Abstract
Fishes of the River Nile are a promising source of animal protein. The catfishes Bagrus bajad Fabricius 1775 and Bagrus docmak Fabricius 1775 are economically important with well-marketable size and use in fish farming. Digenean parasites cause severe damage to the gut tissue of their fish hosts. There are still some questions regarding the taxonomical features of the genus Acanthostomum Looss 1899. The present study has revealed new morphological features that confirmed the identity of the collected worms as Acanthostomum spiniceps Looss 1896 and aided in elucidating the possible functions of different internal organs and surface features. A long-stemmed excretory bladder was detected but there was no evidence of gonotyl. The circumoral spines (28–30) were detected in the early juvenile stage and adult. The ventral sucker has no tegumental folds in juveniles or crescent-shaped tegumental folds in adults but possesses two overlapping lips. In both juveniles and adults, the oral sucker with the associated circumoral spines assumed retracted, and protracted positions reflecting its highest movement activity. Elongation of the anterior region and partial emergence of the oral cavity in living juveniles and adults were described. The possible functions of the forebody glands and the posterior body openings near the anal pores were discussed. Large, non-ciliated, dome-shaped, and small ciliated, button-like papillae were concentrated on the body surface, particularly the oral and ventral suckers. The tegument possesses densely arranged single-pointed and scale-like spines that gradually decrease in size and number as they proceed posteriorly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The River Nile freshwater fishes are important sources of animal protein (Samy-Kamal 2015). The catfish Bagrus bajad Fabricius 1775 and Bagrus docmak Fabricius 1775 (Family: Bagridae) are well distributed and have an economic value due to their good marketing and use in aquaculture (Mashaly et al. 2019). Digeneans are well known for causing severe damage to the alimentary tract of their hosts (Dezfuli et al. 2016). The subfamily Acanthostominae Poche 1926 comprises a group of parasites that infect the alimentary tract of fish and reptiles (Catto and Amato 1993). The genus Acanthostomum Looss 1899 includes at least 21 species recorded from different fish families all over the world. In Africa, Acanthostomum absconditum Looss 1901 and A. spiniceps looss 1896, have been described from B. bajad and B. docmak (Bagriidae) in Egypt (e.g., Fischtal and Kuntz 1963; Moravec 1976; Ibraheem 2006; Gamal and Ibraheem 2019) and in Sudan (e.g., Khalil 1971). Also, these species were reported from Bagrus filamentosus (Bagriidae) and Chrysichthys nigrodigitatus Lacepède 1803 (Claroteidae) in Mali (Dollfus 1932) and Ghana (Thomas 1958). In Egypt, A. spiniceps was also described from B. filamentosus (Bagriidae) by Tadros et al. (1978), from Morone labrax Linnaeus 1758 (Percichthyidae) by El-Shahawi and Al-Bassel (1992), and from Lates niloticus (Latidae) by Morsy et al. (2013) and by Abdel-Gaber et al. (2018).
Now, there are still some questions regarding the taxonomic position of the genus Acanthostomum, in general, and the morphological characters of A. spiniceps in Egypt. Brooks (1980) found that species of the genus Acanthostomum, and those of Proctocaecum Baugh 1957 differ in the absence of a gonotyl in Acanthostomum species. Brooks and Holcman (1993) considered the presence or absence of the gonotyl as homoplasious and introduced a new differentiating character represented by the long-stemmed bladder in Acanthostomum species and short-stemmed bladder in Proctocaecum species. For this reason, it was found necessary to examine the acanthostomine of Bagrus spp. in Egypt to clarify their identity regarding the new proposed diagnostic characters (Brooks and Holcman 1993). Although juveniles and adults of A. spiniceps were described by Ibraheem (2006), some reported features are debatable like the absence of circumoral spines and the presence of tegumental elevations around the ventral sucker of the early juvenile stage. Another unacceptable feature was the presence of crescent-shaped tegumental folds around the ventral sucker of the adult stage (Ibraheem 2006). Added interest is that a complete set of circumoral spines was detected in our preliminary light microscope observations of juveniles of the present acanthostomine. Therefore, the main purpose of this study is to examine juveniles and adults of A. spiniceps using a phase contrast microscope and scanning electron microscope (SEM) to clarify these points. Such a study may also throw light on the possible functions of some internal and surface structures and the role they may play in the biology of both juveniles and adults.
Material and methods
The freshwater catfishes Bagrus bajad and Bagrus docmak were collected from the Damietta branch of the River Nile at Mansoura city from March 2020 to February 2021. Living captured fish were transported in an aerated container, with fresh water, to the parasitology laboratory at Mansoura University and kept alive in an aquarium with aerated water. The parasites were collected and examined following the method of Allam et al. (2023) while their identification was done according to Moravec (1976) and measurements according to Ibraheem (2006). All measurements and scale bars were calculated using OMAX TopView 3.7 program. For scanning electron microscopy (SEM), five living juveniles and 10 living adults were preserved, postfixed, and dehydrated according to El-Naggar et al. (2016). Critical point drying was performed using EMS Q 850 critical point dryer with carbon dioxide as the transition fluid. The specimens were then coated with gold using SPI Sputter Coater and examined with a JEOL JSM 6510 lv electron microscope at the Electron Microscope Unit (EMU) of Mansoura University.
