Abstract
The female of the biting midge Forcipomyia paludis is a dipteran ectoparasite of West Palaearctic damselflies and dragonflies, sucking haemolymph mainly from wing veins of their hosts. This tiny midge remains firmly attached to the wings even during fast flight and aerial fight maneuvers as shown in the present paper by field studies of the large dragonfly, Cordulegaster boltonii. Since individuals of F. paludis firmly attach themselves to the challenging wing surface of their host and can successfully withstand drag and vibrations during flight, we assume that this midge species has specific microstructural adaptations on its legs for attaching to the wing surface. In our morphological study, we used scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM), to study the structure of F. paludis tarsi, as well as the micro morphology of the wing surfaces of their host. Additionally, for the first time, we were able to show attachment devices of the midges dried out in contact with the host’s surface. The spatulae of the plantar setae and especially the empodial setae, are capable of replicating nanoscale wax crystals of the super hydrophobic wing coverage of the dragonfly wing membrane, in order to increase an effective contact area and therefore adhesion. This ability requires extremely soft materials of the spatula, which seems to be rather unique even in comparison to the leg attachment devices of other dipterans and other insect taxa in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female biting midges (Diptera: Ceratopogonidae) are well known to require blood/haemolymph from vertebrates and/or invertebrates to obtain protein for egg production. Among the more than 6000 known ceratopogonid species, a number of them parasitize arthropods such as millipedes, spiders and insects, in order to feed on their haemolymph (Borkent and Dominiak 2020). Their mouthparts are typically well-developed for cutting the integument of its host by saw-like protuberances (Krenn and Aspöck 2012). A few ceratopogonid species, representatives of the genus Forcipomyia, have specialized on damselflies and dragonflies (Odonata) generally attaching to the wings and most likely taking haemolymph from the wing veins (Wildermuth and Martens 2007; Büsse et al. companion). Forcipomyia-species are known from Odonata from both the Old and New World (e.g., Macfie 1932; Orr and Cranston 1997; Naraoka 1999; Martens et al. 2007; Guillermo-Ferreira and Vilela 2013). One of them, Forcipomyia paludis (Macfie 1936), is a widespread biting midge in the Western Palaearctic that occurs between the British Islands and Morocco in the west and the Caucasus in the east (Boudot et al. 2019; Wildermuth 2012; Wildermuth et al. 2019). Currently, more than 80 odonate species are known to host this parasite (Wildermuth 2021). The average parasite load on a single host is rather low (Martens et al. 2007; Vinko et al. 2017), however, in one exceptional case more than 170 midges were found attached to the wings of a teneral Libellula quadrimaculata (Clastrier et al. 1994). Most midges cling to the basal quarter of the wings, sucking from the lower veins of the corrugated wings (Manger 2021).
In general, biting midges are able flyers and infest their host in an imaginal stage during the host’s resting phases. There are documented cases that the female F. paludis infest their host during or shortly after emergence – i.e. ecdysis from larva to adult – when the cuticle is still soft (Wildermuth and Martens 2007; Cordero-Rivera et al. 2019; Wildermuth and Martens 2019: 229). In this situation the midges may also attach to other body parts such as the eyes or the abdomen. However, in adult dragonflies and damselflies, F. paludis cling almost exclusively to the wings (Martens et al. 2007) especially in mature odonates that are infested (e.g. Wildermuth and Martens 2007). However, there are accounts, that other Forcipomyia species also infest parts of the thorax or head of odonates (Trapero-Quintana et al. 2019).
By direct behavioral observations (Wildermuth and Martens 2007) and morphological investigations (Büsse et al. companion) of female midges inserting their proboscis into the host’s wing veins it became evident that F. paludis is an active ectoparasite, although phoresis may not be excluded completely as suggested by Dell’Anna et al. (1995) for F. paludis and Orr and Cranston (1997) for related species.
Highly specialized ectoparasitic species adapted to insect hosts in general, and for F. paludis Odonata imagines as powerful flyers in particular, often show strong morphological adaptations to such a parasitic lifestyle (cf. Jandausch et al. 2018; Petersen et al. 2018; Rebora et al. 2018; Büscher et al. 2021). The functional morphology of its feeding organs, sensory equipment and especially the attachment devices might help to shed light on the biology of this midge species. In this respect the micromorphology of the tarsi would be of special interest. Hitherto, corresponding structures have not been studied in detail and not in respect to the attachment of the parasite to its hosts (Macfie 1936; Cordero-Rivera et al. 2019). Since individuals of F. paludis are able to attach themselves strongly to the challenging wing surface of the host and can successfully withstand drag and vibrations during dragonfly flight, we assume that this midge species show specific microstructural adaptations for attachment on their legs. The surface of adult dragonflies, especially including their wings, is covered by nanoscale crystalline wax (Gorb et al. 2000, 2009), which usually makes surfaces super hydrophobic (Kuitunen et al. 2014; Šigutová et al. 2020) and rather 'unsticky' (Peressadko and Gorb 2004; Gorb et al. 2010; Purtov et al. 2013; Gorb and Gorb 2017; Rebora et al. 2020).
In the present paper, we used photographic documentation of field observations in order to provide evidence that the midges cling firmly to the host’s wings during forward flight and intraspecific fights. As host species we chose Cordulegaster boltonii (Donovan, 1807), a large anisopteran species typically found in small running waters. We deployed scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) to study the tarsal structure of F. paludis and the micromorphology of wing surface of one of its hosts. We discuss the results in comparison to the morphology of attachment devices of other representatives of Diptera and in the context of the parasitic lifestyle of F. paludis. For the first time we show special attachment devices of the midges dried out in contact with the host’s surface, enabling us to formulate functional hypotheses about the adhesion mechanism of this midge species. Therewith, we could underline the behavioral observations indicating a truly parasitic lifestyle to substantiate the role of F. paludis as an ectoparasite of Odonata.
