–12 vs 10–16 µm). From Tahseen et al. (2009), population differs by having a slightly shorter female (vs 602–815 µm). Compared with the population studied by Shokoohi et al. (2014), they differ in body length (vs 574–677 µm in females and 487–675 µm in males), tail length (24–35 vs 13–20 µm in females and 40–43 vs 24–28 µm in males). Compared with the South Korean population (Kang et al. 2019), they differ in body length (vs 0.5–1.1 mm in females and 0.5–1.2 in males).
Pseudacrobeles (Pseudacrobeles) macrocystis De Ley and Siddiqi 1991 (Figs. 2, 3).
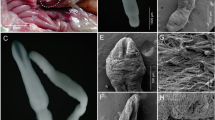
Pseudacrobeles (P.) macrocystis De Ley and Siddiqi 1991 (light microscopy). A Neck (black arrow pointing to the excretory pore, a white arrow pointing to the deirid); B anterior end; C female posterior end (arrow pointing to the phasmid); D entire female; E female reproductive system; F entire male; G lateral field; H male posterior end (black arrows pointing the genital papillae, white arrow pointing the phasmid)
Pseudacrobeles (P.) macrocystis De Ley and Siddiqi 1991 (scanning electron microscopy). A, B Lip region in lateral and frontal views, respectively (red line surrounding one of the labial processes); C excretory pore (arrow); D, E Vulva; F Lateral field; G, J: anus in ventral and lateral views, respectively; H, I female posterior end in lateral and ventral views, respectively (arrow pointing the phasmid); K female phasmid (arrow)
Material examined
Ten females and three males are in a good state of preservation.
Measurements
See Table 2.
Description
Adult
Body 0.61–0.78 mm long. Habitus ventrad curved after fixation, C-shaped in female and J-shaped in male. Cuticle 2–3 µm thickness, with transverse striations; annuli 1–3 μm wide at midbody. Lateral field 4–5 µm wide, occupying 13–16% of midbody diameter, with two alae limited by three longitudinal incisures extending to the phasmids. Lip region with six conoid lips bearing an elongate conoid process; primary axils narrow and smooth, U-shaped, lacking guarding processes; secondary axils width and smooth, V-shaped lacking guarding processes. Labial probolae three, blunt conoid. Stoma cephaloboid; cheilostom with small comma-shaped rhabdia; gymnostom very short with rhabdia poorly discernible; stegostom muscular, elongate, with well-sclerotized rhabdia. Pharynx cephaloboid; pharyngeal corpus subcylindrical, elongate, 3.0–4.0 times isthmus length; isthmus more slender; basal bulb ovoid, bearing well-developed valves. Cardia conoid. Nerve ring at 61–80% of neck length, surrounding pharyngeal corpus at its posterior part. Excretory pore at 68–71% of neck length, at the level of the posterior part of the pharyngeal corpus. Deirids at 75–80% of neck length, at the level of the anterior part of the isthmus.
Female
Reproductive system monodelphic-prodelphic; ovary posteriorly directed, with double flexure posterior to vulva; oviduct short, alveolated; spermatheca well developed, 0.4–1.1 times body diam., with spermatozoa inside; uterus cylindrical, 2.1–3.2 times body diameter long, differentiated in a long distal part with a scarce lumen and thick walls, and a short proximal portion with thinner walls and distinct lumen; post vulval uterine sac 0.6–1.1 times the corresponding body diam. long; vagina straight, 27–41% of body-wide; vulva slightly protruding. Rectum 1.1–2.2 times anal body width long, with three rectal glands. Tail conoid-elongate with acute terminus. Phasmids at 23–35% of tail length.
Male
Reproductive system monorchic. Testis reflexed ventrally anteriorly. Tail ventrally curved, conoid, bearing a thin mucro with acute terminus. Genital papillae three pairs pre-cloacal and five pairs post-cloacal, one anterior subventral, one anterior lateral, two posterior ventral and one posterior subdorsal. Phasmids located posterior to the lateral genital papillae, at 27–33% of tail length. Spicules are ventrally curved, with rounded manubrium, wide calamus, and curved lamina with finely rounded tip. Gubernaculum ventrally curved, 0.5–0.6 times the spicules length, with thin manubrium and corpus with low central cuneus and well-developed lateral crura.
