Abstract
Dendropsophini is a highly diverse clade with a controversial phylogenetic and taxonomic history. Different generic arrangements have been proposed and the monophyly of several clades supported or rejected. Previous evidence suggested that larval morphology could play an important role in our understanding of the evolution and diversification of Dendropsophini, although data are missing for most lineages, including the sister group of Dendropsophus, Xenohyla. Herein we describe the internal morphology of the tadpoles of X. truncata and compare our results with available information for members of Dendropsophini and closely related lineages. We propose that the presence of a fan-like papilla in the buccopharyngeal cavity, a single element suprarostral, and a triangular process at the base of the muscular process are synapomorphies for Dendropsophini; moreover, the presence of a divided m. subarcualis rectus II–IV seems to be a synapomorphy for Pseudini and, the nasal sac insertion of the m. levator lateralis could be a synapomorphy of Dendropsophini + Pseudini.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Faivovich et al. (2005) proposed the tribe Dendropsophini to accommodate species belonging to the genera Dendropsophus Fitzinger, 1843, Lysapsus Cope, 1862, Pseudis Wagler, 1830, Scarthyla Duellman & de Sá, 1988Scinax Wagler, 1830, Sphaenorhynchus Tschudi, 1838, and Xenohyla Izecksohn, 1998. Two putative phenotypic synapomorphies were suggested for that clade: the absence of lingual papillae in the buccopharyngeal cavity of tadpoles—with reversions in Pseudis and Lysapsus—and the absence of nuptial excrescences in adults (Faivovich et al. 2005:89). Nevertheless, the monophyly of Dendropsophini is a controversial issue, since it has been either supported (e.g., Wiens et al. 2010; Jetz and Pyron 2018), or rejected (e.g., Pyron and Wiens 2011; Duellman et al. 2016; Faivovich et al. 2018), and different tribe arrangements have been proposed. Currently, Dendropsophini (111 species) is restricted to Dendropsophus and Xenohyla, whereas Lysapsus, Pseudis and Scarthyla are members of Pseudini, and Sphaenorhynchus (and the recently described, monotypic Gabohyla Araujo-Vieira et al. 2020; Araujo-Vieira et al. 2020) and Scinax are assigned to their own tribes, Sphaenorhynchini and Scinaxini, respectively (Faivovich et al. 2018). The relationships among the tribes are also controversial, with different sister taxa relationships proposed. Two aspects, however, remain constant: the first is the sister relationships of Xenohyla and Dendropsophus that has consistently been recovered in all phylogenetic analyses that included representatives of both genera (e.g., Faivovich et al. 2005; Duellman et al. 2016; Jetz and Pyron 2018; Araujo-Vieira et al. 2019; Orrico et al. 2021); the second is that the absence of information regarding internal morphology of Xenohyla tadpoles precludes the optimization of several characters (Dias et al. 2019; Orrico et al. 2021).
The genus Xenohyla currently comprises two species inhabiting the Brazilian Atlantic Forest (Frost 2022), where adult frogs are usually found in terrestrial bromeliads (Izecksohn 1971; Silva et al. 1988). Although adults of the genus are phenotypically similar and their general morphology is quite unique for anurans (Izecksohn 1959, 1998; Caramaschi 1998), it has been difficult to pinpoint synapomorphies for the genus. A previous suggestion, the presence of a pectoral patch of glands (also referred to as the scars of the large windows of forelimb emergence; Izecksohn 1998; Caramaschi 1998), was not recovered as synapomorphy in a phylogenetic hypothesis that included phenotypic characters (Orrico et al. 2021). To date, there are three putative synapomorphies for the genus: (1) the presence of a small, transverse process in the urostyle (Faivovich et al. 2005) — although interpreted as a teratology by Orrico et al. (2021); (2) the frugivorous habits of the adults (data for X. eugenioi Caramaschi, 1998 are missing; Silva et al. 1989; Faivovich et al. 2005; Orrico et al. 2021); and (3) the curry-like odor and flavor that both species of Xenohyla exude, given that it is unknown for any other hylid species (Orrico et al. 2021).
Currently, only the tadpole of Xenohyla truncata Izecksohn, 1959 has been described (Izecksohn 1998). It is characterized by high tail fins and by its color pattern that resembles that of tadpoles of early diverging lineages of Dendropsophus (such as those of the D. decipiens (Lutz, 1925) and D. ruschii (Weygoldt & Peixoto, 1987) groups; see Dias et al. 2019 and Weygoldt and Peixoto 1987 respectively); no aspects, however, of its internal morphology have been described.
In this study we provide the first description of internal morphology of tadpoles of Xenohyla truncata, including buccopharyngeal cavity, larval cranium, and larval muscle anatomy. Additionally, we provide a redescription of the external morphology, describing some character states not available in the original description, as well as providing new illustrations and images of the living tadpole for the first time; finally, we comment on ontogenetic variation. Moreover, we compare our data with available evidence of other Dendropsophini taxa and discuss its phylogenetic and evolutionary implications.
Materials and methods
All individuals are from the same locality, Restinga de Maricá (42°50′59′′W, 22°57′43′′S), Maricá Municipality, Rio de Janeiro State, Brazil. Individuals were euthanized in 5% lidocaine, preserved in 10% formalin and deposited in the herpetological collections of the Universidade Estadual Paulista, Campus Rio Claro (CFBH), Museu de Zoologia da Universidade Estadual de Santa Cruz (MZUESC), the Tadpole Collection of the Centro de Coleções Taxonômicas, Universidade Federal de Minas Gerais (UFMG), and in the herpetological collection of the Universidade Federal Rural do Rio de Janeiro (UFRRJ). The identification of the tadpoles was performed by direct comparison with the original description (Izecksohn 1998). Also, we also took into consideration the species that occur in the same geographic area where our tadpoles were collected; at the Restinga de Maricá, 15 anuran species have been recorded (Oliveira and Rocha 2015), the larvae of none of which could be confused with X. truncata. The combination between labial tooth row formula 2(2)/3(1), high tail fins, with both fins originating at the body, acute tail tip, and a marbled golden coloration with a black banded pattern at the tail fins uniquely distinguish the tadpoles of X. truncata of all other syntopic species. Additional specimens of other taxa examined are from the Instituto de Ciencias Naturales (ICN), Colombia. Details of the analyzed lots are listed in Appendix.