Materials and methods
The field studies were conducted in July 2013 and 2019 at the Tüfenbach, a small forest brook near Uster, Switzerland (47°18′49’’N, 08°44′30’’E), and additionally on narrow fen ditches near Hinwil, Switzerland (47°17′53’’N, 08°48′48’’E). On sunny days the study site at the Tüfenbach was sunlit for about two hours in late forenoon. There, the male members of Cordulegaster boltonii patrolled regularly up and downstream, sometimes getting in intraspecific fights when two males encountered one another. In order to induce the males for a stopover we put a thin wooden stick 80 cm long at the sunlit water’s edge. While some males just passed by when crossing the small forest clearing, others did stop and used the offered perch for a short rest. All perched individuals were photographed, whenever possible, from the dorsal side using a digital camera (Nikon D7100, Macro objective 105 mm f/2.8) with a special focus on biting midges present on their wings. All dragonfly individuals were identified by their wing vein pattern, especially by the anal triangle. This enabled us to compare the parasite load pattern of every individual documented two or more times; having been flying in between.
Specimens of Forcipomyia paludis (Macfie, 1936) were collected from the wings of Cordulegaster boltonii at the localities mentioned above. In general, the midges detach from the host’s wing when the dragonflies are captured with a sweeping net. To prevent the midges from escaping, a fine-meshed net was used (cf. Schröter 2021). Occasionally single midges remain attached to the dragonfly’s wings (Wildermuth and Martens 2007). Few samples of F. paludis specimens still attached to wing fragments of C. boltonii were preserved for microscopic studies. Samples of ‘solo’ F. paludis were stored in ethanol.
For scanning electron microscopy (SEM) midge specimens stored in 70% ethanol were dehydrated in an ascending ethanol series and critical-point-dried using an automatic Leica EM CPD300 (Leica, Wetzlar, Germany). Dry odonate wings with midges attached were carefully dissected and mounted on the aluminum holders using double-sided adhesive conductive tape. The respective specimens were sputter-coated with a 10 nm layer of Au–Pd (Leica Bal-TEC SCD500). SEM images were taken with a Hitachi S-4800 (Hitachi High- Technologies Corp., Tokyo, Japan) at an acceleration voltage of 3 kV.
For confocal laser scanning microscopy (CLSM) midge specimens stored in 70% ethanol were dissected and their tarsi were embedded in glycerin (99.8%). The scans were conducted using a Zeiss LSM 700 (Carl Zeiss Microscopy GmbH, Jena, Germany), with the wavelengths 405, 488, 555 and 639 nm and emission filters BP420–480, LP490, LP560, LP640 nm (cf. Fig. 1 in Büsse and Gorb 2018). The resulting data was combined into a maximum intensity projection using the program ZEN 2008 (www.zeiss.de/mikroskopie). CLSM in this application makes use of the autofluorescence of the insect cuticle. The mixed fluorescence signals, detected in each pixel, are compiled and visualized in a maximum intensity projection. This allows determining regions of the cuticle with certain auto fluorescences corresponding to different cuticle components. The observed material compositions can generally be subdivided into three main groups (after Michels and Gorb 2012; Appel and Gorb 2014; Michels et al. 2016): (1) sclerotized cuticle showing red auto fluorescence; (2) strong but relatively flexible, chitinous areas showing a range of color through red over green to blue; (3) mostly flexible materials, such as resilin-bearing cuticle, are characterized by a violet or blue color. In the latter case, we refer to the observed areas of blue auto fluorescence as “resilin-supplemented”, leaving room for interpretation on other components of the cuticle.
Male Cordulegaster boltonii infested with female Forcipomyia paludis at the study site. A Patrolling male in flight with 1 midge on the left hindwing (arrow). B Perched male with 1 midge on the right hindwing (arrow and enlarged inset). C–D Male (M4) of C. boltonii perched on 18-vii-2013 at 11:13 h (C) and 11:16 h (B) with a ca 3 min patrol flight in between (solar noon at 13:40h). The dragonfly individual is identified by the vein pattern of the anal triangle on the left and right hind wing. In total, 17 midges are attached to the wings, 9 on the dorsal side (white arrows) and 6 on ventral side (grey arrows) of the wings. The infestation pattern of the midges remained unchanged during the flight
Results
Photographical field study
Biting midges could only be photographically documented once on flying dragonflies (Fig. 1A), yet clearly detected when perched (Figs. 1B–D). They were mainly found on or in the vicinity of the longitudinal veins at the base of the wings, on both the upper and lower side. They clung mostly close to the wing base on the longitudinal wing veins in the valleys of the wing corrugations, usually with the head directed towards the dragonfly’s thorax. The legs were generally spread out radially from the midge’s thorax, the tarsal end is mostly in contact with the wing membrane, sometimes with the wing vein itself. However, due to insufficient digital resolution, this was not always clearly seen. Furthermore, the legs were sometimes covered by other body parts.
Within 300 min total observation time at the study site Tüfenbach on 18-vii-2013, 21-vii-2013 and 27-vii-2013 altogether 37 different male individuals of Cordulegaster boltonii stopped at the implanted perch. These individuals could be photographed and identified, while others just passing by were not registered. In total, 59 perch events were documented for these 37 individuals, i.e., 25 individuals perched only once while 12 males perched two or more times: 6 individuals twice, 4 individuals three times, 1 individual four times and 1 individual six times (Table 1). The period between the first and the last perch event per individual amounted to 3 min at the least and 112 min at the most, respectively. The parasite load varied between 4 and 17 midges per individual. In eight cases with two or more recorded perch events the number and the position of the midges remained unchanged (Fig. 1C, D and supplemental Fig. 1). In one case (M1 21-vii-2013) two midges came in addition between the second and the third perch event, and in two cases (M3 and M11 21-vii-2013) one additional midge settled on the dragonfly’s wings. In only one case the dragonfly (M10 27-vii-2013) lost one midge during the patrol flight.