Remarks
The population examined of P. (P.) macrocystis from South Africa fits well with the original description of this species published by De Ley and Siddiqi (1991) from Malaysia, except for the female body length slightly longer (0.6–0.7 mm vs 0.5–0.6 mm), neck length (168–202 µm vs 153–174 µm), and tail (70–85 µm vs 57–76 µm). Besides, there are no significant differences between the populations studied by De Ley et al. (1993a) from Cameroon and Tanzania. Compared with the Iranian specimens of P. (P.) macrocystis examined by Shokoohi and Abolafia (2012), there are no critical differences, although the male was not reported for the Iranian population. This species is reported for the first time from South Africa.
Molecular characterisation
The nblast result of the 18S rDNA gene fragment of the South African population of P. (P.) macrocystis shows a relationship with other species of the genus with 13 different gaps (99% similarity) with Pseudacrobeles sp. (KU180672), 20 differences (98% similarity) with Pseudacrobeles (Pseudacrobeles) variabilis (AF202150).
Locality and habitat
Both species were recovered from Magoebaskloof (GPS coordinates: S: 23°52′40.368"; E: 29°56′14.459"), Limpopo Province, South Africa, in association with wild grass.
Voucher material
Eight females and three males of P. (P.) macrocystis were deposited in the nematode collection of the Aquaculture Research Unit of the University of Limpopo, South Africa, and two females were deposited in the nematode collection of the University of Jaén, Spain. In addition, two slides of P. oxycercus containing five females and five males were deposited at the Nematology collection of the Aquaculture Research Unit, University of Limpopo, South Africa.
Phylogenetic relationships of Pseudacrobeles (Pseudacrobeles) macrocystis
The phylogenetic analysis of the 18S (Fig. 4) and 28S (Fig. 5) rDNA trees agree with the traditional arrangement of the genera of the subfamily Cephalobinae based on morphological characters (Andrássy 2005), agreeing with other molecular studies (Abolafia et al. 2020, 2021). Thus, the genus Pseudacrobeles Steiner 1938 appears more basal, close to the genus Eucephalobus Steiner 1936, having poorly developed lips and labial probolae (plesiomorphic condition), while genera with complex lips and labial probolae (apomorphic condition) appear in the derived branches in both trees. According to the phylogenetic trees, the evolutionary relationships of Pseudacrobeles appear more or less clear.
Regarding the genus Pseudacrobeles, it was established by Steiner in 1938. Then, the genus Pseudacrobeles divided into two subgenera, including Pseudacrobeles Steiner 1938 and Bunobus De Ley et al. 1993a, b (De Ley et al. 1993a, 1993b). The subgenera differ in the morphology of the lip region. In subgenus Pseudacrobeles, cephalic probolae (setae-like) and labial probolae (conical or low ridges connecting the tips of adjacent lips) are present. Besides, lateral lips are well developed. However, in subgenus Bunobus, cephalic probolae, and labial probolae do not exist. In addition, lateral lips are reduced (see Shokoohi and Abolafia 2012). In conclusion, Pseudacrobeles (P.) macrocystis was observed in various soil types associated with multiple crops in South Africa. This species is bacterivorous; therefore, its ecological role in the soil and its effect on crop production needs to be investigated.
Data availability
This material is the authors’ original work, which has not been previously published elsewhere and has no conflict of interest. Three slides of Pseudacrobeles (Pseudacrobeles) macrocytis and two slides of Poikilolaimus oxycercus were deposited in the Nematology collection of the Aquaculture Research Unit, University of Limpopo, South Africa. Two slides of P. (P.) macrocytis were deposited in the Nematode Collection of the Department of Animal Biology, Plant Biology, and Ecology of the University of Jaén (Spain).
