Abstract
Phytophagous scarab beetles associated with angiosperms have characteristically enlarged lamellate antennae and exhibit a striking morphological variation of sensilla. In this study, we compared the morphology of antennal surface of 62 species Scarabaeoidea using SEM microscopy, particularly also in light of their evolution in association with angiosperms. We investigated the correlation of antennal sensilla morphology, i.e., their structure and distribution, with species diversity and lineage diversification rates. A high diversity of sensilla was observed but also multiple transitional forms, even on the same antennomere. We interpreted this as evidence for a high evolutionary plasticity. We recognized clear patterns of convergence and repeated evolution of certain types of placoid sensilla. One main tendency found in the phytophagous Pleurostict chafers was a shift from sensilla trichodea to placoid-like sensilla, apparently also enhanced by the increase of the lamellate antennal surface, either by size or number of the lamellae. This trend occurred not only in the Pleurosticts, but also in Glaphyridae, a second angiosperm-associated lineage of Scarabaeoidea. However, our results suggest no direct relation between species diversity or the rate of diversification and general sensilla morphology, i.e., the origin of placoid sensilla. This could be explained not only by species-poor lineages also possessing placoid sensilla but also by otherwise successful and species rich groups having sensilla trichodea (e.g., dung beetles). Results further reveal the need to refine current phylogenetic hypotheses by more comprehensive taxon sampling and to expand the molecular characterization of pheromones and odor binding proteins to better understand the role of chemical communication in scarab diversification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiosperm plants and their associated herbivores have an important share of terrestrial biodiversity. Several insect groups tracked the rise of the angiosperms, and close coevolutionary interactions with their angiosperm hosts are regarded as a key factor promoting the extraordinary diversity of insects (Ehrlich and Raven 1964). Others have diversified in conjunction with the subsequent rise of mammals using their dung (e.g., Ahrens et al. 2014). Key factors promoting global diversification were increased productivity and growth rates of angiosperms due to modification of leaf vein density (de Boer et al. 2012); the evolutionary rise of ectomycorrhiza enhancing chemical weathering of soils (Taylor et al. 2011, 2012); and the promotion of soil nutrient release by angiosperm litter that is easily decomposed (Berendse and Scheffer 2009). This autocatalytic ‘litter revolution’ resulted not only in a boom of suitable soil habitats, with abundant food resources under various ecological and biogeographical conditions, but also in the development and diversification a complex intestinal endosymbiont microflora (Zhang and Jackson 2008; Andert et al. 2010; Salem and Kaltenpoth 2022). This general environmental change caused by the rise of the angiosperms is likely also to have affected the intra- and interspecific chemical communication, and in consequence all structures involved in it, such as antennal sensilla in arthropods. Food availability in an increased three-dimensional space provided by the tall angiosperms and deep soil layers, made it more difficult not only to find the right food resources but also mating partners (Leal 1998; Schiestl 2010; Hansson and Stensmyr 2011; Hanks and Millar 2016).
Beetles (Coleoptera) are among the most ecologically diverse insect groups. Phytophagy arose approximately ten times in Coleoptera and may have promoted the largest radiation of beetles, the ‘Phytophaga’ (Hunt et al. 2007; McKenna et al. 2019). While most of the herbivore species (> 100,000) are adapted to specific host plants (e.g., leaf beetles, longhorns and weevils) (Farrell 1998; Marvaldi et al. 2002), Pleurostict scarabs (Scarabaeidae) (Erichson 1847; Ahrens et al. 2014) are polyphagous; nevertheless, they are one of the most diverse phytophagous beetle groups with about 25,000 species (Scholtz and Grebennikov 2005). They belong to the superfamily Scarabaeoidea, which also include stag beetles, dung beetles, and a number of smaller other groups which are all characterized by a club-like, pectinate antenna (Fig. 1BB), a feature which increases the antennal surface. Some of the plant-feeding scarab Pleurostict species can be crop pests (Jackson and Klein 2006). Despite their unspecific phytophagy and rather similar life style of adults as well as larvae, their morphological evolution over millions of years resulted in divergent body shapes—evidence of past evolutionary pressures in consequence of their soil digging activities and specialized feeding habits (Eberle et al. 2014). To find food and conspecifics for reproduction is vital for their survival and evolutionary success (expressed by the patterns of diversification and morphospace divergence). Therefore, we expect similar trends for the evolution of chemical sense and communication being, among others, expressed in the functionality, density, and morphology of antennal sensilla (Meinecke 1975; Leal 1998). However, detailed comparative studies on antennal morphology in relation to their evolution are lacking, as in most other insects.
A Synodendron cylindricum; B Typhoeus typhoeus; C Aphodius fossor; D Onthophagus fracticornis; E, F Odontaeus armiger; G Codocera ferruginea; H Xylonychus piliger; I Automolius angustulus; J, L Liparetrus sp.; K Liparetrus obscurus; M Diphucephala sp.; N, P Camenta innocua; O Triodontella raymondi; Q Tanyproctus sp.; R Miotemna sp.; S Astaena tridentata; T Raysymmela pallipes; U, V Glaresis handlirschi; W, X Orphnus sp.; Y Amphicoma bezdekorum; Z, AA Podolasia pilosa; BB Phaenognatha jenseni. A–T, V, X, Y Antennal lamella surface showing sensilla morphology; U, W, Z second lamella, distal view; AA third lamella, basal view; BB complete antenna
Here, we investigate in detail the morphology of antennae and their sensilla in the context of the evolution of Scarabaeoidea. A particular morphological diversity is known in most herbivorous Pleurostict lineages (Meinecke 1975; Bohacz et al. 2020) being expressed by the size and shape of antennal lamella, the type, number, density, and spatial distribution of sensilla. Therefore, we were interested whether the structure of the antennal sensilla is linked to species diversity and speed of diversification of lineages. We hope to obtain deeper insight into the evolution of scarab beetles and the drivers of their diversification.