(b) Morphological study
The tarsi of F. paludis consist of five tarsomeres. The longest one is the basitarsus (ta1) (150–200 µm), ta2–ta5 are much shorter having rather similar length (30–50 µm). The tarsomeres are strongly covered by cuticular outgrowths of three types. Rather thick spines are situated ventrally at the distal side of each tarsomere except from the terminal one (Fig. 5A–C). Smaller setae (cuticle outgrowths with mobile sockets at the connection to the tarsal cuticle) of varying length with longitudinal grooves and cuticular stoppers at the basis (Fig. 2F) densely cover all sides of all tarsomeres (Fig. 2C–F). Between these setae, short acantae (cuticle outgrowths without mobile sockets) are situated.
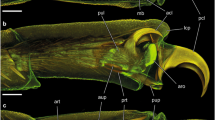
Forcipomyia paludis, SEM images. A–B Dead dried out individuals that remained attached in contact with the wings of the dragonfly Cordulegaster boltonii. A Dorsal aspect of the midge attached to the wing (the base of the wing on the left side of the image). B Ventral aspect of the midge (removed from the wing) with the native position of the legs and tarsi (arrows). C Tarsus. D Pretarsus with attachment devices. F Ventral side of the second and third tarsomere. E Bifurcated claw. cl claw, ep empodium, jt joint between tarsomeres, pt planta, sh setal shafts; st stoppers of tarsal setae, ta1–ta5 first to fifth tarsomeres, ti tibia, ut unguitractor plate, ve wing vein, wm wing membrane
The pretarsus consists of: (i) an unguitractor, (ii) the claws, (iii) the plantae and (iv) the empodium (Fig. 2D). The two latter structures (iii and iv) are covered by tenent (adhesive) setae terminated with spatula-like compressed and widened tips. The claws are strongly curved and bifurcated; showing a narrow gap between these two parts (Figs. 2D, E; 3A, B). In lateral view, the plantae are laterally slightly widened towards the pulvilli, resembling lobes and the empodium is connected to the pretarsus via a joint (Fig. 3A–B, D).
Forcipomyia paludis, SEM images of the pretarsus and attachment devices. A–B Lateral view of the pretarsus. C Empodium, adhesive surface composed of enlarged spatula of the empodial pulvillus. D Transition between empodium and pretarsus. E Setae of the planta lobes (pulvilli). F Basal region of the shafts of empodial setae. G Distal region of empodial setae with spatula that are adhered together and compose adhesive surface. cl claw, ep empodium, jt joint between empodium and pretarsus, pu plantar lobe (pulvillus), pt planta, se setae, sh setal shafts, sp spatula, ta5 fifth tarsomere
The tenent setae on the planta and the empodium are similar in their length (7–10 µm), but show rather strong differences in the structure of their distal regions. Plantar setae are terminated with the spatula with a width of 0.7–1.0 µm (Fig. 3E), whereas empodial spatulae are much wider (5 µm) and are fused into a continuous adhesive surface (Fig. 3C, G). The setal shafts are slightly compressed at their bases with specific uniform orientation of compression planes (Fig. 3D, F). Such a compression of cross-sectional shapes of setae is responsible for an anisotropic bending stiffness of the setae; stiffer in lateral-medial direction and more flexible forwards-backwards. The surfaces of the empodial spatula are slightly corrugated, which is an indication of the soft material they consist of (Fig. 3G).
We were able to study the attachment devices of several specimens of F. paludis that remained attached to the wing surface of the dragonfly (n = 3) and dried out in contact with the wing surface (Fig. 4). The most distant empodial setae are fused together with the spatula (Fig. 4F). Their surface profile is showing strong differences (black and white arrows in Fig. 4): (i) spatulae with no contact to the surface of the wing (exposed to the air), showed a wavy corrugated pattern (Fig. 4E), (ii) spatulae with contact to the surface of the wing, showed an extremely corrugated fine grain pattern (Fig. 4B–D). The dimensions of fine depressions in the surface of the spatula correspond to the dimensions of crystalline wax-protuberances on the wing membrane/veins surface (Fig. 4G), which means that nanoscale depressions represent replicas of the wax crystals. The hidden parts of spatulae are rather smooth, because they are dried in contact with the back surface of neighbouring spatulae (Fig. 4C–D).
Forcipomyia paludis, SEM images of attachment devices that have been dried out in contact with the wing of the dragonfly Cordulegaster boltonii. A Overview of the pretarsus, ventral aspect. B–E Details of the empodium surface. F Empodium, dorsal aspect. G Surface of the wing membrane of the dragonfly C. boltonii, tiny wax crystals are visible. White arrows, spatula surface dried not in contact with the wing surface. Black arrows, spatula surface dried in contact with the wing surface. cl claw, ep empodium, ex exposed area of the spatula, hd hidden area of the spatula, pt planta, sh shaft of the seta, sp spatula
The CLSM results show details of the material composition of the tarsal cuticular structures of the midge (Fig. 5). The tarsomeres demonstrate rather stiff sclerotised cuticle, which is shown in an orange-reddish autofluorescence (Fig. 5A, C). A similar type of cuticle is observed in the ventral spines of tarsomeres ta1–ta4 and in the claws. Between the tarsomeres, in the joint regions, there are regions showing a bluish autofluorescence, corresponding to a soft resilin-enriched membranous cuticle (Fig. 5A, C).
Forcipomyia paludis, CLSM images of attachment devices. A Overview of the tarsus, ventral aspect. B Details of the pretarsus, ventral aspect. C Joint between the third and fourth tarsomeres. D Tenent setae of the planta. cl claw, ep empodium, jt joint, pt planta, sh shafts of the setae, sn spine, sp spatula, ta1–ta5 tarsomeres one to five
The fused empodial spatulae are bluish and can therefore be considered as resilin-rich. The same holds true for the spatula of plantar setae (Fig. 5A, B, D). However, the spatular shafts in empodial and plantar regions are yellowish-greenish, which is an indication of less sclerotised chitinous material with lesser amount of resilin in comparison to that of the spatulae (Fig. 5B, D).