References
Abolafia J (2015) A low-cost technique to manufacture a container to process meiofauna for scanning electron microscopy. Microsc Res Tech 78:771–776. https://doi.org/10.1002/jemt.22538
Abolafia J (2022) Extracción y procesado de nematodos de muestras de suelos de cuevas y otros hábitats. Monografías Bioespeleológicas 16: 6–17. https://drive.google.com/file/d/1ylkw5_qj_Cu5VlQOB14dZ3oAW18M-vxH/view
Abolafia J (2023) Metodología para la realización de análisis morfológicos y morfométricos en nematodos de muestras de suelos de cuevas y otros hábitats. Monografías Bioespeleológicas 17: 1–15. https://drive.google.com/file/d/1UVxczyFPDJ9wBFMn2e9kSXrY-Bup3E3L/view
Abolafia J, Peña-Santiago R (2005) Nematodes of the order Rhabditida from Andalucía Oriental: Pseudacrobeles elongatus (de Man, 1880) comb. n. Nematology 7:917–926
Abolafia J, Peña-Santiago R (2017) On the identity of Chiloplacus magnus Rashid and Heyns, 1990 and C. insularis Orselli and Vinciguerra, 2002 (Rhabditida: Cephalobidae), two confusable species. Nematology 19:1017–1034. https://doi.org/10.1163/15685411-00003104
Abolafia J, Peña-Santiago R (2020) On the identity of Eucephalobus oxyuroides (de Man, 1876) Steiner, 1936 (Rhabditida, Cephalobidae), with an updated taxonomy of the genus and notes about its phylogeny. J Nematol 52:e2020–e2061. https://doi.org/10.21307/jofnem-2020-061
Abolafia J, Peña-Santiago R (2021) Description of Spinocephalus tessellatus n. gen., n. sp. (Rhabditida, Cephalobidae) from Iran, a nematode with a new morphological pattern at lip region. J Nematol 53:e2021–e2078. https://doi.org/10.21307/jofnem-2021-078
Andrássy I (1983) A taxonomic review of the sub-order Rhabditina (Nematoda: Secernentae). Paris, Office de la Recherche Scientifique et Technique, Outre-Mer. Available at http://horizon.documentation.ird.fr/exldoc/pleins_textes/divers11-03/04391.pdf. Accessed 10 Jan 2016
Andrássy I (2005) Free-living nematodes of Hungary (Nematoda errantia). Volume 1. In the series: Csuzdi C. and Mahunka, S. (eds.). Pedozoologica Hungarica, No. 3. Hungarian Natural History Museum, Budapest, 518 pp.
Baermann G (1917) Eine einfache Methode zur Auffindung von Ankylostomum (Nematoden) Larven in Erdproben. Geneeskundig Tijdschrift Voor Nederlandsch-Indië 57:131–137
Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK (1998) A molecular evolutionary framework for the phylum Nematoda. Nature 392:71–75. https://doi.org/10.1038/32160
Borgonie G, Linage-Álvarez B, Ojo A, Shivanbu S, Kuloyo O, Cason ED, Maphanga S, Vermeulen JG, Litthauer D, Ralston CD, Onstott TC, Sherwood-Lollar B, van Heerden E (2015) Deep subsurface mine stalactites trap endemic fissure fluid Archaea, Bacteria, and Nematoda possibly originating from ancient seas. Fron Microbiol 6:833. https://doi.org/10.3389/fmicb.2015.00833
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
De Grisse AT (1969) Redescription ou modification de quelques techniques utilisees dans l’etude des nematodes phytoparasitaires. Mededelingen Van De Rijksfakulteit Landbowwetenschappen Gent 34:351–369
De Ley P, Blaxter ML (2002) Systematics position and phylogeny. In: Lee DE (eds) The biology of nematodes, pp 1–30. Harwood Academic Press, Reading, UK
De Ley P, Siddiqi MR (1991) Description of Pseudacrobeles macrocystis sp. n., with some new observations on the morphology of Cephalobidae (Nematoda). Afro-Asian J Nematol 1:31–40
De Ley P, Siddiqi MR, Boström S (1993a) A revision of the genus Pseudacrobeles Steiner, 1938 (Nematoda: Cephalobidae). Part 1. Subgenus Pseudacrobeles grad. n. Fundamen Appl Nematol 16:219–238