Results
Host: Bagrus bajad (Fabricius 1775) and Bagrus docmak (Fabricius 1775) (Family: Bagriidae).
Locality: Damietta branch of the River Nile at Mansoura City in Dakahlia Governorate, Egypt (coordinates: 31°02'47.2"N, 31°21'12.2"E).
Site of infection: Stomach and intestine.
Infection rate: 25.18%
Light microscopy of A. spiniceps (Figs. 1, 2)
Schematic drawing showing morphological details of adult Acanthostomum spiniceps, a Whole body. Scale bar = 100 µm, bAnterior region. Scale bar = 100 µm. c Middle region. Scale bar = 100 µm. d Posterior region. Scale bar = 100 µm. an Anal opening, at, anterior testis; b base of circumoral spine, cmf circular muscle fibres, cs circumoral spines, e egg, eb excretory bladder, ep excretory pore, fg forebody glands, gp gonopore, ic intestinal caecum, la lateral arm of excretory bladder, lmf longitudinal muscle fibres, ma median arm of excretory bladder, mt metraterm, o ovary, oc oocyte, oes oesophagus, os oral sucker, ph pharynx, pp pars prostatica, pph prepharynx, pt posterior testis, rmf radial muscle fires, sp spermatozoa, sr seminal receptacle, sv seminal vesicle, te terminal pointed end of circumoral spine, u uterus, vd vas deferens, vf vitelline follicle, vs ventral sucker
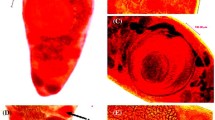
Phase-contrast microscope photographs showing morphological details of juvenile and adult Acanthostomum spiniceps. a Anterior region of juvenile showing anterior portion of oral sucker (os) partially everted outside body but left the circumoral spines (cs) in their position. Spines have wide base (b) and terminal pointed end (te). Scale bar = 50 µm. b Magnified funnel-shaped, highly muscular oral sucker (os) of adult surrounded by (29) circumoral spines (cs) and each spine has wide base (b) and pointed terminal end (te). Scale bar = 50 µm, c Oval-shaped ventral sucker (vs) of adult provided with radial (rmf), circular (cmf), and longitudinal muscle fibres (lmf). Scale bar = 30 µm. d Posterior region of adult showing two large testes, anterior (at) and posterior one (pt). Pear-shaped seminal receptacle (sr) lies between ovary (o) and anterior testis (at). eb, Excretory bladder; ep, excretory pore; vf, vitelline follicles. Scale bar = 100 µm. e Middle body region of adult showing excretory system with 2 main convoluted excretory canals (ec) and uterine loops (u) filled with eggs (eg). vf, Vitelline follicles. Scale bar = 50 µm
Juvenile
Five living juveniles studied. Measurements are in µm. Body small, elongated, (767 ± 45) long and (284 ± 45) wide. Funnel-shaped oral sucker larger than ventral sucker (Fig. 2a), (230 ± 27) long and (206 ± 23) wide; it’s opening with crown of single row of (28–30) nearly equal-sized spines, each (27 ± 6) long and (7 ± 1) wide (Fig. 2a). Prepharynx distinct, (205 ± 79) long. Pharynx large, (87 ± 6) long and (78 ± 18) wide. Oesophagus (84 ± 11) long, two intestinal caeca with two separate posterolateral anal openings. Ventral sucker spherical, (106 ± 21) long and (95 ± 18) wide. Reproductive organs not detected and excretory bladder not clearly visible. Large and small excretory ducts present on each lateral side of body. In some living juveniles, anterior region elongates, and anterior portion of oral sucker partially everts but leaves circumoral spines in their position. In this case, prepharynx becomes extensively elongated, at least twice its real length.
Adult
Thirty living and five stained adults studied. Measurements are in µm. Body elongated, with almost parallel lateral margins and rounded posterior end (Fig. 1a). Average dimensions of 20 well-flattened specimens (1965 ± 371) long and (479 ± 72) maximum width. Oral sucker funnel-shaped, highly muscular, and larger than ventral sucker (Figs. 1a, b, 2b), (332 ± 62) long, and (260 ± 32) wide. Its opening armed with crown of single row of (28–30) large, nearly equal-sized circumoral spines, each (38 ± 8) long and (10 ± 3) wide (Figs. 1a, b, 2b). Oral sucker with circular, longitudinal, and radial muscle fibres (Figs. 1a, b, 2b). Numerous forebody gland cells in anterior body region, particularly around oral sucker. (Fig. 1a, b). General body surface covered with numerous spines, decrease in number posteriorly. Prepharynx relatively thick, and becomes very long, at least twice its original length, in specimens where anterior region elongates. Normal length of prepharynx (196 ± 106) but shorter when contracted. Pharynx oval-shaped with radial muscle fibres, (103 ± 18) long and (136 ± 55) wide (Fig. 1a, b). Ventral sucker oval-shaped (195 ± 25) long and (177 ± 24) wide. Its opening nearly circular and musculature with radial, circular, and longitudinal muscle fibres (Figs. 1c, 2c). Excretory bladder Y-shaped, two anterior arms reach level of anterior extremity of pharynx (Fig. 1a, c) and main arm opens medially at posterior margin of body through terminal excretory pore (Figs. 1d, 2d). Excretory system with two main convoluted excretory canals, one on each lateral side of body and many-branched excretory ducts (Fig. 2e).