Discussion
The firm attachment of the biting midge Forcipomyia paludis during flight activities of its host has hitherto been presumed (Wildermuth and Martens 2019: 871) but not evidenced. In the present study on Cordulegaster boltonii as host species we could show for the first time that the ectoparasitic midges are not flung away even during different flight manoeuvres such as forward flight or vigorous intraspecific fights typical for this dragonfly when patrolling a linear water course (Kaiser 1982). Furthermore, it was documented in three cases that biting midges likely infest dragonflies also during the adult stage and not only during or after emergence as suggested by Cordero-Rivera et al. (2019). In only one case one single midge was lost within the timespan between the second and third perch event of the dragonfly. Presumably, this midge was saturated and therefore disengaged from the dragonfly’s wing (cf. Wildermuth and Martens 2007).
The F. paludis individuals usually select sites near the thorax on or in very close proximity to the wing veins in the concave parts ('valley') of the wing corrugations as shown in different host species (Martens et al. 2007; Manger 2021). (Fig. 2A). This site selection together with head facing position to the wing base (Martens et al. 2007; Manger 2021; Büsse et al. companion) likely reduce drag caused by the flapping wing during the host's flight (Fig. 1). Tarsi of the midge are rather difficult to observe from above, because they are usually hidden below other body parts including the midges' wings, but are usually spread out radially from the center of the body mass (Fig. 2B). This posture may provide rather isotropic resistance to the shear forces that can potentially act from different directions especially during flight.
Presumably, this site selection and body posture also allows for easy piercing of the vein at the sites with softer cuticle (Appel and Gorb 2014; Büsse et al. companion). Attachment forces of midges must be very high, because F. paludis can easily resist forces caused by the flapping wings of the flying dragonflies (Fig. 1). We, therefore, assume that in addition to the action of tarsal attachment devices, an interlocking-like effect of the mouthparts with the vein cuticle might be involved (Büsse et al. companion).
The tarsal end of F. paludis with the deeply cleft claw is typical for this species, and the bottlecap-like empodium has been visualised by Macfie (1936) and Cordero-Rivera et al. (2019), however, without detailed morphological description and morpho-functional interpretations. This gap is filled now by the present study. Tarsal attachment devices of F. paludis consist of several parts working in concert: (1) five tarsomeres richly covered by the cuticular protuberances, (2) bifurcated claws, (3) plantae covered by tenent setae with flattened spatulate tips, and (4) empodium with setal spatulae fused together at their tips. On the tarsomeres, setae with stoppers at the setal base in the vicinity of sockets might be of major importance. The stoppers lead to an increase of the bending rigidity of setae and may contribute to the enhancement of friction/interlocking with the coarse roughness of the substrate. The same function may be expected from the rigid spines situated ventrally at distal margins of ta1–ta4.
The bifurcated claws of F. paludis, having a narrow gap between the two parts are rather similar to the comb-like claws of spiders using these structures for grasping of web threads or draglines (cf. Gorb and Barth 1994). Many plant-associated Coleoptera rely on bifurcated claws, in order to attach to the plant surfaces covered by trichomes (Salerno et al. 2020). Also parasitic flies from different dipteran lineages (Hippoboscidae and Braulidae) have similar solution for attachment on the hairy surface of their vertebrate or insect hosts (Liu et al. 2018; Petersen et al. 2018; Büscher et al. 2021). In general, this kind of hairy substrates, cannot be considered either smooth or rough due to its structural and mechanical specificity. They represent a rather specific kind of substrate that often requires strong specialisations from attachment devices to cope with.
The results of the present study show that in comparison to all other previously studied dipterans, F. paludis exhibit the most strongly specialised pretarsal structures for adhesion. First, the lateral plantar lobes (pulvilli) are strongly reduced in comparison to the majority of flies, but there is much stronger coverage of the plantae by tenent setae. Second, the empodium is strongly transformed from its original form, where the fusion of spatulae led to the appearance of arolium-like medial structure between claws with a continuous smooth adhesive surface. Still this surface remains consisting of discrete partially overlapping islands (spatulae), which have extremely flexible membranous layer capable of intimate contact formation with nanoscale roughness of the host crystalline wax coverage.
In other dipterans, like tipulids (Nematocera), a smooth arolium is present (Rees and Ferris 1939; Hennig 1973; Gorb 2001) and hairy pulvilli have been described in adults of other dipteran groups (Bauchhenss and Renner 1977; Bauchhenss 1979; Walker et al. 1985; Niederegger et al. 2002; Niederegger and Gorb 2003; Gorb et al. 2012; Rebora et al. 2020; Salerno et al. 2020). A lobe like, pulvilliform empodium is present as an additional adhesive structure in tabanoids (Brachycera) and Nematocera excl. Tipulidae (Hennig 1973). Brachyceran and nematoceran pulvilli show soft lobes at the pretarsal plantae and are responsible for attachment to smooth surfaces (Walker et al. 1985). They are covered by tenent setae, sometimes termed tenent hairs, which serve to increase the actual area of contact to the surface. Proximal and distal tenent setae have often different ultrastructure as in some syrphid flies (Gorb 1998), and in some groups the sexual dimorphism is present (Gorb 2001). Both the shape and size of dipteran pulvilli correlate with the weight and biology of the species (Röder 1984a; b).