External morphology
Descriptions, measurements, and proportions were based on a tadpole at stage 35 (CFBH 20807). For analysis of ontogeny variation, 5 individuals between stages 25–27 (UFMG 2581; MZUESC 224471) were also analyzed. Terminology follows Altig and McDiarmid (1999) and Altig (2007). Measurements were taken from digital images to the nearest 0.1 mm with the aid of ImageJ Version 1.50b software (Schneider et al. 2012) and follow Altig and McDiarmid (1999) for total length (TL), body length (BL), internarial distance (IND), interorbital distance (IOD), tail length (TAL), maximum tail height (MTH), tail muscle height (TMH), tail muscle width (TMW); Grosjean (2005) for dorsal fin height (DFH) and ventral fin height (VFH); Lavilla and Scrocchi (1986) for body width (BW), body width at level of nostrils (BWN), body width at eyes level (BWE), body height (BH), eye–nostril distance (END), nostril–snout distance (NSD), eye diameter (ED), nostril diameter (ND), snout–spiracular distance (SSD), oral disk width (ODW); Pinheiro et al. (2012) for dorsal fin insertion angle (DFIA); and Lins et al. (2018) for oral disk position (ODP), spiracle length (SL), spiracular–venter distance (SVD). An illustrative scheme with these measurements is available in Pezzuti et al. (2021). Standardization of character and character states follows Pezzuti et al. (2021). Developmental stages are in accordance with Gosner (1960). Live photos of tadpoles were taken in a small, narrow aquarium using standard macro lens for digital cameras.
Buccopharyngeal cavity
Three individuals (MZUESC 224471 and UFMG 2581) at stage 28 and 29 were manually dissected according to Wassersug (1976) to expose the buccopharyngeal cavity and, after inspection under a stereoscopic microscope with the aid of methylene blue staining, submitted to the protocol of Alcalde and Blotto (2006) for scanning electron microscopy (SEM) using a JEOLJSM-6360LV scanning electron microscope. Buccopharyngeal terminology follows Wassersug (1976, 1980).
Larval muscles and cranial morphology
After external morphology inspection, two tadpoles at stage 35 (CFBH 20807) and 26 (UFMG 2581) were processed following the protocol of Dingerkus and Uhler (1977) of clearing and double staining; the procedure was interrupted after the Alcian Blue step and specimens were manually dissected for inspection of cranial muscles (stained with Lugol’s solution). The skeletal description was based on the same tadpole at stage 35, and complementary information and illustrations were based on a specimen at stage 25 (UFMG 2581). Terminology follows Haas (1995, 2001, 2003), except that we used English names for skeletal structures instead of Latin terms if available. Photographs were taken in a Leica M205 stereomicroscope.
Taxonomy and character evolution
We follow the taxonomic proposal of Faivovich et al. (2018), using Dendropsophini sensu stricto to refer to the clade Xenohyla + Dendropsophus. Furthermore, given the phylogenetic relationships instability between the tribes of Hylinae (e.g., Faivovich et al. 2005, 2018; Duellman et al. 2016; Jetz and Pyron 2018), we compare our results with the available evidence and new observations for Dendropsophini sensu Faivovich et al. (2005; referred here as Dendropsophini sensu lato), i.e., including Lysapsus, Pseudis, Scarthyla, Scinax, and Sphaenorhynchus (and Gabohyla).
We reconstructed the evolutionary history of some characters—(1) presence/absence of lingual papillae; (2) presence/absence of a fan-like papillae; (3) presence absence of the triangular process of palatoquadrate; (4) morphology of the suprarostral cartilage; (5) insertion of the muscle levator mandibulae lateralis; and (6) the configuration of the muscle subarcualis rectus II-IV—with respect to alternative phylogenetic hypotheses (Duellman et al. 2016; Jetz and Pyron 2018; Araujo-Vieira et al. 2019; Orrico et al. 2021) to propose putative synapomorphies. Although we had access to few individuals for dissections and to score the internal morphology characters, available evidence from the literature suggests that polymorphisms in those characters are not common; for instance, studies dealing with musculoskeletal and buccopharyngeal cavities on a broad scale (e.g.,Wassersug 1980; Wassersug and Heyer 1988; Haas 2003) did not report extensive individual variation and polymorphisms accounted few examples.
Transformation series (Hennig 1966; Grant and Kluge 2004) were individualized, and character optimization was performed in T.N.T. v.1.5 (Goloboff and Catalano 2016).
Results
External morphology
Body compressed (BH/BW = 1.06), elliptical in dorsal view, triangular in lateral view, longer than wide (BL/BW = 1.6), longer than high (BL/BH = 1.5) (Fig. 1A). Snout rounded in dorsal view, sloped in lateral view. Nostrils positioned frontally, rounded, closer to the snout tip than to eyes, anteriorly directed, visible in lateral and anterior/frontal views. Eyes lateral (IOD/BWE = 1), laterally directed, 24% of BWE. Spiracle sinistral, lateral (SVD/BH = 0.33), short, directed posterodorsally; centripetal wall present as slight ridge (Fig. 1B). Digestive tract coiled; switchback point slightly dislocated from the center of abdominal region. Vent tube dextral, directed posteriorly, short, distal portion free from ventral fin, positioned above its ventral margin; ventral and dorsal walls same length (Fig. 1C). Tail higher than body (MTH/BH = 1.81); tail muscle height 50% of body height, almost reaching tail tip; tail tip with a distinct flagellum. Dorsal and ventral fins convex, about the same height (DFH/TAL = 0.23; VFH/TAL = 0.23); higher portions between the middle and posterior thirds of the tail. Dorsal fin originating on middle third of body, slightly posterior to the interorbital line, at a high slope (DFIA = 40°), thick proximally; ventral fin originating anteriorly to the vent tube, at the level of intestine switchback point. Lateral line system barely visible in preserved material. Oral disk width 26% of body width, positioned and directed anteriorly (ODP = 48°), not emarginate (Fig. 1D); lips thick, forming a cup-like structure around the mouth; a single row of approximately 70 alternate conical papillae along the margin; wide anterior gap in papillae (AGW/ODW = 0.31); submarginal papillae absent. Two upper tooth ridges: A1 uninterrupted, bearing labial teeth (keratodonts); A2 interrupted in two very short, lateral segments (gap between them about 50% of ODW) and bearing teeth. Three lower tooth ridges: P1 interrupted by a wide gap and always bearing teeth; P2 and P3 uninterrupted, progressively shorter. Jaw sheaths wide, finely serrated, upper sheath slightly W‑shaped, lower sheath V‑shaped; upper wider than lower.