Testes oval-shaped, tandem in position, posterior to ovary near posterior end of body (Figs. 1a, d, 2d). Anterior testis (243 ± 31) long and (193 ± 68) wide. Posterior testis (258 ± 60) long and (194 ± 46) wide. Mature spermatozoa clearly seen in each testis (Figs. 1a, d, 2d). Seminal vesicle long, coiled tube below ventral sucker (Fig. 1a, c), more dilated at its posterior region but narrower anteriorly. Pars prostatica present and genital pore found immediately in front of ventral sucker (Fig. 1a, c). No evidence detected for cirrus.
Ovary spherical or subspherical, at right side in front of testes, (159 ± 36) long and (132 ± 41) wide, (Figs. 1a, d, 2d). Seminal receptacle bear-shaped, immediately behind ovary (Figs. 1a, d, 2d), (183 ± 29) long and (98 ± 49) wide. The vitelline follicles distributed laterally at posterior half of body, from level of nearly middle region of anterior testis to region behind vesicula seminalis (Figs. 1a–d, 2e). Uterine loops entirely located anterior to ovary and occupy space from ventral sucker to ovary (Figs. 1a, 2e). Mature eggs operculated, yellow to brown in colour, (22 ± 3) long and (12 ± 2) wide (Fig. 2e).
Scanning electron microscopy (SEM) (Figs. 3, 4, 5)
Juvenile
The body (less than 750 µm in length) is elongated with a funnel-shaped oral sucker and a posterior rounded end. (Fig. 3a). In most specimens examined, two positions (retracted and protracted) of the oral sucker and associated circumoral spines are detected (Fig. 3a–e). In the retracted position, the oral sucker opening is nearly circular or oval in outline and completely opened while the circumoral (rudimentary) spines are completely buried, inside the body, in tegumental sockets covered by spiny tegumental elevations (Fig. 3a–c). The tegument covering the inner surface of the oral sucker is smooth (Fig. 3b). In some specimens with retracted spines, the bases of these spines partially protrude outside their tegumental sockets and a circumoral tegumental collar is clearly visible (Fig. 3d). There was no evidence of a spiny tegument covering the bases of these rudimentary spines. In the protracted position, the circumoral spines are completely everted and exposed outside the body although their bases are still covered with smooth tegument (Fig. 3e). The outer border of the circumoral tegumental collar is clearly visible and the circumoral spines form a crown of a single uninterrupted row (28–30 spines), located at nearly equal intervals, on the internal rim of the oral sucker opening (Fig. 3e). Each circumoral spine measures (25 ± 1) and consists of a wide base and a distal pointed end (Fig. 3e). The ventral sucker is fully everted with a wide base and a terminal shallow circular opening covered with radial tegumental ridges (Fig. 3f). The tegument covering its inner and outer surfaces has no spines. However, presumed sensory dome-shaped papillae, arranged in 2 circles (outer and inner), are clearly visible on its outer tegumental surface (Fig. 3f). Moreover, few dome-shaped sensory papillae are seen on its inner surface (Fig. 3f). There is no evidence of a genital pore on the region located in front of the ventral sucker. Numerous tegumental spines are still hidden in the tegument while most elevated spines are single-pointed. Posteriorly, where the tegumental spines are absent, the tegument shows numerous presumably gland duct openings at the ventral surface close to the lateral anal and excretory openings (Fig. 3g). The excretory pore is subterminal, rounded, and surrounded by tegumental papilla (Fig. 3g).