The tenent setae of F. paludis are relatively soft structures, as known for dipterans in general. In calliphorid flies, for example, their tips are usually compressed, widened, and bent at an angle of 60° to the hair shaft (Bauchhenss and Renner 1977; Bauchhenss 1979). When walking on smooth surfaces, these hairs produce fluid secretion, which is essential for attachment (Gorb 2001; Kovalev et al. 2012, 2013; Peisker and Gorb 2012; Peisker et al. 2014), especially on rough surfaces. Some authors have hypothesised that different forces may therefore contribute to the resulting attachment force: capillary adhesion and intermolecular van der Waals forces. The action of intermolecular forces is possible only at very close proximity between surfaces. The distance decreases and the contact force increases when the contacting surfaces slide against each other (Filippov et al. 2011). This may explain why flies sitting on a smooth ceiling always move their legs in a lateral-medial direction (Wigglesworth 1987). During these movements, pulvilli slide over the surface obtaining optimal intimate contact with the substrate. This is most likely one of the main reasons why F. paludis females attach firmly to odonate wings also during fast flight maneuvres of the host. Furthermore, the spatulated plantar setae of F. paludis resemble smooth adhesive organs of orthopterans, phasmatopterans, hymenopterans, etc. (Fig. 3C, G), also known for reliable attachment.
Even more, it was previously suggested that fly setae are composed of flexible cuticle, and are able to replicate a surface profile (Gorb et al. 2001; Gorb et al. 2012). The results of freezing-substitution experiments show that the area of the setae tips becomes larger when the pulvillus is in contact with the surface. This deformation is best seen in the middle of the pulvillus, whereas on the side parts, the setae are often not in contact. The whole pulvillus becomes compressed and elongated, while contact is made (Gorb 2001). Setal spatulae of Calliphora are highly flexible structures that form contact with the surface by bending their tips in the distal direction. The mid-part of the spatulae is filled with a secretion that is released into the contact area in the region of contact. After initial wet contact is formed, the thin membraneous central region of the endplate presumably comes into contact with the substrate due to attractive forces, acting similar to the wet thin foil, so that a solid–solid contact is presumably formed in the centre of the plate (Gorb et al. 2012). This fact might be the most important factor for the strong adhesion in F. paludis as the most distant empodial setae are fused together, making firm contact with the hosts' wing.
It is well known that large scale roughness affects animal adhesion less, due to the good mechanical interaction with the claws (Dai et al. 2002). In contrast to the situation on fine-scale roughness in the range of 0.1–2.0 µm (Peressadko and Gorb 2004; Voigt et al. 2008; Bullock and Federle 2011), where claws do not interlock and adhesive pads do not adhere (Wolff and Gorb 2012). Many plant surfaces, covered with crystalline waxes having similar dimensions, can effectively reduce insect adhesion (Gorb and Gorb 2002; Gorb et al. 2014a, b; Rebora et al. 2020; Salerno et al. 2020).
A similar situation is present in F. paludis, here, the attachment capability must be highly reduced due to the nanostructured superhydrophobic surface of the odonate wing (Kuitunen et al. 2014; Šigutová et al. 2020). We assume that the solution for adhesion enhancement on the fine-scale roughness of the dragonfly wing is a very soft and adaptable membranous surface of the midges' spatulae. This functional adaptation in F. paludis partially relies on the gradient of resilin concentration in the setae, with its stronger concentration on the setal tip (spatula). Similar gradients have been previously reported in beetles and interpreted as an adaptation to contact enhancement with various substrate profiles and to the reduction of setal clusterisation (Peisker et al 2013; Gorb and Filippov 2014; Heepe et al. 2018).
We do not know much about the possible role of the fluid in setal adhesion of F. paludis yet. More experimental studies with living midge individuals are required. Also, the adhesion force measurements in the future will shed more light on the functionality of different parts of the midge pretarsus.
Some Ceratopogonidae spp. suck haemolymph also from other insects such as lacewings, beetles or butterflies (e.g. Lane 1977; Havelka 1979; Dobosz 1991). However, to our knowledge, no micromorphological studies on the tarsal adaptation to the attachment on the host’s surface are available. Only in two African Forcipomyia species specific structural modifications are known, enabling the claws to attach on the butterfly’s wing scales (Lane 1977). It would be worth to investigate and compare the tarsal devices of various host specific Ceratopogonidae that parasitize arthropods.
Data availability
All data supporting our findings are presented in the paper and the supplementary material respectively. The raw data can be made available on reasonable request.