A. Tadpole of Xenohyla truncata, stage 35 (CFBH 20807), in lateral, dorsal and ventral views. B. Detail of the spiracle. C. Detail of the vent tube (right side). D. Detail of the oral disk. E–F. Tadpoles of X. truncata, stages 25 and 26 (UFMG 2581), detail of the developing oral disk. A anterior labial row, N nare, P posterior labial row, RA anterior labial ridge, RP posterior labial ridge, S spiracle, VT vent tube. Scale bars = 1 cm (A), 0.1 cm (D), 0.05 cm (E), and (F) 0.02 cm
Coloration
In life, body marbled with golden and black spots (Fig. 2). Dorsally, body yellowish to golden with regularly scattered black spots; at the snout, a golden transverse band, medially interrupted, extends between nostrils above the oral disk; laterally, a narrow black longitudinal stripe extends from lateral area of the oral disk to posterior portion of body, passing through the eye; from below eye level to venter, body marbled with large golden and black blotches. Spiracle distal portion golden. Iris reddish; anterior, posterior, dorsal, and ventral areas of iris dark. Tail with a banded pattern consisting of a blackish background and three longitudinal wide irregular golden stripes; fins translucent in the flagellum region.
Measurements (in millimeters)
BH = 8.3; BL = 12.4; BW = 8.0; BWE = 8.0; BWN = 4.8; DFH = 5.4; ED = 1.9; END = 3.1; IND = 4.3; IOD = 8.0; MTH = 14.3; ND = 0.3; NSD = 1.7; ODW = 2.3; SL = 0.7; SSD = 8.5; SVD = 2.4; TAL = 23.6; TMH = 4.0; TL = 36; TMW = 3.5; VFH = 5.4.
Variation
Five individuals at stages 25–26 from Maricá, Rio de Janeiro, Brazil (UFMG 2615) present fewer (i.e., 23–31) marginal papillae; upper tooth ridges are formed but lack labial teeth; among lower rows, P1 is present as two small segments bearing 3–7 teeth and P2 17–19 teeth; in the older specimen, P3 ridge is developed but lacks teeth (Fig. 1E, F). Upper jaw sheaths vary from straight to concave.
Buccopharyngeal cavity
Buccal floor triangular (i.e., narrow anteriorly, wide posteriorly; Fig. 3A). Single pair of large, globose, infralabial papilla (IL) (Fig. 4A). Tongue anlage (TA) elliptical, lacking lingual papillae; three rounded, pustulation-like projections present (Fig. 4B). Buccal floor arena U-shaped, laterally delimited by single fan-shaped buccal floor arena papilla (Fig. 4C), with few pustulations. Buccal pockets (BP) obliquely oriented. Ventral velum (VV) with spicular support, arch-shaped, with irregular margin, lacking marginal projections; large secretory pits scattered along the margin and adjacent region (Fig. 4D). Medial notch present and well-marked; glottis (GL) exposed (Fig. 4D). Branchial basket triangular, shallow, bearing three evident filter cavities.
Buccopharyngeal cavity of a tadpole of Xenohyla truncata (MZUESC 224,471) at stage 29. A. Buccal floor. B. Buccal roof. BFAP buccal floor arena papilla, BP buccal pocket, GL glottis, GZ glandular zone, IL infralabial papillae, IN internal nare, LRP lateral ridge papilla, MR median ridge, P pustulation, TA tongue anlage, VV ventral velum. Scale bars = 200 µm
Buccal roof triangular (Fig. 3B), longer than wide. Prenarial arena long and wide, rounded, with a transverse protuberance. Internal nares (IN) elliptical, arranged perpendicular to the anteroposterior axis; prenarial papillae absent; narial valve poorly developed or absent. Postnarial arena rectangular, devoid of papillae; few, scattered, round pustulations present. Median ridge (MR) trapezoid, low, with irregular margin. Short conical lateral ridge papillae (LRP) present. Buccal roof arena absents with rounded pustulations scattered along buccal roof. Glandular zone not evident. Dorsal velum with smooth margin, interrupted medially, lacking papillae.
Larval cranium
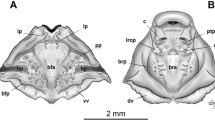
Neurocranium longer than wide; greatest width at the subocular bar level (Fig. 5A, B). Suprarostral cartilage (Fig. 5C) formed by a single element; suprarostral alae and completely fused; dorsal posterior process of the suprarostral ala well developed, triangular. Ethmoidal region short; trabecular horns short, parallel to each other. Basicranial fenestra weakly chondrified, partially occluded by a thin membrane. Frontoparietal fenestra large, rectangular. Orbital cartilage low. Otic capsules robust, rhomboidal in dorsal view, representing ca. 1/4 of chondrocranium length; a thin synotic tectum connects the two capsules. Palatoquadrate thin in lateral view, attached to neurocranium through a wide anterior quadratocranial commissure and an almost perpendicular ascending process. Articular process long. Muscular process triangular, well-developed, and curved dorsomedially; triangular process at the base of the muscular process. Connection between the tip of the muscular process and the neurocranium through a thick ligament. In the lower jaw (Fig. 5D), Meckel’s cartilage sigmoid, transversely oriented, almost perpendicular to the chondrocranium longitudinal axis. Infrarostral cartilages rectangular in frontal view, curved, joined at the symphysis.