Chemical communication influences the antennal morphology of insects through selection pressures (Elgar et al. 2018). As in all insects, the antennae are the primary sensory structures in scarabs (Crowson 1981; Chapman 1998). Olfactory sensilla are located on the (unilateral) lamellae, i.e., leaf-like extensions of the terminal three to seven antennomeres (club) which are spread only by haemolymph pressure (Pass 1980). The number of antennal lamellae may differ between lineages, species, and sex, particularly, in herbivore Pleurostict scarabs (Ahrens and Vogler 2008). Odorant signals can be detected from conspecifics (Nikonov et al. 2002), from microbial processes and from secondary plant metabolisms (de Bruyne and Baker 2008). It is known that several of the used pheromones are produced by intestinal endosymbiont bacteria of the beetles (Hoyt et al. 1971).
If the successful evolution of scarabs in association with angiosperms is mediated by chemical communication and thus by the evolution of advanced antennal sensilla, we would expect particular morphological tendencies in sensilla in association with herbivorous feeding. Furthermore, species diversity and speed of diversification in such lineages with advanced and modified sensilla would be higher compared to that of lineages with unmodified, sensilla trichodea.
Materials and methods
Sampling, preparation, and scanning electron microscopy
We examined 62 species including all major lineages of Scarabaeoidea (Suppl. Table 1). All species examined in this study are deposited in the collections of the Zoological Museum Alexander Koenig Bonn (ZFMK). The antennal lamellae were prepared for SEM as follows: specimens were separately softened in warm water overnight. The following day the antennae were separated from the head with a forceps. For subsequent dehydration, the separated antennal lamellae were treated in an ascending alcohol series (with ethanol solutions of 50%, 70%, 90% and 100%). applying each ethanol solution for five minutes. Some samples were cleaned in an ultrasonic cleaner (Emmi 30; EN IOS 9001:2000). Thereafter each antenna was placed in an acetone filled vial for three minutes, air dried on a microscope slide, and mounted on pin stubs. Finally, they were coated in a Cressington sputter coater (108 auto). SEM photographs of coated specimens were taken with a Zeiss Gemini (SmartSEM, V05.00.05, SEM Type: Supra 55 VP). Some SEMs were obtained with a Zeiss EVO 40 XVP Scanning Electron Microscope at MUSE (Trento, Italy) after gold coating on pieces previously cleaned using a sonicator. Sensilla terminology and classification used here follows Bohacz et al. (2020) and Meinecke (1975).
Patterns of antennal sensilla morphology and its relation to species diversity and diversification rates
The link between plasticity pattern of antennal sensilla morphology (Suppl. Tables 2, 3) and their successful co-evolution with angiosperms was investigated at two levels. In the absence of species level phylogenies, diversification is often modeled using two different approaches: diversification rates and raw values of species richness (Magallón and Sanderson 2001). We calculated the correlation between the general type of sensilla variation (hair-shaped sensilla vs. placoid sensilla vs. both occurring types mixed; Table 1) with, and a) extant species numbers (of examined lineages) and b) the net diversification rates (Magallón and Sanderson 2001) of selected lineages (of those we were able to examine the sensilla morphology and of which we had molecular data to include the lineages into the phylogeny). In the context of a birth-and-death model of diversification and since extinction is negligible (due to the lack of data), the maximum-likelihood estimate of diversification rate was obtained as r = log(n)/t for the stem group age (Magallón and Sanderson 2001). We did not consider crown group ages for the calculation of diversification rates, since sampling of molecular phylogenies is yet very incomplete, and current sampling (Ahrens et al. 2014; Gunter et al. 2016; Šípek et al. 2016; Neita Moreno et al. 2019; Eberle et al. 2019) does not reflect complete extant crown groups (but only limited parts of them).
For this purpose, the antennal sensilla morphology was mapped on the recent molecular tree of Neita Moreno et al. (2019) which represents an extended sampling compared to the analysis of Ahrens et al. (2014) which includes four markers widely used in beetle systematics (Hunt et al. 2007; Bocak et al. 2014). We did not consider more recent trees with phylogenomic data (Zhang et al. 2018; McKenna et al. 2019; Cai et al. 2022), due to their more limited taxon sampling. Coding for sensilla and their distribution was very roughly done as follows (Table 1): (1) sensilla trichodea (covering almost 100% of the lamellar surface), (2) placoid sensilla (covering almost 100% of the lamellar surface); (3) both types (covering in relation of ca 50–50% of the lamellar surface); (4) polyedric sensilla; (5) undefined sensilla type (homology uncertain). Sensilla types can be characterized even at finer scales (Altner and Prillinger 1980; Meiniecke 1975; Bohacz et al. 2020; see Suppl. Tables 2, 3), however, many lineages and taxa share multiple minor or transitional types, even within the same species, which make a summarizing analysis in relation to successful evolution very complex. Therefore, we applied for this study a more simplified scheme (see also Scholtz 1990), to reveal prominent and principal patters.