SEM photomicrographs of juvenile Acanthostomum spiniceps. a Whole body (ventral view). an, Posterolateral anal opening; ep, excretory pore; os, oral sucker; vs, ventral sucker. Scale bar = 100 µm. b Frontal view of oral sucker (os), in retracted position. Oral sucker opening (oso) is nearly oval in outline and completely opened while rudimentary circumoral spines (rcs) are buried in sockets (arrows) covered by spiny tegumental elevations (ste). Scale bar = 30 µm. c Enlarged spiny tegumental elevations (ste) and bases of rudimentary circumoral spines(brcs). arrows, Tegumental sockets. Scale bar = 10 µm. d Oral sucker (os) with bases of rudimentary circumoral spines (brcs) partially protruding outside their tegumental sockets and circumoral tegumental collar (c) is visible. oso, Oral sucker opening. Scale bar = 20 μm. e Oral sucker (os) in protracted position with circumoral spines (cs) completely everted and circumoral tegumental collar (c) is visible. oso, Oral sucker opening. Scale bar = 20 μm. f Enlarged fully everted ventral sucker (vs) with a wide base and terminal shallow circular opening covered with radial tegumental ridges (rtr). Dome-shaped, papillae (dp) are arranged in two circles on its outer tegumental surface. Scale bar = 10 µm. g Posterior end with excretory pore (ep) surrounded by papillae (*). Note presence of gland duct openings (go) and absence of spines. Scale bar = 5 μm
SEM photomicrographs of whole body and oral sucker of adult Acanthostomum spiniceps. a Whole body in a ventrolateral view. cs, Circumoral spines; ep, excretory pore; os, oral sucker; vs, ventral sucker. Scale bar = 100 µm. b Magnified oral sucker (os) in retracted position with its opening (oso) lined by a non-spiny lip (l) provided with radial tegumental ridges (rtr) and dome-shaped papilla (dp). Circumoral spines are hidden underneath the tegumental collar (c) except for their bases(bcs) which remain exposed. Scale bar = 10 µm. c Bases of circumoral spines (bcs) of retracted oral sucker covered with smooth tegument (smt) penetrated by numerous gland duct openings (go) with secretory bodies (sb). Scale bar = 5 µm. d Magnified portion of bases of circumoral spines(bcs) covered by smooth tegument(smt) penetrated by gland duct openings (go) without secretory bodies. Scale bar = 2 µm. e Protracted oral sucker (os) with circumoral spines (cs) protruding completely outside circumoral tegumental collar(c) and part of its internal portion (*) including buccal cavity(bc) emerges outside body. ms, Mucoid secretion. Scale bar = 20 µm. fEnlarged protracted circumoral spines (cs) covered with highly microvillus tegument (mt) penetrated by numerous gland duct openings(go). sb, Secretory bodies. Scale bar = 10 µm
SEM photomicrographs of ventral sucker, posterior body region and distributed spines of adult Acanthostomum spiniceps. a Ventral sucker (vs) in retracted position with oval-shaped opening surrounded by two successive lips, outer lip (ol) and inner lip(il). dp, Non-ciliated, dome-shaped papillae; gp, gonopore; Scale bar = 20 µm. b Magnified tegumental region around ventral sucker with small ciliated, button-like papillae (cbp). Scale bar = 1 µm. c Posterior body region (ventrolateral view) with lateral anal opening (an) and excretory pore (ep). Scale bar = 20 µm. d Posterior body region with conspicuous constriction(arrow) and circular wheel-like structure containing excretory pore (ep) in its centre. Scale bar = 20 µm. e Tegument of dorsal body surface covered with numerous single-pointed spines (ps). Scale bar = 2 µm. f Tegument of lateral body surface with few pointed spines (ps). Scale bar = 5 µm. g Spines (sp) still embedded in highly folded tegument(ft). Scale bar = 2 µm. h Incompletely developed scale-like spines (sc). Scale bar = 5 µm. i Enlarged multidigitate scales (sc), each possessing wide base arising from shallow depression of tegument (arrow) and its concave surface forms multiple parallel (2–6) digits (di). Scale bar = 3 µm
Adult
The body (more than 750 µm in length) is elongated with a funnel-shaped oral sucker and a posterior rounded end (Fig. 4a). As in juveniles, two positions of the oral sucker and associated circumoral spines are detected (retracted and protracted) (Fig. 4b, e). In the retracted position, the oral sucker opening is nearly circular in outline and lined by a non-spiny lip provided with fine radial tegumental ridges and dome-shaped, presumed sensory papilla (Fig. 4b). In this case, the circumoral tegumental collar is clearly visible while the circumoral spines are hidden in tegumental sockets underneath the tegumental collar except for their bases which are exposed but completely covered with a smooth tegumental layer and penetrated by gland duct openings (Fig. 4b–d). The tegument of the circumoral collar appears slightly elevated on the position of the underneath spines. In the protracted position, the opening of the oral sucker is greatly widened, and circular in outline and the associated circumoral spines protrude completely outside the circumoral tegumental collar with their bases still embedded in the tegument covering the opening of the sucker (Fig. 4e). Moreover, part of the internal portion of the sucker protrudes outside the body (Fig. 4e). The tegument covering the inner surface of the oral sucker is highly folded, non-microvillus and has no spines (Fig. 4e), while that covering the circumoral spines and the regions in between is microvillus and penetrated by gland duct openings (Fig. 4f). Some openings are small and contain spherical secretory bodies while others are large and have no secretions (Fig. 4f). In addition to these gland duct openings, the general body surface of some specimens, particularly at the anterior region, is covered with a great number of larger secretory bodies of different sizes which probably represent mucoid secretions of the host intestinal mucoid cells (Fig. 4e).
The ventral sucker appears in a retracted position with an oval-shaped opening surrounded by two successive lips, outer and inner (Fig. 5a). The whole surface of the ventral sucker including the outer and inner lips is covered by a non-spiny tegument and several large, non-ciliated, dome-shaped papillae are detected on the tegument covering both the inner and outer lips (Fig. 5a). Moreover, small ciliated, button-like papillae are seen on the regions surrounding the sucker (Fig. 5b). The genital pore lies immediately in front of the anterior extremity of the sucker (Fig. 5a). The posterior region of the body is elongated and has few or no tegumental spines (Fig. 5c). The two lateral anal openings are slit-like structures, and the excretory pore is terminal (Fig. 5c). In a single specimen, the posterior body region forms a conspicuous constriction around the body and subsequently, the region of the body lying posterior to it becomes greater in size and assumes a circular wheel-like structure containing the excretory pore in its center (Fig. 5d).