Larval cranial skeleton of Xenohyla truncata at stage 25 (UFMG 2581). A–B. Neurocranium, dorsal and lateral views. C. Suprarostral cartilage, frontal view. D. Lower jaw, frontal view. E. Hyobranchial skeleton, ventral view. ABP anterior branchial process, ALPH anterolateral process, AP articular process, APH anterior process, AS ascending process, BB basibranchial, BF basicranial fenestra, BH basihyal, CB ceratobranchial, CH ceratohyal, HP hypobranchial plate, IC infrarostral, MC Meckel’s cartilage, MP muscular process, OC otic capsule, ORC orbital cartilage, PQ palatoquadrate, QC anterior quadratocranial commissure, RAP retroarticular process, SA suprarostral ala, SC suprarostral corpus, SF subocular fenestra, TH trabecular horn. Scale bars = 0.05 cm A, B, and E and 0.02 cm C and D
Ceratohyals (Fig. 5E) long, flat, and subtriangular; anterior margin with well-developed anterior and anterolateral processes; posterior processes triangular and long. Ceratohyals confluently joined by a chondrified pars reuniens. Basibranchial rectangular, with rounded urobranchial process present. Small basihyal present. Hypobranchial plates long, triangular, contacting each other along their anterior half. Branchial basket with four curved ceratobranchials bearing numerous lateral projections. Ceratobranchial I with a triangular anterior branchial process, continuous with the hypobranchial plate. Ceratobranchials II and III also fused to the hypobranchial plates, with less pronounced processes. Four long, curved spicules projecting dorsally from the ceratobranchials. Ceratobranchials distally joined by terminal commissures.
Muscles
We identified 33 cranial muscles in the tadpoles of Xenohyla truncata (Fig. 6; Table 1). Most muscles followed general patterns of origin and insertion, with some interesting modifications. The interhyoideus posterior is present and well developed. The subarcualis rectus II–IV spans between ceratobranchials I and IV, and some fibers attach to the branchial process II. The levator mandibulae lateralis extends between the articular process of palatoquadrate and the well-developed, nasal sac.
Larval cranial musculature of Xenohyla truncata at stage 35 (CFBH 20807). A–B. Dorsal view, showing levatores mandibulae muscles. C. Ventral view, showing mandibular, hyoid and hyobranchial muscles. D. Detail of the oral disk. E. Detail of the anterior hyobranchial muscles. F. Lateral view, showing hyoid muscles. G. Detail of the lateral, rostral region showing insertions of levatores mandibulae muscles. CB constrictor branchialis, GH geniohyoideus, HA hyoangularis, IH interhyoideus, IM intermandibularis, LMA levator mandibulae articularis, LMEP levator mandibulae externus profundus, LMES levator mandibulae externus superficialis, LMI levator mandibulae internus, LML levator mandibulae lateralis, LMLP levator mandibulae longus profundus, LMLS levator mandibulae longus superficialis, ML mandibulolabialis, OH orbitohyoideus, RC rectus cervicis, SA suspensorioangularis, SAR subarculalis rectus, SH suspensoriohyoideus, SO subarcualis obliquus
Discussion
Recently, Dias et al. (2019) reviewed the larval morphology of Dendropsophus tadpoles and discussed the evolution of some characters in the context of Dendropsophini sensu lato. They suggested that several character states could be synapomorphies for Dendropsophus or for Dendropsophini sensu stricto but stressed that the character states observed in Xenohyla could affect character state optimizations. We shall discuss these characters (Fig. 7) in light of our new findings and under different tree topologies.
Tadpoles of Xenohyla truncata share general external morphology characters states with Dendropsophus larvae of all species groups (Orrico et al. 2021), such as the nostrils positioned near the snout tip, the eyes lateral and proportionately large, and the small oral disk anteriorly directed and typically with thick lips (with or without papillae) that form a cup-like arrangement with a dorsal gap in papillation (e.g., Mijares-Urrutia 1990; Rivera-Correa and Gutiérrez-Cárdenas 2012; Pezzuti et al. 2021), although the origin of the ventral fin at the posterior third of the body seems to be autapomorphic for X. truncata, with secondary origin in some species of Dendropsophus (e.g., D. seniculus). Xenohyla truncata tadpoles are particularly similar to tadpoles of Dendropsophus minutus (Peters, 1872) and D. parviceps (Boulenger, 1882) groups by the triangular body in lateral view (Bokermann 1963; Santos et al. 1998), D. parviceps, D. marmoratus (Laurenti, 1768), and D. minutus groups by the dorsal fin insertion at mid-body length (Bokermann 1963; Santos et al. 1998; Peixoto and Gomes 1999), and D. molitor (Schmidt, 1857), D. marmoratus, and D. minutus groups by the ventral fin insertion, which is anterior to the vent tube (Bokermann 1963; Mijares-Urrutia 1990; Peixoto and Gomes 1999). The darkish longitudinal stripes in the tail are also found in tadpoles of D. ruschii and D. decipiens groups (Bokermann 1963; Weygoldt and Peixoto 1987), suggesting that it can be a plesiomorphic character state in Dendropsophini sensu stricto.
The diversity of oral disk configurations in Dendropsophini sensu stricto is astonishing. This includes not only variations in number and arrangement of tooth rows but also the apparent decoupling of tooth ridge (soft tissue) and labial teeth (keratinization of individual cells) development. A synthetic description of these disks via a LTRF then seems inaccurate: e.g., some tadpoles in this study could be assigned with a LTRF 2/3 if ridges are considered, or less if only ridges with teeth are considered. Considering ridges, the larval configuration in X. truncata (based on specimens at advanced larval stages) is LTRF 2(2)/3(1) (Izecksohn 1998; this study). Configurations in early larvae are incomplete stages to this formula that develops gradually as in tadpoles in general (e.g.,Thibaudeau and Altig 1988; Vera Candioti et al. 2011). Taking this into account, comparisons with species of Dendropsophus should be made with much attention to detail. Nevertheless, to our knowledge only a single species in this genus, D. anceps, develops a third posterior row, whereas the vast majority attains at most two rows (Orrico et al. 2021). This already constitutes an important difference among genera, surely related to heterochronic shifts during oral disk development. One might speculate that the evolution of oral disk morphology in Dendropsophus is marked by a truncation in the development of the oral disk.