Speciation rates of the clades including stem lineages were calculated based on the time tree (and divergence times) of Ahrens et al. (2014) and number of extant species from Schoolmeesters (2021) and Ahrens (unpublished data). Speciation or diversification rates are an estimate of the rate of change in species numbers over time using clade age and species richness data, typically modeled as some form of birth–death process (Nee et al. 1994; Morlon 2014). While such analyses try to capture speciation and extinction processes more accurately, they can produce misleading results when diversity is not constant or unbounded through evolutionary time (Rabosky 2009; Wiens 2011), particularly when the fossil record is poor (Magallón and Sanderson 2001). This is known to be the case for insect diversification where there have been rapid bursts of speciation through time (Cornwallis et al. 2021).
Results
Our results demonstrate that the majority of early lineages of Scarabaeoidea have trichoid antennal sensilla (sensilla trichodea; Fig. 1A–H; Suppl. Fig. 3). This includes Lucanidae, Geotrupidae, Hybosoridae, Ochodaeidae, Belohinidae, Pleocomidae, and Passalidae (Suppl. Figs. 1A–H, 3A–C, M–R, 5I–N). In most cases, all antennomeres of the antennal club have such sensilla, on both sides. In Bolboceratidae and Glaresidae, we found, in addition to trichoid antennal sensilla, highly modified sensilla, which were in case Glaresis polyedric with fine pores (Fig. 1U, V; Suppl. Fig. 3D–I; see also Anton and Beutel 2012) and multi-auricular in Bolboceratidae (Fig. 2F; Suppl. Fig. 5A–H; see also Meinecke 1975). In some genera of Ceratocanthinae (Hybosoridae) the sensilla trichodea occur in different shapes, with more specialized shorter setae often lumped inside alleged "sensory areas" such as the case of the genus Pterorthochaetes (Fig. 3S–T). In Belohinidae there is a distinctive U-shaped sensory area composed of setae-like sensilla on the outer side of the last antennomere (Suppl. Fig. 3M–N). The highest variation of sensilla morphology we found in Scarabaeidae, of which, however, the monophyly (Timmermanns et al. 2016; Zhang et al. 2018; Song and Zhang 2018; McKenna et al. 2019; Ayivi et al. 2021) is still subject to some controversy since some molecular phylogenies did not confirm monophyly (Hunt et al. 2007; Bocak et al. 2014; Ahrens et al. 2014; Cai et al. 2022; see Fig. 3 based on Neito Moreno et al. 2019).
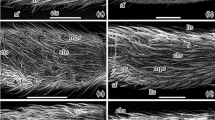
A–G Valgus hemipterus; B–D Prodoretus rhodesianus; E Anomala dubia; F Pentodon punctatus; H Osmoderma sp.; I Hoplia philanthus; J Schizonycha ruficollis; K Amphimallon assimile; L Cetonia aurata; M Melolontha melolontha; N Trichius sp.; O Gnorimus variabilis; P Pachypus sp; Q Photyna ornata; R Euchirus longimanus; S Pachytrichus sp.; T Aclopus sp. A second lamella, distal view; B third lamella, distal view; C first lamella, basal view; D–T—antennal club surface showing sensilla morphology
Antennal and sensilla morphology mapped onto the phylogeny of the Scarabaeoidea (Neita et al. 2019), with reference to the number of known species per lineage shown (simplified). Several nodes are simplified by a cartoon-like triangle, single branches were represented in that analysis only by a single species. Colored dots with letters refer to prevailing general sensilla type and illustrated species: A Glarsis handlirschi; B Trox hispidus; C Sinodendron cylindricum; D Typhaeus typhoeus; E Passalus striolatus; F Odontaeus armiger; G Onthophagus fracticornis; H Aphodius fossor; I Codocera ferruginea; J Amphicoma bezdekorum; K Hybosorus illigeri; L Orphnus sp.; M Arctodium vulpinum; N Neophaenognata jenseni; O Liparetrus obscurus; P Camenta innocua; Q Astaena tridentata; R Maladera holosericea; S Euchirus longimanus; T Hoplia philanthus; U Photyna ornata; V Schizonycha ruficollis; W Melolontha melolontha; X Amphimallon assimile; Y Tanyproctus sp.; Z Valgus hemipterus; AA Pachypus sp.; BB Prodoretus rhodesianus; CC Anomala dubia; DD Pentodon idiota
Within Scarabaeidae, saprophagous and dung-associated taxa (the clade Scarabaeinae + Aphodiinae) still possess sensilla trichodea (Fig. 3H, G). In Pleurostict lineages including its sister clade Orphninae [+ Allidiostomatinae according to Neito Moreno et al. (2019), supposed sister to Orphninae, but their sensilla could not being yet examined here] we see a clear shift to strong modifications of sensilla morphology from sensilla trichodea to an auriculate and/or placoid style (Fig. 3; Suppl. Fig. 2A–I). This strongly coincides with the transition to herbivorous feeding, i.e., the direct association with angiosperms (Fig. 3, arrow; see also Ahrens et al. 2014). In a few lineages throughout Scarabaeoidea, the sensilla are situated unilaterally on the lamellae (e.g., Acanthocerodes; Suppl. Fig. 3Q–R) or in a kind of closable pocket (e.g., in Codocera ferruginea; Suppl. Fig. 1A–F.). External faces of the club or also the basal face of a lamella may have a reduced coverage with sensilla or an entire lack of them (Fig. 2B, C), a pattern that is found in almost all lineages (with numerous exceptions, however). Among Pleurosticts, in Liparetrinae (see Table 1) and Aclopinae we observed a transition from sensilla trichodea to placoid sensilla in which very different shapes of trichoid to auriculate and round placoid sensilla can be encountered in some taxa on the same lamella. In these cases, placoid and trichoid sensilla, or their intermediate forms, have often similar frequencies (almost 50:50; see Table 1). Other Liparetrinae have entirely sensilla trichodea only (e.g., Colymbomorphini, Sercoidini; Bohacz et al. 2020), a state which is not encountered anymore in the sister clade (Melolonthinae + Rutelinae + Cetoniinae + Dynastinae). For this reason, we assume that this transformation series is likely: sensilla trichodea > scale-like sensilla > auriculate sensilla > placoid sensilla (Fig. 1I–M). In some lineages, such as Hopliini and Macrodactylini, these auriculate sensilla are preserved in mixture with normal placoid sensilla (Figs. 2I, 3T; see also Romero-López et al. 2013, 2017).