The general body surface of is covered with numerous tegumental spines which, according to their size and morphological appearance, can be categorized into two types: single-pointed spines measuring (2 ± 0.2) µm in length (Fig. 5e, f) and numerous multidigitate, scale-like structures measuring (3.5 ± 1) µm in length and (2.5 ± 0.3) μm in width (Fig. 5h, i). Spines are numerous at the anterior region but decrease in size and number as they proceed posteriorly. The single-pointed spines are seen on the dorsal, lateral, and ventral surfaces of nearly the posterior two-thirds of the body (Fig. 5e, f) while the multidigitate scales are found on the dorsal, ventral, and lateral surfaces of the anterior third of the body (Fig. 5h, i). Each multidigitate pointed scale-like structure has a concave and a convex surface and its base arises from a shallow depression of the tegument (Fig. 5i). The base of each scale is a compact piece and its distal surface forms multiple parallel 2–6 digits (Fig. 5i). In many scales, the digits are not distinct (Fig. 5h). In most body regions, the tegument surrounding the scales and spines is highly folded while in some areas, the spines or scales do not emerge above the folded tegumental surface (Fig. 5g).
Discussion
Based on the present detailed morphological observations on the juveniles and adults Acanthostomum spiniceps, it was possible to compare precisely the present collected specimens with those described elsewhere in other regions of Egypt and African countries and confirm their identity (Moravec 1976; Fernandes et al. 2002; Ibraheem 2006; Morsy et al 2013; Al Ghamdi 2018). Brooks (1980) reported that species of the genus Acanthostomum, and those of Proctocaecum are closely related but differ from each other in the absence of a gonotyl in Acanthostomum species. Accordingly, the digenean species described herein belongs to the genus Acanthostomum. Moreover, the present study showed a long-stemmed, Y-shaped excretory bladder, a feature that comes in agreement with the new diagnostic characteristic proposed by Brooks and Holcman (1993). Martínez-Aquino et al. (2017) using Bayesian inference and Maximum likelihood analyses of combined 28S rDNA and ITS1 C 5.8S C ITS2 sequences, concluded that the genus Acanthostomum is monophyletic and the subfamily Acanthostominae is a paraphyletic taxon, in contrast with previous classifications (Brooks 1980, 2004; Brooks and Caira 1982; Brooks and Holcman 1993) that considered subfamily Acanthostominae a monophyletic taxon based on the morphological data. Therefore, molecular, and phylogenetic analysis is still required to be done for the intestinal Acanthostomum spp. of the freshwater Bagrus spp. in Egypt.
The acanthostomine trematodes are characterized by the presence of a single row of large circumoral spines around the oral sucker (Tkach and Snyder 2003). The number of spines in the present study in both juveniles and adults was (28–30) but was (27) in the study of Al Ghamdi (2018) and Fernandes et al. (2002) and (23) in the study of Morsy et al. (2013) and Abdel-Gaber et al. (2018). At the light microscope level, the morphological characteristics of adult A. spiniceps, in the present study, resemble those previously described for the same species by other authors in Egypt and other African countries, with few differences. The morphometrical data of adult A. spiniceps described by Fischthal and Kuntz (1963) from Bagrus spp. in Egypt and Fernandes et al. (2002) from Astroscopus in Brazil are closely similar but they are considerably greater than those of the present specimens, those of Moravec (1976) from River Nile at Cairo, Ibraheem (2006) from the River Nile at El-Menia city, upper Egypt and Morsy et al. (2013) from Lates niloticus of the River Nile at Beni Suef Governorate (see Table 1). These variations in the size of these specimens and their internal organs are intraspecific and could be attributed to the different methods of preparation, the number of specimens examined and the environment harboring the fish host.
Ibraheem (2006) found that the early juveniles of both A. absconditum and A. spiniceps have no developed circumoral spines on the tegument overlaying the oral sucker and concluded that this could be an adaptation that helps the sucker to make a smooth seal with the intestinal epithelium before suction. In the present study, circumoral (rudimentary) spines could be seen on the oral sucker opening. Using phase-contrast and SEM, the oral sucker with the associated circumoral spines of both juveniles and adults was detected in 2 main positions, retracted and protracted. In the retracted position, the circumoral spines are buried in tegumental sockets covered by spiny tegumental elevations while in the protracted position, the circumoral spines are completely everted over the oral sucker opening. Retraction of the entire oral sucker along with its circumoral spines into the anterior part of the body, in only adults of A. spiniceps and A. absconditum, was documented by Ibraheem (2006). Also, elongation of the head region and partial emergence of the oral cavity were also observed in both living juveniles and adults of A. spiniceps. This behaviour may reflect the high movement activity of the head region including the oral sucker. It seems probable that retraction of the circumoral spines is done when the anterior body region moves forewords searching either for a sheltered or healthy site. To ensure a firm attachment of the oral sucker, the circumoral spines protract and insert into the intestinal epithelium. The emergence of the anterior region of the buccal cavity might serve to capture and suck masses of the epithelial tissues of the intestine. The presence of (rudimentary) circumoral spines in some retracted positions indicates that the worm is in an early juvenile stage and completely developed circumoral spines are not yet formed. This may interpret the absence of the circumoral spines in early juveniles of A. spiniceps examined by Ibraheem (2006). Tkach and Snyder (2003) found that the circumoral spines with the associated muscles and tegumental surface of adult Acanthostomum macroclemidis form a terminal collar-like structure that can be retracted into the anterior part of the body and suggested that the position of the oral sucker and circumoral spine collar depends on the worm physiological state upon fixation while the circumoral spines change their orientation slightly when the oral sucker retracts. Also, Tkach and Snyder (2003) reported the ability of Proctocaecum coronarium to retract the oral sucker and associated circumoral spines completely into the anterior part of the body. As far as our knowledge is concerned, retraction of the oral sucker and circumoral spines into the anterior body region was not observed in other digenean groups possessing similar spines.