De Ley P, Siddiqi MR, Boström S (1993b) A revision of the genus Pseudacrobeles Steiner, 1938 (Nematoda Cephalobidae). Part 2. Subgenus Bunobus subgen. n., problematical species, discussion and key. Fundamen Appl Nematol 16:289–308
De Ley P, van de Velde MC, Mounport D, Baujard P, Coomans A (1995) Ultrastructure of the stoma in Cephalobidae, Panagrolaimidae and Rhaditidae, with a proposal for a revised stoma terminology in Rhabditida (Nematoda). Nematologica 41:153–182. https://doi.org/10.1163/003925995X00143
De Ley P, Felix AM, Frisse LM, Nadler SA, Sternberg PW, Thomas WK (1999) Molecular and morphological characterization of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1:591–612. https://doi.org/10.1163/156854199508559
de Man JG (1881) Die Einheimischen, frei in der reinen Erde und im süssen Wasser lebende Nematoden. Vorläufiger Bericht und descriptivsystematischer Theil. Tijdschrift Van De Nederlandse Dierkundige Vereeniging 5:1–104
de Man JG (1895) Description of three species of Aguillulidae, observed in diseased pseudo-bulbs of tropical orchids. Proc Transact Liverpool Biol Soc 9:76–94
Dougherty EC (1955) The genera and species of the subfamily Rhabditinae Micoletzky, 1922 (Nematoda). A nomenclatorial analysis—including an addendum on the composition of the family Rhabditidae Örley, 1880. J Helminthol 29:105–152
Filipjev IN (1934) The classification of the free-living nematodes and their relation to the parasitic nematodes. Smithson Misc Collect 89:1–63 + 8 plates.
Holovachov O, Boström S, De Ley IT, Nadler SA, De Ley P (2009) Description of Penjatinema novaezeelandiae sp. n. (Rhabditida: Cephalobidae) from New Zealand—a second species of a rare genus. J Nematode Morph Syst 12:7–18
Koohkan M, Shokoohi E, Mullin P (2015) Phylogenetic relationships of three families of the suborder Mononchina Kirjanova & Krall, 1969 inferred from 18S rDNA. Nematology 17:1113–1125. https://doi.org/10.1163/15685411-00002928
Kang H, Jongmin S, Kim D, Bae C, Kim Y, Choi I (2009) First report of five free living nematode species(Nematoda: Rhabditida) from Korea. J Species Res 8(3):259–267 https://doi.org/10.12651/JSR.2019.8.3.259
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Larget B, Simon DL (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol 16:750–759. https://doi.org/10.1093/oxfordjournals.molbev.a026160
Rambaut A (2018) Figtree, a graphical viewer of phylogenetic trees, version 1.4.4. Institute of Evolutionary Biology, University of Edinburgh. Available at https://github.com/rambaut/figtree/releases/tag/v1.4.4. Accessed 25 Nov 2018
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MRBAYES 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Syst Biol 61:539–542
Shokoohi E (2022) Observation on Hemicriconemoides brachyurus (Loof, 1949) Chitwood & Birchfield, 1957 associated with grass in South Africa. Helminthologia 59:210–216. https://doi.org/10.2478/helm-2022-0019
Shokoohi E, Abolafia J (2012) Nematodes of the order Rhabditida from Tehran province, Iran. The genus Pseudacrobeles Steiner, 1938 with description of Pseudacrobeles (Pseudacrobeles) iranicus sp. n. Ann Zool 62:331–340
Shokoohi E, Abolafia J (2019) Soil and freshwater Rhabditid nematodes (Nematoda, Rhabditida) from Iran: a compendium. University of Jaen, Spain, p 226
Shokoohi E, Abolafia J, Kheiri A (1937) Zad J (2007) Nematodes of the order Rhabditida from Tehran province (Iran). The genus Chiloplacus Thorne. Russ J Nematol 2(15):129–151