Lingual papillae are present in most anuran tadpoles (e.g., Rada et al. 2019; Dias et al. 2021) with few exceptions (e.g., microhylids; Vera Candioti 2007). Tadpoles of Xenohyla truncata lack lingual papillae, but small pustulations at the tongue anlage could represent vestigial papillae, as described in other hylids (Wassersug 1980). The absence of lingual papilla was suggested by Faivovich et al. (2005) as a putative synapomorphy of Dendropsophini sensu lato. Besides X. truncata, tadpoles of Dendropsophus, Scinax, and Scarthyla goinorum lack lingual papillae, but these are present in Pseudis, Lysapsus, and Sphaenorhynchus (e.g., Vera Candioti 2007; Pedro HS Dias personal observation; Fig. 8); this renders absence of lingual papillae ambiguous at this level in all topologies (Duellman et al. 2016; Jetz and Pyron 2018; Araujo-Vieira et al. 2019; Orrico et al. 2021). Based on the tree topology of Duellman et al. (2016), Dias et al. (2019) suggested that the absence of lingual papillae could be a synapomorphy of Dendropsophus or Dendropsophini sensu stricto. It is worth noting that tadpoles of Sca. vigilans (Solano, 1971) possess lingual papillae (Pedro HS Dias personal observation); as such, assuming a sister relationship with Sca. goinorum (Bokermann, 1962), the lack of lingual papillae optimizes as a synapomorphy for Dendropsophini sensu stricto and Scinax, independently, being also an autapomorphy of Sca. goinorum in the topology proposed by Duellman et al. (2016).
In the buccal floor of several Dendropsophus species, there is a fan-like papilla located near the buccal pockets (Dias et al. 2019). These structures likely represent coalescence or failed-to-divide individual buccal floor arena papillae typical of other related tadpoles (e.g., Vera Candioti 2007). Kaplan and Ruíz-Carranza (1997) were the first to notice this feature and it was suggested as a synapomorphy for Dendropsophus by Dias et al. (2019), with reversion in some less inclusive taxa (e.g., D. nanus). The same fan-like papillae are present in X. truncata, suggesting it to be a synapomorphy for Dendropsophini sensu stricto.
Dias et al. (2019) suggested that the presence of two pairs of infralabial papillae could be a synapomorphy of the D. garagoensis (Kaplan, 1991) group, with an independent gain in D. decipiens. The presence of a single pair of infralabial papilla in Xenohyla truncata does not affect that character state optimization although it suggests that each pair seems to be of independent origin. We stress, nevertheless, that the more data on the number of infralabial papillae in other Dendropsophus species might change this interpretation because the taxonomic coverage of the knowledge on larval oral morphology is heavily biased to the clade that contains the D. leucophyllatus (Beireis, 1783), D. microcephalus (Cope, 1886), and the D. minutus groups.
The morphology of the larval musculoskeleton also offers some interesting features to discuss. Tadpoles of Dendropsophus have a triangular process at the base of the muscular process (e.g., Vera Candioti 2007). Dias et al. (2019) suggested that this could represent a synapomorphy for the genus, but the presence in Xenohyla truncata suggests that it is a synapomorphy of Dendropsophini sensu stricto. The same is true for the suprarostral cartilage as a single element, present in X. truncata and in all described Dendropsophus, except for D. ebraccatus (Cope, 1874) (Haas 2003).
In tadpoles of the Dendropsophus microcephalus group, Lysapsus, and Pseudis, the subarcualis rectus II–IV muscle has been reported to consist of two slips extending from ceratobranchial I to branchial process III (anterior slip) and from branchial process III to ceratobranchial IV (posterior slip) (Haas 2003; Alcalde and Barg 2006; Vera Candioti 2007). Conversely, like X. truncata, D. ebraccatus and D. decipiens have a single, continuous slip (Haas 2003; Dias et al. 2019). This suggests that the presence of an interrupted muscle is a synapomorphy for Lysapsus + Pseudis (Pseudini), with independent transformation within some species of Dendropsophus, such as D. nanus and D. microcephalus. Further evidence from other taxa may resolve whether this is a synapomorphy of a less inclusive clade or not.
Finally, the levator mandibulae lateralis muscle of Dendropsophini sensu stricto (except for Dendropsophus ebraccatus; Haas 2003) and Pseudini tadpoles inserts on the nasal sac (e.g., Haas 2003; Vera Candioti 2007; Pedro HS Dias personal observation). This state optimizes ambiguously in the Duellman et al. (2016) phylogenetic hypothesis yet is recovered as a synapomorphy of Dendropsophini + Pseudini according to the Jetz and Pyron (2018) scheme, and as a synapomorphy for a larger clade containing Dendropsophini, Sphaenorhynchini, and Pseudini in Araujo-Vieira (2019) and Orrico et al. (2021) hypotheses, respectively. Intriguingly, a nasal insertion of this muscle is also reported in tadpoles of the Lophyohylini Phyllodytes Wagler, 1830 (Vera Candioti et al. 2017).
Dendropsophini sensu stricto is a speciose and diverse clade with distinctive larvae. Dendropsophus tadpoles, for example, have been known to show large interspecific variation in their internal and external morphology (e.g., Gomes and Peixoto 1991; Schulze et al. 2015; Dias et al. 2019); however, only few studies have been conducted so far. Our data on Xenohyla truncata tadpoles shed light on some questions regarding the evolution of Dendropsophini sensu stricto tadpoles, but many others remain unanswered. Particularly, data on internal morphology are available for few species only. Including this study, the buccopharyngeal cavity is known for 12 species (Wassersug 1980; Echeverría 1997; Kaplan and Ruíz-Carranza 1997; Vera Candioti 2007; Dias et al. 2019) and musculoskeletal elements for 5 species (Haas 2003; Vera Candioti 2007; Arenas-Rodríguez et al. 2018; Dias et al. 2019)—and it is very likely that the poor taxon sampling is concealing the true diversity of the tribe.
Data availability
All relevant data are within the manuscript.