References
Appel E, Gorb SN (2014) Comparative functional morphology of vein joints in Odonata. Schweizerbart Science Publishers, Zoologica Stuttgart, pp 1–104
Bauchhenss E (1979) Die Pulvillen von Calliphora erythrocephala Meig. (Diptera, Brachycera) als Adhäsionsorgane. Zoomorphology 93:99–123
Bauchhenss E, Renner M (1977) Pulvillus of Calliphora erythrocephala Meig. (Diptera; Calliphoridae). Int J Insect Morphol Embryol 6:225–227
Borkent A, Dominiak P (2020) Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa 4787(1):1–377
Boudot J-P, Havelka P, Martens A (2019) The biting midge Forcipomyia paludis as a parasite of Odonata in North Africa (Diptera: Ceratopogonidae). Notul Odonatol 9(4):164–168
Bullock JMR, Federle W (2011) The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci 18(3):298–304
Büscher TH, Petersen DS, Bijma NN, Bäumler F, Pirk CWW, Büsse S, Heepe L, Gorb SN (2021) The exceptional attachment ability of the ectoparasitic bee louse Braula coeca (Diptera, Braulidae) on the honeybee. Physiol Entomol 19:170. https://doi.org/10.1111/phen.12378
Büsse S, Gorb SN (2018) Material composition of the mouthpart cuticle in a damselfly larva (Insecta: Odonata) and its biomechanical significance. R Soc Open Sci 5(6):172117. https://doi.org/10.1098/rsos.172117
Büsse S, Wildermuth H, Gorb SN (companion) Morphological adaptations of the mouthparts to the ectoparasitic lifestyle of the biting midge Forcipomyia paludis (Diptera: Ceratopogonidae), specialized in dragonflies (Odonata) - Submitted as "companion paper" to Zoomorpholgy as well. https://doi.org/10.1007/s00435-022-00564-6
Clastrier J, Grand D, Legrand J (1994) Observations exceptionnelles en France de Forcipomyia (Pterobosca) paludis (Macfie), parasite des ailes de libellules (Diptera, Ceratopogonidae et Odonata). Bull Soc Entomol Fr 99:127–130
Cordero-Rivera A, Barreiro AR, Otero MC (2019) Forcipomyia paludis (Diptera: Ceratopogonidae) in the Iberian Peninsula, with notes on its behavior parasitizing Odonates. Bol SEA 64:243–250
Dai Z, Gorb SN, Schwarz U (2002) Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J Exp Biol 205:2479–2488
Dell’Anna L, Utzeri C, Sabatini A, Coluzzi M (1995) Forcipomyia (Pterobosca) paludis (Macfie, 1936) (Diptera, Ceratopogonidae) on adult dragonflies (Odonata) in Sardinia, Italy. Parassitologia 37:79–82
Dobrosz R (1991) Forcipomyia eques Joh. (Diptera, Ceratopogonidae), an ectoparasite of lace-wings (Planipennia, Chrysopidae) in Poland. Ann up Siles Mus 2:235–237
Filippov A, Popov VL, Gorb SN (2011) Shear induced adhesion: contact mechanics of biological spatula-like attachment devices. J Theor Biol 276:126–131
Gorb SN (1998) The design of the fly adhesive pad: distal tenent setae are adapted to the delivery of an adhesive secretion. Proc Roy Soc B 265:747–752
Gorb SN (2001) Attachment devices of insect cuticle. Kluwer Academic Publishers, Dordrecht, Boston, London, p 305
Gorb SN, Barth FGB (1994) Locomotor behavior during prey-capture of a fishing spider, Dolomedes plantarius (Araneae: Araneidae): galloping and stopping. J Arachnol 22:89–93
Gorb SN, Filippov AE (2014) Fibrillar adhesion with no clusterisation: Functional significance of material gradient along adhesive setae of insects. Beilstein J Nanotech 5:837–846
Gorb EV, Gorb SN (2002) Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol Exp App 105(1):13–28
Gorb EV, Gorb SN (2017) Anti-adhesive effects of plant wax coverage on insect attachment. J Exp Bot 68(19):5323–5337. https://doi.org/10.1093/jxb/erx271
Gorb SN, Kesel A, Berger J (2000) Microsculpture of the wing surface in Odonata: evidence for cuticular wax covering. Arthropod Struc Develop 29:129–135
Gorb SN, Gorb EV, Kastner V (2001) Scale effects on the attachment pads and friction forces in syrphid flies (Diptera, Syrphidae). J Exp Biol 204:1421–1431
Gorb SN, Tynkkynen K, Kotiaho JS (2009) Crystalline wax coverage of the imaginal cuticle in Calopteryx splendens (Odonata: Calopterygidae). Int J Odonatol 12:205–221
Gorb EV, Hosoda N, Miksch C, Gorb SN (2010) Slippery pores: anti-adhesive effect of nanoporous substrates on the beetle attachment system. J R Soc Interface 7:1571–1579
Gorb SN, Schuppert J, Walther P, Schwarz H (2012) Contact behaviour of setal tips in the hairy attachment system of the fly Calliphora vicina (Diptera, Calliphoridae): a cryo-SEM approach. Zoology 115:142–150
Gorb EV, Böhm S, Jacky N, Maier L-P, Dening K, Pechook S, Pokroy B, Gorb SN (2014a) Insect attachment on crystalline bioinspired wax surfaces formed by alkanes of varying chain lengths. Beilstein J Nanotech 5:1031–1041
Gorb EV, Purtov J, Gorb SN (2014b) Adhesion force measurements on the two wax layers of the waxy zone in Nepenthes alata pitchers. Sci Rep 4:1–7
Guillermo-Ferreira R, Vilela DS (2013) New records of Forcipomyia (Pterobosca) incubans (Diptera: Ceratopogonidae) parasitizing wings of Odonata in Brazil. Biota Neotrop 13:1–3
Havelka P (1979) Atrichopogon lucorum (Meigen, 1818) (Diptera, Ceratopogonidae) – ein euer temporärer, canthariphiler Ektoparasit am Ölkäfer Meloe violaceus MRSH, 1802 (Coleoptera, Meloinae). Zeitschrift der Arbeitsgemeinschaft Österr. Entomologen 30(3/4):117–119
Heepe L, Höft S, Michels J, Gorb SN (2018) Material gradients in fibrillar insect attachment systems: the role of joint-like elements. Soft Matter 14(34):7026–7033. https://doi.org/10.1039/C8SM01151F
Hennig W (1973) Diptera (Zweiflügler). In: Beier M (ed) Handbuch der zoologie. Walter de Gruyter, Berlin, New York, pp 1–335
Jandausch K, Beutel RG, Pohl H, Gorb SN, Büsse S (2018) The legs of “spider associated” parasitic primary larvae of Mantispa aphavexelte (Mantispidae, Neuroptera) – attachment devices and phylogenetic implications. Arthropod Struct Dev 47:449–456