In most Pleurostict lineages can be found different types of placoid sensilla which are either mixed with each other (Fig. 2D–F, H) or sorted in different zones on each lamella (Fig. 2A, G). However, often transitions between different sensilla "types" can be rather fluent in one and the same lamella (see also Meinecke 1975). In several lineages, particularly in the clade Cetoniinae + Rutelinae, sensilla on the distal face of the lamella are situated in deepened pockets or sulci, which are encountered also in Hopliini, Melolonthinae or Pachytrichini. The dimension and number of these pockets can vary from group to group (see Suppl. Table 3). The round placoid sensilla can transform into elongate placoid sensilla which can be 20 times as long as wide, independently from the length of the antennal lamella (Fig. 1N–S). Such elongate placoid sensilla originated at least twice independently from each other, one time in Sericini + Ablaberini, and one time in Tanyproctini (and Acoma whose systematic placement is considered somewhat uncertain; Evans and Smith 2020). Interestingly, similar elongate placoid sensilla occur also in Glaphyridae (Fig. 1Y) which show no transitional steps from the sister group Ochodaeidae. In these cases, no other sensilla types are mixed with these elongate placoid sensilla, except in the lineage of Neotropical Sericini (Pacheco et al. 2022) in which we find, as if complexity was not enough, a stepwise reversal from elongate placoid to almost round placoid sensilla (Fig. 1T). In Ablaberini and Old World Sericini, no such reversals have been found. Furthermore, in the Neotropical Sericini, placoid sensilla can be mixed with other placoid sensilla which have pores, similar to those found in Dynastinae.
While adopting Meinecke's (1975) typification of sensilla (Suppl. Tables 2, 3, Bohacz et al. 2020) and studying more species in particular several species of Liparetrinae and Aclopinae, we realized that incorporating the immense amount of infraspecific sensilla variation into a character matrix with multistate character coding would be rather complicate (e.g., Figs. 1I, K, 2D, S). Therefore, the correlation analysis was based on simpler assumptions (see methods). Nevertheless, neither the correlation analysis of pure species diversity (r = − 0.329; p = 0.066) nor the diversification rate (r = 0.048; p = 0.794) could reveal a significant relation between the morphological constitution between sensilla type and evolutionary success of the lineages. Even if we excluded the evolutionary very successful dung beetles, which had also an indirect angiosperm follow-up associated with the mammals (Ahrens et al. 2014) and which possess antenna uniformly covered with sensilla trichodea (Fig. 1C, D), there was no change in levels of significance (species diversity: r = − 0.149; p = 0.431/ diversification rate: r = 0.054; p = 0.778).
Discussion
While comparative studies on antennal sensilla morphology in insects are rare (Hallberg and Hansson 1999), we attempt here to relate the comparative morphology of antennal sensilla, as a proxy for the evolutionary impact of underlying chemical communication, with species diversity and diversification rates. General morphology of antennal sensilla is rather uniform in most insects, mainly composed of sensilla trichodea (e.g., Hallberg and Hansson 1999; Nowińska and Brożek 2017; Yuvaraj et al. 2018); this could be one cause for the lack of overarching studies in beetles and other insect groups. This, however, is not the case in scarabaeiform beetles in which at least 44 different sensilla types have been reported so far (Meinecke 1975; Scholtz 1990; Bohacz et al. 2020).
The general patterns of antennal sensilla morphology reported here suggest that there is an apparent link between the evolution of placoid sensilla and the association with angiosperms (including phytophagous feeding) (Fig. 3). This trend is possibly even parallel, as many taxa in the Liparetrinae + Aclopinae, sister to the remainder Pleurosticti have mostly sensilla trichodea (Fig. 3; Suppl. Figs. 6, 8; Bohacz et al. 2020), while ancestral state of the latter remains uncertain yet since in all ancestral lineages already prevail placoid sensilla, either round or elongate ones.
No direct relation between species diversity or rate of diversification and general sensilla morphology was found, instead. This could be explained by the fact that some lineages with placoid sensilla include only few species belonging often phylogenetically isolated rogue lineages of uncertain systematic placement (e.g., Phaenomeridinae, Euchirini, Pachypodini), which were addressed in this study too. Many more were not yet included or just poorly sampled due to either insufficient available specimens (e.g., the polyphyletic Tanyproctini; Eberle et al. 2019) or the lack of DNA data for certain lineages, thus being not represented yet in the available molecular phylogenies (Hunt et al. 2007; Bocak et al. 2014; Ahrens et al. 2014; Timmermanns et al. 2016; Zhang et al. 2018; Song and Zhang 2018; McKenna et al. 2019; Neito Moreno et al. 2019; Ayivi et al. 2021; Cai et al. 2022). Moreover, there are also several lineages with exclusively trichoid sensilla that are quite diverse such as scarab dung beetles (Scarabaeinae, Aphodiinae) or stag beetles (Lucanidae) (Table 1).