It is noteworthy that adult A. spiniceps contains numerous fore-body glands in the anterior region with their ducts opening onto the tegumental surface of the oral sucker and this agrees with the observations reported by Gamal and Ibraheem (2019) in the same species. Martins et al. (2016) also reported granules in Acanthostomum gnerii in the form of multiple agglomerations of glandular cells intermediating the proteinaceous spines. It seems probable that secretions of these glands may serve in the attachment of the oral sucker to the host tissue and/ or share in the extracorporeal digestion of the host tissues during feeding as suggested by (EL-Naggar et al. 1991, 1992, 1993) in the intestinal digeneans Astiotrema reniferum, Eumazenia spp., and Orientocreadium batrachoides. Ligasová et al. (2011) hypothesized that the secretory fore-body glands provide material (phospholipids) that may be involved in the process of penetration or immune modulation of the host. Acetylcholinesterase was demonstrated in the forebody glands of Microphallus and may interfere with the local peristalsis in the host gut and so prevent parasite expulsion (Davies 1979). In addition to these gland duct openings, the general body tegument of some specimens of A. spiniceps, particularly that covering the anterior region, is covered with a great number of larger secretory bodies of different sizes. There is no evidence that these bodies are coming out of the gland duct openings. Therefore, they are certainly of a host origin and may represent mucoid secretions of the host intestinal mucoid cells produced as an immune response of the host towards the attacking parasite. The SEM has revealed numerous posterior openings on the body surface near the anal openings. Although secretory bodies were not observed getting out of these openings, they may either represent openings of gland cells located in the posterior region of the body or shallow pits in the outer tegumental layer. Similar gland duct openings or shallow tegumental pits were observed in the same region of adult A. absconditum living in the intestine of the same host, Bagrus spp. (unpublished data). If these openings are the outlets of posterior body glands, they might produce anti-immune secretions that suppress the host’s immune response. However, if they are shallow tegumental pits, then it is possible that their secretion is coming out of the tegument and used for adhesion or protection of the body surface from the destructive effect of host digestive enzymes.
Ibraheem (2006) described the ventral sucker of A. spiniceps juveniles as being either submerged inside a tegumental elevation or extended at the level of the surrounding elevated tegument, a feature which was not observed in the present study. Moreover, Ibraheem (2006) described an elevated, crescent-shaped naked tegumental fold surrounding the ventral sucker of adult A. spiniceps. Such a feature has not been seen in the present study or in other previously described Acanthostomum spp. (e.g. Moravec 1976; Fernandes et al. 2002; Tkach and Snyder 2003; Morsy et al. 2013; Abdel-Gaber et al. 2018). In contrast, the ventral sucker of the present study possesses an oval-shaped opening surrounded by two successive (overlapping) clearly visible non-spiny lips (outer and inner). Therefore, it seems likely that what has been described by Ibraheem (2006) as tegumental elevation or crescent-shaped naked tegumental fold is a remarkably actual part of the ventral sucker covered externally by a non-spiny tegument. Ibraheem (2006) considered the ventral sucker in adults A. spiniceps and A. absconditum as being week enough to be the principal adhesive organ. In the present study, the ventral sucker is highly muscular (radial, longitudinal, and circular muscles) and possesses two successive elevated lips and conspicuous sensory papillae on its outer and inner surfaces. Such features suggest a strong role of the sucker in maintaining permanent and firm attachment in conjunction with the oral sucker. During retraction of the sucker, the lips with their smooth tegument could share in producing a strong suction mechanism by creating intimate contact with the host intestinal surface without irritation. The sensory papillae of the sucker surface would probably serve as contact receptors that control the mechanism of sucker attachment. Further ultrastructural studies are needed in this field to get a detailed structure of the types of sucker muscle fibres and their orientation. Such data would make it possible to postulate or precisely understand the suction mechanism of the sucker.
The present investigation has revealed the presence of two types of presumed sensory structures namely large, non-ciliated, dome-shaped papillae and small ciliated, button-like papillae, on the body surface, particularly on the oral and ventral suckers. Ibraheem (2006) reported six sensory papillae on the ventral sucker of juvenile A. spiniceps. Gamal and Ibraheem (2019) found two types of sensory papillae, ciliated and non-ciliated, in the juvenile stages of A. absconditum. Using both light microscopy and SEM, Tkach and Snyder (2003) recorded numerous prominent sensory papillae on the tegument covering the region of the oral and ventral suckers of A. macroclemidis. According to Brooks and Overstreet (1977), the presence of these papillae around the ventral sucker and the nearby genital pore is characteristic of some acanthostomines and may indicate the use of these papillae in the process of copulation to ensure the close apposition of the genital pores.