Kaiser H (1982) Do Cordulegaster males defend territories? A preliminary investigation of mating strategies in Cordulegaster boltoni (Donova) (Anisoptera: Cordulegastridae). Odonatologica 11:139–152
Kovalev AE, Varenberg M, Gorb SN (2012) Wet versus dry adhesion of biomimetic mushroom-shaped microstructures. Soft Matter 8:7560–7566
Kovalev AE, Filippov AE, Gorb SN (2013) Insect wet steps: loss of fluid from insect feet adhering to a substrate. J Roy Soc Interface 10(78):1–8
Krenn HW, Aspöck H (2012) Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct Dev 41:101–118
Kuitunen K, Kovalev A, Gorb SN (2014) Sex-related effects in the superhydrophobic properties of damselfly wings in young and old Calopteryx splendens. PLoS ONE 9(2):1–11
Lane RP (1977) Ectoparasitic adaptations in Forcipomyia from butterflies with two new African species (Ceratopogonidae). Syst Entomol 2:305–312
Liu S-P, Friedrich F, Petersen DS, Büsse S, Gorb SN, Beutel RG (2018) The thoracic anatomy of the swift lousefly Crataerina pallida (Diptera) - functional implications and character evolution in Hippoboscoidea. Zool J Linn Soc 185:111–131. https://doi.org/10.1093/zoolinnean/zly032
Macfie JWS (1932) Ceratopogonidae from wings of dragonflies. Tijdsch Entomol 75:265–283
Macfie JWS (1936) Two new species of Ceratopogonidae (Diptera) from the wings of dragonflies. Proc Roy Entomol Soc B 5:62–64
Manger R (2021) Odonata wing vein preferences in haemolymph sucking Forcipomyia paludis (Diptera: Ceratopogonidae). Libellula Supplement 16:189–200
Martens A, Ehmann H, Peitzner G, Peitzner P, Wildermuth H (2007) European Odonata as hosts of Forcipomyia paludis (Diptera: Ceratopogonidae). Int J Odonatol 11:59–70
Michels J, Gorb SN (2012) Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J Microsc 245:1–16. https://doi.org/10.1111/j.1365-2818.2011.03523.x
Michels J, Appel E, Gorb SN (2016) Functional diversity of resilin in Arthropoda. Beilstein J Nanotech 7:1241–1259. https://doi.org/10.3762/bjnano.7.115
Naraoka H (1999) On the Forcipomyia (Pterobosca) tokunagai Oka and Asahina (Dipera: Ceratopogonidae). J Nat Hist Aomori 4:17–21 (in Japanese with English title)
Niederegger S, Gorb SN (2003) Tarsal movements in flies during leg attachment and detachment on a smoothe substrate. J Insect Physiol 49:611–620
Niederegger S, Gorb SN, Jiao Y (2002) Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J Comp Physiol A 187:961–970
Orr AG, Cranston PS (1997) Hitchhiker or parasite? A ceratopogonid midge and its odonate host. J Nat Hist 31:1849–1858
Peisker H, Gorb SN (2012) Evaporation dynamics of tarsal liquid footprints in flies (Calliphora vicina) and beetles (Coccinella septempunctata). J Exp Biol 215:1266–1271
Peisker H, MichelsJ J, Gorb SN (2013) Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat Com 4(1661):1–7
Peisker H, Heepe L, Kovalev A, Gorb SN (2014) Comparative study of the fluid viscosity in tarsal hairy attachment systems of flies and beetles. J Roy Soc Interface 11(99):1–7
Peressadko A, Gorb SN (2004) Surface profile and friction force generated by insects. In: Boblan I, Bannasch R (eds) Bionik, vol 249(15). VDI Verlag, Fortschritt-Berichte VDI, Düsseldorf, pp 257–263
Petersen DS, Kreuter N, Heepe L, Büsse S, Wellbrock AHJ, Witte K, Gorb SN (2018) Holding tight to feathers - structural specializations and attachment properties of the avian ectoparasite Crataerina pallida (Diptera, Hippoboscidae). J Exp Biol 221(13):jeb179242. https://doi.org/10.1242/jeb.179242
Purtov J, Gorb EV, Steinhart M, Gorb SN (2013) Measuring of the hardly measurable: adhesion properties of anti-adhesive surfaces. Appl Phys A 111(1):183–189
Rebora M, Michels J, Salerno G, Heepe L, Gorb EV, Gorb SN (2018) Tarsal attachment devices of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J Morph 279:660–672
Rebora M, Salerno G, Piersanti S, Gorb E, Gorb SN (2020) Role of fruit epicuticular waxes in preventing Bactrocera oleae (Diptera: Tephritidae) attachment in different cultivars of Olea europaea. Insects 11:1–17
Rees BE, Ferris GF (1939) The morphology of Tipula reesi Alex. Microentomology 4:143–178
Röder G (1984a) Morphologische Untersuchungen an Prätarsen von Diptera and Mecoptera (Insecta). Nürnberg: Dissertation
Röder G (1984b) Zur Morphologie des Prätarsus der Diptera and Mecoptera. Zool Jb Anat 144:465–502
Salerno G, Rebora M, Piersanti S, Gorb E, Gorb SN (2020) Mechanical ecology of fruit-insect interaction in the adult Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Zoology 139:125748. https://doi.org/10.1016/j.zool.2020.125748
Schröter A (2021) Eine Gnitzenjagd in Georgien. Libellennachrichten 46:12–17
Šigutová H, Šigut M, Kovalev A, Gorb SN (2020) Wing wettability gradient in a damselfly Lestes sponsa (Odonata: Lestidae) reflects the submergence behavior during underwater oviposition. Roy Soc Open Sci 7(12):201258. https://doi.org/10.1098/rsos.201258
Trapero-Quintana A, Torres-Cambas Y, Rivas-Torres A, Ferreira S, Cordero-Rivera A (2019) The first record of parasitism by Forcipomyia (Diptera: Ceratopogonidae) in Cuban odonates. Novit Carib 14:105–110. https://doi.org/10.33800/nc.v0i14.202
Vinko D, Kulijer D, Billqvist M, Martens A (2017) The biting midge Forcipomyia paludis (Macfie, 1936) (Diptera: Ceratopogonidae) in Slovenia, Bosnia and Herzegovina, Croatia and Sweden. Nat Slov 19:5–21
Voigt D, Schuppert JM, Dattinger S, Gorb SN (2008) Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J Insect Physiol 54:765–776
Walker G, Yule AB, Ratcliffe J (1985) The adhesive organ of the blowfly, Calliphora vomitoria: a functional approach (Diptera: Calliphoridae). J Zool Lond 205:297–307
Wigglesworth VB (1987) How does a fly cling to the under surface of a glass sheet? J Exp Biol 129:363–367
Wildermuth H (2012) Die Verbreitung der an Libellen (Odonata) parasitierenden Gnitze Forcipomyia paludis (Macfie, 1936) in der Schweiz (Diptera: Ceratopogonidae). Entomo Helvetica 5:71–83