References
Alcalde L, Barg M (2006) Chondrocranium and cranial muscle morphology in Lysapsus and Pseudis tadpoles (Anura: Hylidae: Hylinae). Acta Herpetol 87:91–100. https://doi.org/10.1111/j.1463-6395.2006.00205.x
Alcalde L, Blotto BL (2006) Chondrocranium, cranial muscles and buccopharyngeal morphology on tadpoles of the controversial Leptodactylidae frog Limnomedusa macroglossa (Anura: Leptodactylidae). Amphibia-Reptilia 27:241–253. https://doi.org/10.1163/156853806777239959
Altig R (2007) A primer for the morphology of anuran tadpoles. Herpetol Conserv Biol 2:71–74
Altig R, McDiarmid RW (1999) Tadpoles the biology of anuran larvae. The University of Chicago Press, Chicago
Araujo-Vieira K, Blotto BL, Caramaschi U, Haddad CFB, Faivovich J, Grant T (2019) A total evidence analysis of the phylogeny of hatchet-faced treefrogs (Anura: Hylidae: Sphaenorhynchus). Cladistics 35:469–486. https://doi.org/10.1111/cla.12367
Araujo-Vieira K, Luna MC, Caramaschi U, Haddad CFB (2020) A new genus of lime treefrogs (Anura: Hylidae: Sphaenorhynchini). Zool Anz 286:81–89. https://doi.org/10.1016/j.jcz.2020.04.002
Arenas-Rodríguez A, Vargas JFR, Hoyos JM (2018) Comparative description and ossification patterns of Dendropsophus labialis (Peters, 1863) and Scinax ruber (Laurenti, 1758) (Anura: Hylidae). PeerJ 6:e4525. https://doi.org/10.7717/peerj.4525
Beireis GC (1783) Beschreibung eines bisher unbekannt gewesenen amerikanischen Froschen, welcher sich in der Naturaliensammlung des Herrn Hofraths Beireis in Helmstädt befindet. Schriften Berlin Ges Naturf Freunde. 4:178–182
Bokermann WCA (1962) Cuatro nuevos hylidos del Brasil. Neotropica La Plata 8:81–92
Bokermann WCA (1963) Girinos de anfíbios brasileiros—I (Amphibia, Salientia). An Acad Bras Cienc 35:465–474
Boulenger GA (1882) Catalogue of the Batrachia Salientia s Ecaudata in the collection of the British museum, 2nd edn. Taylor and Francis, London
Caramaschi U (1998) Description of a second species of the genus Xenohyla (Anura: Hylidae). Amphibia-Reptilia 19:377–384. https://doi.org/10.1163/156853898X00043
Cope ED (1862) On some new and little known American Anura. Proc Acad Nat Sci Philadelphia 14:151–159
Cope ED (1874) Description of some species of reptiles obtained by Dr. John F. Bransford, assistant surgeon United States Navy, while attached to the Nicaraguan surveying expedition in 1873. Proc Acad Nat Sci Philadelphia 26:64–72
Cope ED (1886) Thirteenth contribution to the herpetology of tropical America. Proc Am Philos Soc 23:271–287
Dias PHS, Araujo-Vieira K, Carvalho-e-Silva AMPT, Orrico VGD (2019) Larval anatomy of Dendropsophus decipiens (A Lutz, 1925 (Anura: Hylidae: Dendropsophini) with considerations to the larvae of this genus. PlosOne. 17:e0219716. https://doi.org/10.1371/journal.pone.0219716
Dias PHS, Vera Candioti F, Sabbag AF, Colaço G, Silva HR, Haddad CFB, Carvalho-e-Silva AMPT, Grant T (2021) Life on the edge: Tadpoles of cycloramphidae (Amphibia; Anura), anatomy, systematics, functional morphology, and comments on the evolution of semiterrestrial tadpoles. J Zoolog Syst Evol Res 59:1297–1321. https://doi.org/10.1038/s41559-018-0515-5
Dingerkus G, Uhler LD (1977) Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol 52:229–232. https://doi.org/10.3109/10520297709116780
Duellman WE, de Sá R (1988) A new genus and species of South American hylid frog with a highly modified tadpole. Trop Zoo 1:117–136. https://doi.org/10.1080/03946975.1988.10539408
Duellman WE, Marion AB, Hedges B (2016) Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arborana). Zootaxa 4104:1–109. https://doi.org/10.1646/zootaxa.4104.1.1
Echeverría DD (1997) Microanatomy of the buccal apparatus and oral cavity of Hyla minuta Peters, 1872 (Anura, Hylidae), with data on feeding habits. Alytes 15:26–36
Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheeler WC (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist 294:1–240. https://doi.org/10.5531/sd.sp.12
Faivovich J, Pereyra MO, Luna MC, Hertz A, Blotto BL et al (2018) On the monophyly and relationships of several genera of Hylini (Anura: Hylidae: Hylinae), with comments on recent taxonomy changes in hylids. S Am J Herpetol 13:1–32. https://doi.org/10.2994/SAJH-D-17-00115.1
Fitzinger LJFJ (1843) Systema reptilium. fasciculus primus. Braumüller et Seidel, Wien
Frost DR (2022) Amphibian species of the world: an online reference. version 6.1. Electronic database. https://amphibiansoftheworld.amnh.org/index.php. Accessed 3 July 2022.