We refrained here from a sister group comparison (e.g., Slowinski and Guyer 1993) due to two reasons: (1) existence of only one clear major shift from sensilla trichodea to placoid-like sensilla at the root of the clade (Melolonthinae + Rutelinae + Cetoniinae + Dynastinae) Pleurostict chafers (green dot, Fig. 3), while (2) the shift among Liparetrinae and Aclopinae is rather patchily known yet and in the known cases often quite gradual which requires a much denser lineage sampling for being able to link the sensilla morphology with exact number of species diversity or the rate of evolution. Yet, Pleurostict chafers, particularly the clade (Melolonthinae + Rutelinae + Cetoniinae + Dynastinae), are the most diverse lineage of Scarabaeoidea. Thus, the connection of chemical communication/sensilla morphology (i.e., a tendency to placoid sensilla) and diversification success continues being needed to be explored further in detail, particularly also because Zhang et al. (2018) reported, in comparison to all other scarabaeiform beetles, significantly increased diversification rates for Scarabaeidae, which in their tree included dung beetle lineages (Scarabaeinae + Aphodiinae) and phytophagous Pleurosticts.
We expect that chemical communication is rather more complex than it would become apparent from the simple examination of sensilla morphology. Sensilla trichodea prevail also in all hyperdiverse phytophagous beetle lineages of Phytophaga with no increased antennal surface (e.g., Ritcey and McIver 1990; Ranger et al. 2017; Vera and Bergmann 2018; Di Palma et al. 2019; Dong et al. 2020), thus high performance in chemical sensing is not necessarily linked with complicated or highly modified sensilla structure (e.g., Symonds et al. 2011). For instance, Rutelinae and Melolonthinae, although having in many lineages quite similar placoid sensilla, differ completely in their pheromone compounds (Melolonthinae: amino acid derivatives and terpenoids; Rutelinae: fatty acid derivatives; Leal 1998). Since "Melolonthinae" referred in Leal (1998) to unrelated lineages of Liparetrini, Rhizotrogini and Melolonthini, it is uncertain, whether these compounds could be part of an "ancestral" phylogenetical pattern or if they do represent lineage specific components. Chapman (1982) suggested that insects with a generalist diet require a greater number of sensilla than species with a more specialized diet, a hypothesis which is, however, only moderately supported by more recent studies (Lopez et al. 2014).
Last but not least, the high plasticity of sensillar morphology, which was already noticed by Meinecke (1975) based on histological analyses, makes the extraction of categorical data and discrete character states difficult in a comparative context. However, such plasticity is less common in the much less diverse early lineages of Scarabaeoidea (besides prominent exceptions such as in Glaresidae, Bolboceratidae, Glaphyridae, or Ochodaeidae; Fig. 3). This might be a starting point for further hypotheses to test, particularly when exploring sensilla variation in the context of pheromone diversity which is so far rather fragmentarily known.
Interestingly, placoid sensilla (although with much inferior morphological diversity) are also known in other, less diverse beetle lineages with antenna that have an increased antennomere surface (Ramsey et al. 2015). However, increased antennomere surface is not generally linked to the presence of placoid sensilla, as e.g., in Drilidae with pectinate antenna yet only sensilla trichodea occur (Faucheux and Kundrata 2017). Nevertheless, Ramsey et al. (2015) argue that the elaborate lamellate antennae, such as in male Rhipicera beetles, increases the surface area, which changes the airflow across the antennae, and thus the likelihood of odorant–receptor interactions (Jaffar-Bandjee et al. 2020). The latter is supposed to have an impact, in consequence, to the sensilla morphology and their efficacy.
Conclusions
While there seem to be a clear connection between the sensillar morphology and the polyphagous–phytophagous feeding traits of Pleurostict chafers, simple linear statistics do not confirm this hypothesis. Simple evolutionary connections appear to be obscured by other additional patterns such as evolutionary opportunities and competition, in combination with dispersal chances. Some lineages of the diverse Pleurosticts include only a relatively low number of species (e.g., Liparetrinae + Aclopinae) and are obviously restricted to the southern world (exception Podolasiini?). Also, among other Pleurosticts (i.e., the paraphyletic "Melolonthinae", Cetoniinae, and Rutelinae), there are some quite species-poor lineages and slowly diverging lineages with "modern" sensilla (Table 1). Hence, we see the here presented hypotheses and results as a starting point to refine our phylogenetical understanding of the Scarabaeoidea but most of all to open up for a molecular characterization of antennal sensilla functionality, regarding pheromones and odor binding proteins of sensilla (e.g., Leal 2001; Nikonov et al. 2002; González-González et al. 2019) to improve our understanding of their chemical communication, ecology and evolution.