Wildermuth H (2021) Die Libellengnitze Forcipomyia paludis – eine kurze Entdeckungsgeschichte. Libellennachrichten 46:6–11
Wildermuth H, Martens A (2007) The feeding action of Forcipomyia paludis (Diptera: Ceratopogonidae), a parasite of Odonata imagines. Int J Odonatol 10:249–255
Wildermuth H, Martens A (2019) Die Libellen Europas. Quelle & Meyer, Wiebelsheim, p 958
Wildermuth H, Schröter A, Kohl S (2019) The West Palearctic biting midge Forcipomyia paludis (Diptera: Ceratopogonidae): first evidence as a parasite on Odonata wings from the Caucasus ecoregion. Notul Odonatol 9:158–163
Wolff JO, Gorb SN (2012) Surface roughness effects on attachment ability of the spider Philodromus dispar (Araneae, Philodromidae). J Exp Biol 215:179–184
Acknowledgements
We are thankful for the support by the members of the functional morphology and biomechanics group at Kiel University. SNG was supported through the DFG grant GO995/46-1 in the framework of the Special Priority Program “Physics of Parasitism”. SB was directly supported through the DFG grants BU3169/1-2. Special thanks to Andreas Martens for valuable comments and corrections of an earlier version of the manuscript. Furthermore, we want to thank one unknown reviewer and Beny Wipfler for their effort and valued contribution. We are also grateful to Victoria Kastner for language polishing.
Funding
Open Access funding enabled and organized by Projekt DEAL. SNG was supported through the DFG grant GO995/46–1 in the framework of the Special Priority Program “Physics of Parasitism”. SB was directly supported through the DFG grants BU3169/1–2.
Author information
Authors and Affiliations
Contributions
SB, HW and SNG designed the project and developed the concept of the study. SNG did the SEM analysis and post- processing. SB carried out the CLSM analysis and post- processing. HW and SK did the field study and respective photography. SB did the post-processing of the field studies photographs. SNG, HW and SB wrote the original manuscript. All authors edited the manuscript as well as read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no known competing interests.
Consent to participate and publication
We all agree(ed).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
435_2022_561_MOESM1_ESM.png
Supplementary Figure 1: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M1 on 21-vii-2013 (21-vii.M1). Showing three different perching events in a timespan of 112min. Change of the parasite load was recognized (+2; see Table 1). Supplementary file1 (PNG 2601 KB)
435_2022_561_MOESM2_ESM.png
Supplementary Figure 2: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M3 on 21-vii-2013 (21-vii.M1). Showing four different perching events in a timespan of 77min. Change of the parasite load was recognized (+1; see Table 1). Supplementary file2 (PNG 3660 KB)
435_2022_561_MOESM3_ESM.png
Supplementary Figure 3: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M9 on 21-vii-2013 (21-vii.M1). Showing six different perching events in a timespan of 34min.No change of the parasite load was recognized (±0; see Table 1). Supplementary file3 (PNG 3757 KB)
435_2022_561_MOESM4_ESM.png
Supplementary Figure 4: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M10 on 21-vii-2013 (21-vii.M1). Showing two different perching events in a timespan of 16min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file4 (PNG 3662 KB)
435_2022_561_MOESM5_ESM.png
Supplementary Figure 5: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M11 on 21-vii-2013 (21-vii.M1). Showing two different perching events in a timespan of 3min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file5 (PNG 3325 KB)
435_2022_561_MOESM6_ESM.png
Supplementary Figure 6: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M12 on 21-vii-2013 (21-vii.M1). Showing two different perching events in a timespan of 6min. Change of the parasite load was recognized (+1; see Table 1). Supplementary file6 (PNG 4326 KB)
435_2022_561_MOESM7_ESM.png
Supplementary Figure 7: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M1 on 27-vii-2013 (21-vii.M1). Showing three different perching events in a timespan of 5min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file7 (PNG 2732 KB)
435_2022_561_MOESM8_ESM.png
Supplementary Figure 8: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M10 on 27-vii-2013 (21-vii.M1). Showing three different perching events in a timespan of 6min. Change of the parasite load was recognized (-1; see Table 1). Supplementary file8 (PNG 9728 KB)
435_2022_561_MOESM9_ESM.png
Supplementary Figure 9: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M11 on 27-vii-2013 (21-vii.M1). Showing two different perching events in a timespan of 34min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file9 (PNG 3836 KB)
435_2022_561_MOESM10_ESM.png
Supplementary Figure 10: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M13 on 27-vii-2013 (21-vii.M1). Showing two different perching events in a timespan of 38min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file10 (PNG 3786 KB)
435_2022_561_MOESM11_ESM.png
Supplementary Figure 11: Male Cordulegaster boltonii infested with Forcipomyia paludis. Individual M15 on 27-vii-2013 (21-vii.M1). Showing three different perching events in a timespan of 38min. No change of the parasite load was recognized (±0; see Table 1). Supplementary file11 (PNG 2841 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gorb, S.N., Wildermuth, H., Kohl, S. et al. Tarsal attachment structures of the biting midge Forcipomyia paludis (Diptera: Ceratopogonidae), a specialized ectoparasite of Odonata imagines. Zoomorphology 141, 297–306 (2022). https://doi.org/10.1007/s00435-022-00561-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00561-9