Goloboff PA, Catalano SA (2016) TNT version 1.5, including a full implementation on phylogenetic morphometrics. Cladistics 32:221–238. https://doi.org/10.1111/cla.12160
Gomes MDR, Peixoto OL (1991) Larvas de Hyla do grupo ‘‘leucophyllata’’com a descrição da de H. elegans Wied, 1824 e notas sobre a variação do padrão de colorido do adulto nesta espécie (Anura, Hylidae). Rev Bras Biol 51:257–262
Gosner KL (1960) A simplified table for staging anurans embryos and larvae with notes on identifications. Herpetologica 16:183–190
Grant T, Kluge AG (2004) Transformation series as an ideographic character concept. Cladistics 20:23–31. https://doi.org/10.1111/j.1096-0031.2004.00003.x
Grosjean S (2005) The choice of external morphological characters and developmental stages for tadpole-based anuran taxonomy: a case study in Rana (Sylvarana) nigrovittata (Blyth, 1855) (Amphibia, Anura, Ranidae). Contrib Zool 74:61–76. https://doi.org/10.1163/18759866-0740102005
Haas A (1995) Cranial features of Dendrobatidae larvae (Amphibia: Anura: Dendrobatidae). J Morphol 224:241–264. https://doi.org/10.1002/jmor.1052240302
Haas A (2001) Mandibular arch musculature of anurans tadpoles, with comments on homologies of Amphibian jaw muscles. J Morphol 247:1–33. https://doi.org/10.1002/1097-4687(200101)247:1%3c1::AID-JMOR1000%3e3.0.CO;2-3
Haas A (2003) Phylogeny of frogs as inferred from primarily larval characters (Amphibia: Anura). Cladistics 19:23–89. https://doi.org/10.1111/j.1096-0031.2003.tb00405.x
Hennig W (1966) Phylogenetic systematics. University of Illinois Press, Champaign
Izecksohn E (1959) Uma nova espécie de “Hylidae” da Baixada Fluminense, Estado do Rio de Janeiro, Brasil. Rev Bras Biol 19:259–264
Izecksohn E (1971) Sobre a distribuição de alguns anfíbios anuros descritos da Baixada Fluminense, Estado do Rio de Janeiro, Brasil. Arq Unive Fed Rur Rio De Janeiro 1:5–7
Izecksohn E (1998) “1996”) Novo gênero de Hylidae brasileiro (Amphibia, Anura). Rev Univer Rur Ser Ciênc Vida 18:47–52
Jetz W, Pyron RA (2018) The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat Ecol Evol 2:850–858. https://doi.org/10.1038/s41559-018-0515-5
Kaplan M (1991) A new species of Hyla from the eastern slope of the Cordillera oriental in northern Colombia. J Herpetol 25:313–316. https://doi.org/10.2307/1564684
Kaplan M, Ruíz-Carranza PM (1997) Two new species of Hyla from the andes of central Colombia and their relationships to the other small Andean Hyla. J Herpetol 31:230–244. https://doi.org/10.2307/1565391
Laurenti JN (1768) Specimen Medicum, Exhibens Synopsin Reptilium Emendatum cum Experimentis Circa Venena et Antidota Reptilium Austriacorum. Wien, Austria: Joan
Lavilla EO, Scrocchi GJ (1986) Morfometría larval de los géneros de Telmatobiinae (Anura: Leptodactylidae) de Argentina y Chile. Physis 44:39–43
Lins ACR, De Magalhães RF, Costa RN, Brandão RA, Py-Daniel TR et al (2018) The larvae of two species of Bokermannohyla (Anura, Hylidae, cophomantini) endemic to the highlands of central Brazil. Zootaxa 4527:5019–5520. https://doi.org/10.11646/zootaxa.4527.4.3
Lutz A (1925) Batraciens du Brésil. Comptes Rendus et Mémoires Hebdomadaires des Séances de la Société de Biologie et des ses Filiales. Paris 93:211–214
Mijares-Urrutia A (1990) El renacuajo de Hyla meridensis (Anura: Hylidae) de los Andes de Venezuela. Rev Biol Trop 1990:2319–3234
Oliveira JCF, Rocha CFD (2015) Journal of coastal conservation: a review on the anurofauna of Brazil’s sandy coastal plains. how much do we know about it? J Coast Conserv 19:35–49
Orrico VGD, Grant T, Faivovich J, Rivera-Correa M, Rada MA et al (2021) The phylogeny of dendropsophini (Anura: Hylidae: Hylinae). Cladistics 37:73–105. https://doi.org/10.1111/cla.12429
Peixoto OL, Gomes MDR (1999) The tadpole of Hyla nahdereri Lutz and Bokermann, 1963. J Herpetol 33:477–479
Peters WCH (1872) Über eine Sammlung von Batrachiern aus Neu Freiburg in Brasilien. Monatsberichte Der Königlichen Preussische Akademie Des Wissenschaften Zu Berlin 1872:680–684
Pezzuti TL, Leite FSF, Rossa-Feres DDC, Garcia PCA (2021) The tadpoles of the iron quadrangle, Southeastern Brazil: a baseline for larval knowledge and anuran conservation in a diverse and threatened region. S Am J Herpetol 22(sp1):1–107. https://doi.org/10.2994/SAJH-D-20-00042.1
Pinheiro PDP, Pezzuti TL, Garcia PCA (2012) The tadpole and vocalizations of Hypsiboas polytaenius (Cope, 1870) (Anura, Hylidae, Hylinae). S Am J Herpetol 7:123–133. https://doi.org/10.2994/057.007.0202
Pyron A, Wiens JJ (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol 61:543–583. https://doi.org/10.1016/j.ympev.2011.06.012
Rada M, Dias PHS, Pérez-Gonzalez JL, Anganoy-Criollo M, Rueda-Solano LA et al (2019) The poverty of adult morphology: bioacoustics, genetics, and internal tadpole morphology reveal a new species of glassfrog (Anura: Centrolenidae: Ikakogi) from the sierra nevada de santa marta. Colombia Plosone 14:e0215349. https://doi.org/10.1371/journal.pone.0215349
Rivera-Correa M, Gutiérrez-Cárdenas PDA (2012) A new highland species of treefrog of the Dendropsophus columbianus group (Anura: Hylidae) from the andes of Colombia. Zootaxa 3486:50–62. https://doi.org/10.11646/zootaxa.3486.1.2
Santos CS, Alves ACR, Silva SPC (1998) Description of the tadpoles of Hyla giesleri and Hyla microps from Southeastern Brazil. J Herpetol 32:61–66
Schmidt O (1857) Diagnosen neuer Frösche des zoologischen cabinets zu Krakau. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Math Naturwissenschaftliche Classe 24:10–15
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 Years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Schulze A, Jansen M, Köhler G (2015) Tadpole diversity of Bolivia’s lowland anuran communities: molecular identification, morphological characterization, and ecological assignment. Zootaxa. https://doi.org/10.11646/zootaxa.4016.1.1