References
Ahrens D, Vogler AP (2008) Towards the phylogeny of chafers (Sericini): analysis of alignment-variable sequences and the evolution of segment numbers in the antennal club. Mol Phylog Evol 47:783–798
Ahrens D, Schwarzer J, Vogler AP (2014) The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc R Soc, B 281:20141470
Altner H, Prillinger L (1980) Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Intern Rev Cytol 67:69–139
Andert J, Marten A, Brandl R, Brune A (2010) Inter- and intraspecific comparison of the bacterial assemblages in the hind gut of humivorous scarab beetle larvae (Pachnoda spp.). FEMS Microbiol Ecol 74:439–449. https://doi.org/10.1111/j.1574-6941.2010.00950.x
Anton E, Beutel RG (2012) The head morphology of Dascillus (L.) (Dascilloidea: Dascillidae) and Glaresis Erichson (Scarabaeoidea: Glaresidae) and its phylogenetic implications. Arthrop Syst Phyl 70:3–42
Ayivi SPG, Tong Y, Storey KB, Yu D-N, Zhang J-Y (2021) The mitochondrial genomes of 18 new Pleurosticti (Coleoptera: Scarabaeidae) exhibit a novel trnQ-NCR-trnI-trnM gene rearrangement and clarify phylogenetic relationships of subfamilies within Scarabaeidae. Insects 12:1025. https://doi.org/10.3390/insects12111025
Berendse F, Scheffer M (2009) The angiosperm radiation revisited, an ecological explanation for Darwin’s ‘abominable mystery.’ Ecol Lett 12:865–872. https://doi.org/10.1111/j.1461-0248.2009.01342.x
Bocak L, Barton C, Crampton-Platt A, Chesters D, Ahrens D, Vogler AP (2014) Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. SYst Entomol 39:97–110
Bohacz C, Harrison J, Ahrens D (2020) Comparative morphology of antennal surface in Pleurostict scarab beetles (Coleoptera). Zoomorph 139:327–346
Cai C, Tihelka E, Giacomelli M, Lawrence JF, Ślipiński A, Kundrata R, Yamamoto S, Thayer MK, Newton AF, Leschen RAB, Gimmel ML, Lü L, Engel MS, Huang D, Pisani D, Donoghue PCJ (2022) Integrated phylogenomics and fossil data illuminate the evolution of beetles. R Soc Open Sci 9:211771. https://doi.org/10.1098/rsos.211771
Chapman RF (1982) Chemoreception: the significance of receptor numbers. Adv Insect Physiol 16:247–356
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge
Cornwallis CK, Padje AV, Ellers J, Klein M, Jackson R, Kiers ET, West SA, Henry LM (2021) Symbiont-driven niche expansion shaped the adaptive radiation of insects. Preprint https://doi.org/10.21203/rs.3.rs-1063949/v1
Crowson R (1981) The biology of the Coleoptera, vol xii. Academic Press, London, UK, p 802
de Boer HJ, Eppinga MB, Wassen MJ, Dekker SC (2012) A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat Commun 3:1221. https://doi.org/10.1038/ncomms2217
De Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. J Chem Ecol 34:882–897
Di Palma A, Pistillo M, Griffo R, Garonna AP, Germinara GS (2019) Scanning electron microscopy of the antennal sensilla and their secretion analysis in adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae). Insects 10(4):88. https://doi.org/10.3390/insects10040088
Dong Z, Dou F, Yang Y, Wickham JD, Tang R, Zhang Y et al (2020) First description and comparison of the morphological and ultramicrocharacteristics of the antennal sensilla of two fir longhorn beetles. PLoS ONE 15(10):e0241115. https://doi.org/10.1371/journal.pone.0241115
Eberle J, Myburgh R, Ahrens D (2014) The evolution of morphospace in phytophagous scarab chafers: no competition - No divergence? PLoS ONE 9(5):e98536
Eberle J, Sabatinelli G, Cillo D, Bazzato E, Šípek P, Sehnal R, Bezděk A, Král D, Ahrens D (2019) A molecular phylogeny of Melolonthinae chafer beetles revisits the polyphyly of Tanyproctini (Coleoptera, Scarabaeidae). Zool Scr 48:349–358
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608. https://doi.org/10.2307/2406212
Elgar MA, Zhang D, Wang Q, Wittwer B, Thi Pham H, Johnson TL, Freelance CB, Coquilleau M (2018) Insect antennal morphology: the evolution of diverse solutions to odorant perception. Yale J Biol Med 91(4):457–469
Erichson WF (1847) Naturgeschichte der Insecten Deutschlands. Erste Abtheilung. Coleoptera, vol. 3, Lieferung 4. Nicolaische Buchhandlung, Berlin, pp. 481–640.