Data availability
All data used in this study are available upon personal request to the authors.
References
Abdel-Gaber R, Abdel-Ghaffar F, Mehlhorn H, Al Quraishy S, Morsy K, Maher S (2018) Light microscopic study of four plagiorchiid trematodes infecting marine fish in the south-eastern Mediterranean Sea, Alexandria city, with descriptions of two new species. Parasitol Res 117:1341–1356. https://doi.org/10.1007/s00436-018-5811-0
Al Ghamdi A (2018) Redescription of Acanthostomum spiniceps (Digenea) Infecting Lates niloticus (Perciformes: Latidae) on the basis of light microscopy. World App Sci J. https://doi.org/10.5829/idosi.wasj.2018.619.623
Allam HE, Mashaly MI, El-Naggar MM (2023) Ecological studies on the helminth parasites of catfishes Bagrus spp. (Bagridae) and Chrysichthys auratus (Claroteidae) inhabiting Damietta branch, River Nile. Egypt. J Basic App Sci 10(1):55–68
Brooks DR (1980) Revision of the Acanthostominae Poche, 1926 (Digenea: Cryptogonimidae). Zool J Linn 45:53–56
Brooks DR (2004) Comments on the gonotyl of Proctocaecum macroclemidis (Tkach and Snyder, 2003) n. comb. (Digenea: Acanthostomidae: Acanthostominae), with a key to the genera of Acanthostominae and a new phylogenetic for Proctocaecum Baugh, 1957. J Parasitol 90:594–597. https://doi.org/10.1645/GE-133R
Brooks DR, Caira JN (1982) Atrophecaecum lobacetabulare n. sp. (Digenea: Cryptogonimidae: Acanthostominae) with discussion of the generic status of Paracanthosthomum Fischthal and Kuntz, 1965 and Ateuchocephala Coil and Kuntz, 1960). Proc Biol Soc Wash 95:223–231
Brooks DR, Holcman B (1993) Revised Classification and Phylogenetic hypothesis for the Acanthostominae Looss, 1899 (Digenea: Opistorchiformes: Cryptogonomidae). Proc Biol Soc Wash 106:207–220
Brooks DR, Overstreet RM (1977) Acanthostome digeneans from the American alligator in the southeastern United States. Proc Biol Soc Wash 90:1016–1029
Catto JB, Amato JFR (1993) Digenetic trematodes (Cryptogonomidae: Acanthostominae) parasites of the Caiman, Caiman Crocodilus yacare (Reptilia, Crocodylia) from the Pantanal Mato-Grossense, Brazil, with the description of a new species. Mem Inst Oswaldo Cruz 88:435–440. https://doi.org/10.1590/S0074-02761993000300014
Davies C (1979) The forebody glands and surface features of the metacercariae and adults of Microphallus similis. Int J Parasitol 9(6):553–564
Dezfuli BS, Bosi G, DePasquale JA, Manera M, Giari L (2016) Fish innate immunity against intestinal helminths. Fish Shellfish Immunol 50:274–287. https://doi.org/10.1016/j.fsi.2016.02.002
Dollfus RP (1932) Mission saharienne Augi- dras-Draper, 1927–1928. Trematodes de mam- mifbres, oiseaux et poissons. Bull Mus Natl Hist Nat, Paris, 2 ser. 4, 555–563.
El-Naggar MM, Ibrahim HA, Hamada SF (1991) Redescription of Astiotrema reniferum (looss 1898 Stossich 1904, a digenean intestinal parasite of Clarias lazera. J Egypt Soc Parasitol 40:245–252
El-Naggar MM, Ibrahim HA, Hamada SF (1992) Redescription of Eumasenia bangweulensis, a digenean intestinal parasite of Clarias lazera in Egypt. J Basic Appl Zool 9(D):357–366
El-Naggar MM, Ibrahim HA, Hamada SF (1993) Re-description of Orientocreadium batrachoides Tubangui, 1931, a digenean intestinal parasite of Clarias gariepinus in Egypt. J Basic Appl Zool 10:157–170
El-Naggar MM, Cable J, Arafa SZ, El-Abbassy SA, Kearn GC (2016) Scanning and transmission electron microscopy of the histopathological impact of Macrogyrodactylus clarii (Monogenea: Gyrodactylidae) on the gills of catfish Clarias gariepinus. Folia Parasitol. https://doi.org/10.14411/fp.2016.017
El-Shahawi GAZ, Al-Bassel DA (1992) A general survey of the helminth parasites infecting common fishes in some inland water in Egypt. Proc Zool Soc A R Egypt 23:227–241
Fernandes BMM, Pinto RM, Cohen SC (2002) Report on two species of Digenea from marine fishes in Brazilian. J Biol 62(3):459–462. https://doi.org/10.1590/S1519-69842002000300009
Fischtal JH, Kuntz RE (1963) Trematode parasites of fishes from Egypt. part V. Annotated record of some previously described forms. J Parasitol 49:91–98. https://doi.org/10.2307/3275682
Gamal S, Ibraheem MH (2019) Morphology of adult and juvenile Acanthostomum absconditum (Looss, 1901) (Cryptogonimidae: Acanthostominae) from Bagrus bayad at El-Minia. Egypt El-Minia Sci 30:1–5
Ibraheem MH (2006) On the morphology of Acanthostomum spiniceps (Looss, 1896) and A absconditum (Looss, 1901) (Digenea: Cryptogonimidae: Acanthostominae) with particular reference to the juvenile stage. Acta Zoo 87(3):159–169. https://doi.org/10.1111/j.1463-6395.2006.00227.x
Khalil LF (1971) Checklist of the Helminth parasites of African freshwater fishes. Technical Communication No. 42 of the Commonwealth Institute of Helminthology St. Albans. Commonwealth Agricultural Bureaux.England 80.