Silva HR, Britto-Pereira MC, Caramaschi U, Cerqueira R (1988) Utilização de Neoregelia cruenta (Bromeliaceae) como abrigo diurno por anfíbios anuros na restinga de Maricá-Rio de Janeiro. An Sem Reg Ecol S Carlos 6:307–318
Silva HR, de Britto-Pereira MC, Caramaschi U (1989) Frugivory and seed dispersal by Hyla truncata, a neotropical treefrog. Copeia 1989:781–783
Solano H (1971) Una nueva especie del genero Hyla (Amphibia: Anura) de Venezuela. Acta Biol Venez Caracas 7:211–218. https://doi.org/10.1590/S0034-71081998000400013
Thibaudeau DG, Altig R (1988) Sequence of ontogenetic development and atrophy of the oral apparatus of six anuran tadpoles. J Morphol 197:63–69. https://doi.org/10.1002/jmor.1051970106
Vera Candioti MF (2007) Anatomy of anuran tadpoles from lentic water bodies systematic relevance and correlation with feeding habits. Zootaxa 1600:1–175. https://doi.org/10.11646/zootaxa.1600.1.1
Vera Candioti MF, Haad MB, Baldo JD, Kolenc F, Borteiro C, Altig R (2011) Different pathways are involved in the early development of the transient oral apparatus in anuran tadpoles (Anura: Leiuperidae). Biol J Linn Soc 104:330–345. https://doi.org/10.1111/j.1095-8312.2011.01727.x
Vera Candioti F, Haas A, Altig R, Peixoto O (2017) Cranial anatomy of the amazing bromeliad tadpoles of Phyllodytes gyrinaethes (Hylidae: Lophyohylini), with comments about other gastromyzophorous larvae. Zoomorphology 136:61–73
von Tschudi JJ (1838) Classification der Batrachier mit Berücksichtigung der fossilen Thiere dieser Abtheilung der reptilien. Petitpierre, Neuchâtel
Wagler J (1830) Natürliches System der Amphibien, mit vorangehender classification der Säugthiere und Vogel. Ein Beitrag zur vergleichenden Zoologie. München, Stuttgart and Tübingen
Wassersug RJ (1976) Oral morphology of anuran larvae: terminology and general descriptions. Occ Pap Mus Nat Hist Univ Kansas 48:1–23
Wassersug RJ (1980) Internal oral features of larvae from eight anuran families: functional, systematic, evolutionary, and ecological consideration. Misc Publ Mus Nat Hist Univ Kansas 68:1–148. https://doi.org/10.5962/bhl.title.16230
Wassersug RJ, Heyer, (1988) A survey of internal oral features of leptodactylid larvae. Smiths Contr Zool 457:1–99
Weygoldt P, Peixoto LO (1987) Hyla ruschii n. sp., a new frog from the Atlantic forest domain in the state of Espirito Santo, Brazil (Amphibia, Hylidae). Stud Neotrop Fauna Environ 22:237–247. https://doi.org/10.1080/01650528709360736
Wiens JJ, Kuczynski CA, Hua X, Moen DS (2010) An expanded phylogeny of treefrogs (Hylidae) based on nuclear and mitochondrial sequence data. Mol Phylogenet Evol 55:871–882. https://doi.org/10.1016/j.ympev.2010.03.013
Acknowledgements
We thank the Center for Electron Microscopy (UFPR) for the use of the scanning electron microscope. We thank Célio FB Haddad and John D Lynch for granting access to the specimens under their care. Pedro H Dias thanks the Marie Sklodowska-Curie Actions (MSCA-IF-2020, MEGAN; 101030742). Victor GD Orrico is a CNPq fellow (#PQ 310467/2017-9; PQ 310256/2020-8; UNIVERSAL 431772/2018-5). Katyuscia Araujo-Vieira thanks Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP proc. # 2019/24979-2) and Agência Nacional de Promoción Científica y Tecnológica, Argentina (ANPCyT, 346/2019). Tiago L Pezzuti acknowledges CAPES for his PNPD fellowship (8887.468027/2019-00).
Funding
This work was supported by Marie Sklodowska-Curie Actions (MSCA-IF-2020, MEGAN; 101030742), CNPq (#PQ 310467/2017-9; PQ 310256/2020-8; UNIVERSAL 431772/2018-5), FAPESP (proc. # 2019/24979-2), ANPCyT (346/2019), and CAPES (8887.468027/2019-00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Open Access Publication funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Pedro HS Dias, Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review and editing, Project administration. Bárbara C Marcondes, Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review and editing. Tiago L. Pezzuti, Funding acquisition, Conceptualization, Methodology, Writing – original draft, Writing – review and editing. Florencia Vera Candioti, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review and editing. Katyuscia Araujo-Vieira, Funding acquisition, Writing – original draft, Writing – review and editing. Maritana M Prodocimo, Methodology Writing – original draft, Writing – review and editing. Hélio R da Silva, Writing – review and editing. Victor GD Orrico, Funding acquisition, Writing – original draft, Writing – review and editing, Conceptualization, Methodology. Alexander Haas Writing – original draft, Writing – review and editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated vertebrate species. Tadpoles were collected under the permanent permit # 48034-1issued to Victor G.D. Orrico by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Additional tadpoles studied. All specimens are deposited in herpetological collection of the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá, Colombia.
Scarthyla goinorum: ICN 45601, 45603: Letícia, Amazonas, Colombia.
Scarthyla vigilans: ICN 46079: Arauquita, Arauca, Colombia.
Spahenorhynchus dorisae: ICN 45612: Letícia, Amazonas, Colombia.
Sphaenorhynchus lacteus: ICN 45627: Letícia, Amazonas, Colombia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
dos Santos Dias, P.H., Marcondes, B.C., Pezzuti, T.L. et al. The missing piece of the puzzle: larval morphology of Xenohyla truncata (Anura: Hylidae: Dendropsophini) and its implication to the evolution of Dendropsophini tadpoles. Zoomorphology 142, 111–126 (2023). https://doi.org/10.1007/s00435-022-00575-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00575-3