Evans AV, Smith ABT (2020) On the tribal classification of the Nearctic Melolonthinae (Coleoptera: Scarabaeidae), with descriptions of new species of Acoma Casey, 1889. Zootaxa 4748(1):51–77. https://doi.org/10.11646/zootaxa.4748.1.3
Faucheux MJ, Kundrata R (2017) Comparative antennal morphology of male Drilini with special reference to the sensilla (Coleoptera: Elateridae: Agrypninae). Zoolog Anz 266:105–119. https://doi.org/10.1016/j.jcz.2016.11.002
Farrell BD (1998) ‘Inordinate Fondness’ explained: why are there so many beetles? Science 281:555–559. https://doi.org/10.1126/science.281.5376.555
González-González A, Rubio-Meléndez ME, Ballesteros GI, Ramírez CC, Palma-Millanao R (2019) Sex- and tissue-specific expression of odorant-binding proteins and chemosensory proteins in adults of the scarab beetle Hylamorpha elegans (Burmeister) (Coleoptera: Scarabaeidae). PeerJ 7:e7054. https://doi.org/10.7717/peerj.7054
Gunter NL, Weir TA, Slipinksi A, Bocak L, Cameron SL (2016) If dung beetles (Scarabaeidae: Scarabaeinae) arose in association with Dinosaurs, did they also suffer a mass co-extinction at the K-Pg boundary? PLoS ONE 11(5):e0153570. https://doi.org/10.1371/journal.pone.0153570
Hallberg E, Hansson BS (1999) Arthropod sensilla: morphology and phylogenetic considerations. Microsc Res Tech 47(6):428–439
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of Cerambycid beetles: basic science and practical applications. J Chem Ecol 42(7):631–654. https://doi.org/10.1007/s10886-016-0733-8
Hansson BS, Stensmyr MC (2011) Evolution of insect olfaction. Neuron 72(5):698–711. https://doi.org/10.1016/j.neuron.2011.11.003 (PMID: 22153368)
Hoyt CP, Osborne GO, Mulcock AP (1971) Production of an insect sex attractant by symbiotic bacteria. Nature 230:472–473
Hunt T, Bergsten J, Levkanicova Z, Papadopoulou A, St John O, Wild R, Hammond PM, Ahrens D, Balke M, Caterino MS, Gómez-Zurita J, Ribera I, Barraclough TG, Bocakova M, Bocak L, Vogler AP (2007) A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318:1913–1916
Jackson TA, Klein MG (2006) Scarabs as pests: a continuing problem. Col Bull 60:102–119
Jaffar-Bandjee M, Krijnen G, Casas J (2020) Challenges in modeling pheromone capture by pectinate antennae. Integr Comp Biol 60(4):876–885. https://doi.org/10.1093/icb/icaa057
Leal WS (1998) Chemical ecology of phytophagous scarab beetles. Ann Rev Ent 43:39–61. https://doi.org/10.1146/annurev.ento.43.1.39
Leal WS (2001) Molecules and macromolecules involved in chemical communication of scarab beetles. Pure Appl Chem 73(3):613–616. https://doi.org/10.1351/pac200173030613
López MF, Armendáriz-Toledano F, Sámano JE, Shibayama-Salas M, Zúñiga G (2014) Comparative study of the antennae of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytinae): sensilla types, distribution and club Shape. Ann Entomol Soc Am 107:1130–1143
Magallón S, Sanderson MJ (2001) Absolute diversification rates in angiosperm clades. Evolution 55:1762–1780
Marvaldi AE, Sequeira AS, O’Brien CW, Farrell BD (2002) Molecular and morphological phylogenetics of weevils (Coleoptera: Curculionoidea): do niche shifts accompany diversification? Syst Biol 51:761–785
McKenna DD, Shin S, Ahrens D, Balke M, Beza C, Clarke DJ, Donath A, Escalona HE, Friedrich F, Letsch H, Liu S, Maddison D, Mayer C, Misof B, Murin PJ, Niehuis O, Peters RS, Podsiadlowski L, Pohl H, Scully ED, Yan EV, Zhou X, Ślipiński A, Beutel RG (2019) The evolution and genomic basis of beetle diversity. PNAS 116(49):24729–24737
Meinecke CC (1975) Riechsensillen und Systematik der Lamellicornia (Insecta, Coleoptera). Zoomorph 82:1–42
Morlon H (2014) Phylogenetic approaches for studying diversification. Ecol Lett 971:508–525
Nee S, May RM, Harvey PH (1994) The reconstructed evolutionary process. Phil Trans R Soc Lond B 344:305–311
Neita Moreno JC, Agrain FA, Eberle J, Ahrens D, Pereyra V (2019) On the phylogenetic position and systematics of extant and fossil Aclopinae (Coleoptera: Scarabaeidae). Syst Entomol 44:709–727
Nikonov AA, Peng G, Tsurupa G, Leal WS (2002) Unisex pheromone detectors and pheromone-binding proteins in scarab beetles. Chem Sens 27:495–504
Nowińska A, Brożek J (2017) Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorph 136:327–347. https://doi.org/10.1007/s00435-017-0354-y
Pacheco TL, Monné ML, Vaz-de-Mello FZ (2022) Ahrens D (2022) First non-feeding species of Sericini chafers (Coleoptera, Scarabaeidae): new genus and phylogenetic position. Org Divers Evol. https://doi.org/10.1007/s13127-022-00555-x
Pass G (1980) The anatomy and ultrastructure of the antennal circulatory organs in the cockchafer beetle Melolontha melolontha L. (Coleoptera, Scarabaeidae). Zoomorph 96:77–89
Rabosky DL (2009) Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol Lett 12:735–743
Ramsey A, Houston TF, Ball AD, Goral T, Barclay MV, Cox JP (2015) Towards an understanding of molecule capture by the antennae of male beetles belonging to the genus Rhipicera (Coleoptera, Rhipiceridae). Anat Rec 298:1519–1534. https://doi.org/10.1002/ar.23188
Ranger CM, Horst L, Barnett J, Reding ME, Anderson B, Krause CR (2017) Comparative morphology and distribution of antennal sensilla on Xylosandrus germanus and Xylosandrus crassiusculus (Coleoptera: Curculionidae: Scolytinae). Ann Ent Soc Amer 110(2):172–188. https://doi.org/10.1093/aesa/saw069
Ritcey GM, McIver SB (1990) External morphology of antennal sensilla of four species of adult flea beetles (Coleoptera: Chrysomelidae : Alticinae). Int J Ins Morph Embryol 19(2):141–153. https://doi.org/10.1016/0020-7322(90)90024-J