Shokoohi E, Abolafia J, Kheiri A, Zad J (2008) Nematodes of the order Rhabditida from Tehran province (Iran). Some known species of the family Cephalobidae. J Nematode Morph Syst 11:67–85
Shokoohi E, Mehdizadeh S, Amirzadi N, Abolafia J (2014) Four new geographical records of rhabditid nematodes (Nematoda: Rhabditida: Rhabditomorpha) from Iran with a note on phylogenetic position of the genus Pelodera Schneider, 1866. Russ J Nematol 22:49–66
Shokoohi E, Swart A, Marais M, Moyo NAG, Abolafia J (2023) Characterization of Acrobeloides longiuterus (Rashid & Heyns, 1990) Siddiqi, De Ley & Khan, 1992 (Rhabditida: Cephalobidae) from South Africa including the SEM study of the species. Zoomorphology 142:13–25. https://doi.org/10.1007/s00435-022-00583-3
Steiner G (1936) Opuscula miscellanea nematologica, III. Proc Helminthol Soc Wash 3:16–22
Steiner G (1938) Opuscula miscellanea nematologica, VII. Proc Helminthol Soc Wash 5:35–40
Sudhaus W (1980) Systematisch-phylogenetische und biologish-ökologische Untersuchungen and Rhabditis (Poikilolaimus) Arten als Beitrag zu Rassenbildung und Parallelevolution bei Nematoden. Zoologische Jahrbücher (systematik) 107:287–343
Sudhaus W, Fitch D (2001) Comparative studies on the phylogeny and systematics of the Rhabditidae (Nematoda). J Nematol 33:1–70
Tahseen Q, Hussain A, Sultana R, Khan R (2009) Descriptions of the Indian species of Poikilolaimus Fuchs, 1930 (Nematoda: Rhabditidae) with P. jodhpurensis (Khera, 1969) Sudhaus, 1980 revisited and a brief discussion on relationship with other congeners. J Nematode Morph Syst 12:27–40
van der Linde WJ (1938) A contribution to the study of nematodes. Entomo Memoirs 2:3–40
Acknowledgements
The authors thank to “University of Jaén/Caja Rural Jaén Foundation” for financial support received for the project entitled “Filogeografía de nematodos rabdítiidos (Nematoda, Rhabditida) en ambientes xerofíticos del sur de la península ibérica” (UJA2014/03/01) and the research activities “PAIUJA 2017/2018: EI_RNM02_2017”, “PAIUJA 2019/2020: EI_RNM02_2019” and “POAIUJA 2021/2022: EI_RNM02_2021” of the University of Jaén, Spain. SEM pictures were obtained with the assistance of technical staff (Amparo Martínez-Morales) and equipment of “Centro de Instrumentación Científico-Técnica (CICT)” from the University of Jaén.
Funding
Open access funding provided by University of Limpopo. This study was financially supported by the University of Jaén (Spain) for the SEM photographs. The authors also appreciate the Aquaculture Research Unit for the soil sampling.
Author information
Authors and Affiliations
Contributions
ES collected the samples. ES and JA identified the species, and analysed the data, wrote and revised the manuscript. JA took LM and SEM of the species. All the authors revised the manuscript and contributed to the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The paper reflects the authors’ own research and analysis in a truthful and complete manner.
Consent to participate
All the authors have been personally involved in substantive work leading to the manuscript and contributed to preparing the final draft of the manuscript.
Consent for publication
The manuscript has not been published in whole or in part elsewhere and is not currently being considered for publication in another journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shokoohi, E., Abolafia, J. Morphological and molecular characterisation of two free-living bacterivorous nematodes belonging to Rhabditida from South Africa. Zoomorphology 142, 299–311 (2023). https://doi.org/10.1007/s00435-023-00604-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00604-9