Ligasová A, Bulantová J, Šebesta O, Kašný M, Karel K, Libor M (2011) Secretory glands in cercaria of the neuropathogenic schistosome Trichobilharzia regenti - ultrastructural characterization, 3-D modelling, volume and pH estimations. Parasit Vectors 4:162. https://doi.org/10.1186/1756-3305-4-162
Martínez-Aquino A, Vidal-Martínez VM, Aguirre-Macedo ML (2017) A molecular phylogenetic appraisal of the acanthostomines Acanthostomum and Timoniella and their position within Cryptogonimidae (Trematoda: Opisthorchioidea). Peer. https://doi.org/10.7717/peerj.4158
Martins ML, Tancredo KR, Marchiori NC, Pereira J, Castro LAS, Garcia P (1824) Esquivel J (2016) acanthostomum Gnerii Szidat, 1954 (Digenea: Cryptogonimidae) from Silver Catfish Rhamdia quelen (Quoy Gaimard. Neotro Helminthol 10(2):189–203
Mashaly MI, El-Naggar AM, Hagras AE, Alshafei HA (2019) Microhabitat selection of ectoparasitic Monogenean populations of the Nile Catfish Clarias gariepinus. Jordan J Biol Sci 12(5):573–580
Moravec F (1976) On two acanthostomatid trematodes, Acanthostomum spiniceps Looss, 1896 and A. absconditum Looss, 1901 from African bagrid fishes. Folia Parasitol 28:201–206
Morsy K, El-fayoumi H, Ali S (2013) Acanthostomum Spiniceps (digenea: cryptogonimidae: acanthostominae), a parasite of the African snook Lates niloticus (Perciformes: latidae) a light and scanning electron microscopic study. J Egypt Soc Parasitol 43(3):697–704
Samy-Kamal M (2015) Status of fisheries in Egypt reflections on past trends and management challenges. Fish Biol Fish 25:631–649. https://doi.org/10.1007/s11160-015-9404-z
Tadros G, Iskandar AR, Wassef NA (1978) On some intestinal trematodes from the Nile and Red Sea fishes with a histopathological study of their habitat. J Egypt Soc Parasitol 8:383–392
Thomas JD (1958) Two new digenetic trematodes, Heterorchis protopteri, n. sp. (Fellodistomidae) and Acanthostomum bagri, n. sp. (Acanthostomidae: Acanthostominae) from West Africa. Proc Helminthol Soc Wash 25:8–14
Tkach VV, Snyder SD (2003) Acanthostomum macroclemides n. sp. (Digenea: Cryptogonimidae: Acanthostominae) from the alligator snapping turtle. Macroclemys Temmincki J Parasitol 89:159–167. https://doi.org/10.1645/0022-3395(2003)089[0159:AMNSDC]2.0.CO;2
Acknowledgements
This paper is part of a PhD thesis to be submitted to the Zoology Department, Faculty of Science, Mansoura University, Egypt. The paper was carried out with joint financial support from The Ministry of Social Solidarity. We are grateful to Dr Mohamed Shaheen, Electron Microscope Unit of Mansoura University, for his great assistance in SEM.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was carried out with joint financial support from The Ministry of Social Solidarity to Hend Allam Grant No: MSS202.
Author information
Authors and Affiliations
Contributions
HA, MM and Mel-N made a substantial contribution to the conception and design of the study. All authors contributed to data acquisition. HA collected fish, examined the parasites, photographed them. HA, MM and MEl-N analyzed and interpreted the data. HA has written the first draft of the manuscript and all authors revised it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No experiments were performed on live fish. All applicable institutional, national, and international guidelines for the care and use of animals were followed. Mansoura University ethics approval no Sci-Z-Ph-2020–9.
Consent to participate
Authors declare that they have participated in this work.
Consent for publication
Authors declare that they know the content of this manuscript and agreed to submit it to Zoomorphology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allam, H.E., Mashaly, M.I. & EL-Naggar, M.M. Light and scanning electron microscope observations of the digenean intestinal parasite Acanthostomum spiniceps Looss 1896 (Cryptogonimidae) from the catfish Bagrus bajad and B. docmak. Zoomorphology 142, 285–298 (2023). https://doi.org/10.1007/s00435-023-00600-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00600-z