Romero-López AA, Carrillo-Ruiz H, Morón MA (2013) Morphological diversity of antennal sensilla in Hopliinae (Coleoptera: Scarabaeoidea: Melolonthidae). Acad J Entomol 6:20–26
Romero-López AA, Benítez-Herrera LN, Martínez-Bonilla OK, Yanes-Gómez G, Aragón-Sánchez M (2017) Comparative study of distribution of antennal chemoreceptors of Macrodactylus of Mexico. Southwest Entomol 42:111–119. https://doi.org/10.3958/059.042.0110
Salem H, Kaltenpoth M (2022) Beetle–bacterial symbioses: endless forms most functional. Annu Rev Entomol 67:201–219. https://doi.org/10.1146/annurev-ento-061421-063433
Schiestl FP (2010) The evolution of floral scent and insect chemical communication. Ecol Lett 13:643–656. https://doi.org/10.1111/j.1461-0248.2010.01451.x
Scholtz CH (1990) Phylogenetic trends in the Scarabaeoidea (Coleoptera). J Nat Hist 24(4):1027–1066. https://doi.org/10.1080/00222939000770631
Scholtz CH, Grebennikov VV (2005) Scarabaeoidea Latreille, 1802. In Coleoptera, Beetles. vol. 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). Handbook of zoology. vol. IV Arthropoda: Insecta, part 38 (eds RG Beutel, RAB Leschen), pp. 367–426. New York, NY: W. de Gruyter-Berlin
Schoolmeesters P (2021) World Scarabaeidae Database. In: O Bánki, Y Roskov, L Vandepitte, R E DeWalt, D Remsen, P Schalk, T Orrell, M Keping, J Miller, R Aalbu, R Adlard, E Adriaenssens, C Aedo, E Aescht, N Akkari, M A Alonso-Zarazaga, B Alvarez, F Alvarez, G Anderson, et al. Catalogue of Life Checklist (Version 2021-10-04). https://doi.org/10.48580/d4sz-38g
Šípek P, Fabrizi S, Eberle J, Ahrens D (2016) A molecular phylogeny of rose chafers (Coleoptera: Scarabaeidae: Cetoniinae) reveals a complex and concerted morphological evolution related to their flight mode. Mol Phylog Evol 101:163–175. https://doi.org/10.1016/j.ympev.2016.05.012
Slowinski JB, Guyer C (1993) Testing whether certain traits have caused amplified diversification: an improved method based on a model. Am Nat 134:907–921
Song N, Zhang H (2018) The mitochondrial genomes of phytophagous scarab beetles and systematic implications. J Ins Sci 18(6):1–11
Symonds MR, Johnson TL, Elgar MA (2011) Pheromone production, male abundance, body size, and the evolution of elaborate antennae in moths. Ecol Evol 2:227–246
Taylor L, Banwart S, Leake J, Beerling DJ (2011) Modeling the evolutionary rise of ectomycorrhiza on sub-surface weathering environments and the geochemical carbon cycle. Am J Sci 311:369–403. https://doi.org/10.2475/05.2011.01
Taylor LL, Banwart SA, Valdes PJ, Leake JR, Beerling DJ (2012) Evaluating the effects of terrestrial ecosystems, climate and carbon dioxide on weathering over geological time: a global-scale process-based approach. Phil Trans R Soc B 367:565–582. https://doi.org/10.1098/rstb.2011.0251
Timmermans MJTN, Barton C, Haran J, Ahrens D, Culverwell CL, Ollikainen A, Dodsworth S, Foster PG, Bocak L, Vogler AP (2016) Family-level sampling of mitochondrial genomes in Coleoptera: compositional heterogeneity and phylogenetics. Genome Biol Evol 8:161–175. https://doi.org/10.1093/gbe/evv241)
Vera W, Bergmann J (2018) Distribution and ultrastructure of the antennal sensilla of the grape weevil Naupactus xanthographus (Coleoptera: Curculionidae). Microsc Res Tech 81:590–598. https://doi.org/10.1002/jemt.23014
Wiens JJ (2011) The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits.’ Q Rev Biol 86:75–96
Yuvaraj JK, Andersson MN, Anderbrant O, Löfstedt C (2018) Diversity of olfactory structures: a comparative study of antennal sensilla in Trichoptera and Lepidoptera. Micron 111:9–18. https://doi.org/10.1016/j.micron.2018.05.006
Zhang H, Jackson TA (2008) Autochthonous bacterial flora indicated by PCR-DGGE of 16S rRNA gene fragments from the alimentary tract of Costelytra zealandica (Coleoptera: Scarabaeidae). J Appl Microbiol 105:1277–1285. https://doi.org/10.1111/j.1365-2672.2008.03867.x
Zhang SQ et al (2018) Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat Commun 9:205
Acknowledgements
We are thankful to Olaf Jäger (Senckenberg Zoological Museum Dresden) for the loan of Pleocoma specimens and to Karin Ulmen (LIB Bonn) for the help at the SEM and Nicola Angeli and Valeria Lencioni (MUSE, Trento) again for help with SEM. The study was part of a project funded by the German Science Foundation (AH175/10-1). We appreciate the helpful comments of an anonymous reviewer of an earlier version of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
DA: conceptualization; data curation; formal analysis; investigation; methodology; resources; funding acquisition; supervision; validation; visualization; writing—original draft; writing—review and editing. TLP: conceptualization; data curation; formal analysis; writing—original draft; writing—review and editing. CB: data curation; investigation; writing—review and editing. PS: data curation; writing—review and editing. AB: investigation; validation; writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors have seen and agree with the contents of the manuscript and there is no conflict of interest, including specific financial interest and relationships and affiliations relevant to the subject of manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pacheco, T.L., Bohacz, C., Ballerio, A. et al. Revisiting trends in morphology of antennal sensilla in scarabaeoid beetles. Zoomorphology 141, 315–326 (2022). https://doi.org/10.1007/s00435-022-00565-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00565-5