Abstract
New material and new methodologies substantially widen the anatomical knowledge on cocculinid limpets. We first provide 3D-anatomies of Fedikovella caymanensis and Teuthirostria cancellata based on serial sections. Both species differ in several major points (mainly the gill-type and several features of the alimentary tract) from typical cocculinids, accordingly they are classified in a new clade, Teuthirostriidae fam. nov. Specimens studied by McLean and Harasewych (LA County Mus Contrib Sci 453:1–33, 1995) under “Fedikovella beanii” probably represent another species new to science. Additional investigations of original (type) section series of Cocculina laevis Thiele, 1904 (type species of Paracocculina Haszprunar, 1987) and of Cocculina radiata Thiele, 1904 (type species of Coccocrater Haszprunar, 1987) imply some nomenclatorial revisions: Cocculina cervae Fleming, 1948 is designated as type species of Pedococculina gen. nov. Anatomical characters confirm the subsequent placement of Cocculina viminensis Rocchini, 1990 into Coccopigya Marshall, 1986, whereas the original generic status of the whale-fall inhabitant Cocculina craigsmithi McLean, 1992 is confirmed despite the unusual habitat. The latter species probably has symbiotic bacteria in the midgut gland; if so this might be due to the environmental and feeding conditions at whale cadavers or hydrothermal vents. Contrary to Lepetelloidea, the Cocculiniformia cannot be included in Vetigastropoda. Recent molecular data support a sistergroup relationship of Cocculiniformia with Neomphalida, and we add the phenotypic perspective on this so-called “Neomphaliones”-hypothesis. In particular, more phylogenomic data are needed to specify the position of Cocculinida among the rhipidoglossate Gastropoda.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wide separation of the limpet families Cocculinidae Dall (1882) and Pseudococculinidae Hickman (1983), which until the 1980s were placed in a single family, is now undoubtedly founded on phenotypic and genotypic characters: The Lepetelloidea (with nominally 8 families and more than 20 genera) are nowadays regarded as an offshoot of the Vetigastropoda (Ponder and Lindberg 1997; Lindberg 2008; Kano 2008; Kano et al. 2016; Cunha and Giribet 2019; Ponder et al. 2020), possibly close to Lepetodrilidae (Lee et al. 2019). In contrast, the Cocculiniformia (Cocculinidae and Bathysciadiidae) are mostly considered as an independent offshoot of rhipidoglossate Gastropoda with still unclear sistergroup relationships (Aktipis and Giribet 2012; Kano and Warén 2013), but possibly related to Neomphalida (e.g., Lee et al. 2019). Recent phylogenomic analyses did not contain cocculiniform representatives (Zapata et al. 2014, 2015; Cunha and Giribet 2019).
Also the generic division of the Cocculinidae is still uncertain and awaits molecular confirmation (Lee et al. 2018): all characters used so far for generic and specific definition show mosaic evolution, i.e. there is a very low degree of coincidence between the morphological characters (Strong et al. 2003), and molecular data are still scarce (Lindberg 2008) or in progress (Lee et al. 2018). In general cocculinid genera are weakly defined at present and can often be diagnosed only by combination of character states (Moskalev 1976; Marshall 1986; Haszprunar 1987; McLean 1987, 1992; McLean and Harasewych 1995; Sasaki 1998; Leal and Harasewych 1999; Strong and Harasewych, 1999; Strong et al. 2003; Ardila and Harasewych 2005; Zhang and Zhang 2018).
One obvious problem is the scarcity of detailed morphological data: up to now out of about 80 nominal species the soft part anatomy of only 11 species (Thiele 1904b; Moskalev 1976; Haszprunar 1987; Sasaki 1998; Strong and Harasewych 1999; Strong et al. 2003; Chen and Linse 2020) and the hard parts by SEM of about 30 species have been studied. Herein we significantly widen the anatomical data available for cocculinoid limpets: (1) up to now the anatomy of the genera Teuthirostria Moskalev, 1976 and Fedikovella Moskalev, 1976 is unknown. Paratypes from the Moscow Museum of Natural History (see Acknowledgements) made it possible to study these genera by means of serial sectioning and 3D graphical treatment. (2) A gift of well-preserved specimens of Coccopigya viminensis (Rocchini 1990) added the first Mediterranean species to the list of anatomically studied species. (3) Though accurate, the anatomical descriptions by Thiele (1904b) lack data on certain important anatomical details. Loans of Thiele´s original sections from the Humboldt Museum in Berlin (see Acknowledgements) enabled one of us (GH) to fill these gaps. (4) Several cocculinid species inhabit so-called whale falls, but up to now possible modifications of the alimentary tract, which may shed light on functional aspects, remain unknown. Preserved animals of Cocculina craigsmithi McLean, 1992 were provided by the late Dr. James H. McLean; this species lives and feeds on decaying whale bone (McLean 1992), but has also been reported from the vicinity of Juan de Fuca hydrothermal vents (Tunnicliffe in Bennet et al. 1994: 219).
About a decade ago Hartmann et al. (2011) first provided detailed anatomical and histological data on the sistergroup Bathysciadiidae. The recent anatomical data of the Antarctic hot-vent-inhabitant Cocculina enigmadonta by Chen and Linse (2020) and in particular the new data presented herein improve and modify the diagnoses of the Cocculinidae and its nominal genera as previously established (Haszprunar 1987, 1988b, 1998; Strong et al. 2003). In addition, the increasing knowledge of the anatomical variation within the family also influences ideas on the phylogenetic relationships of the family as a whole. The most recent molecular data suggest a sistergroup relationship between Neomphalida and Cocculinida among the rhipidoglossate Gastropoda. Based on the new data we provide a phenotypic evaluation of this so-called “Neomphaliones”-hypothesis.
Material and methods
Fedikovella caymanensis Moskalev, 1976: two section series of paratypes from the locus typicus: Cayman Trough, 19° 00′ 6 N, 80° 29′ 5 W; depth 6800 m; 14th cruise of ‘Akademik Kurchatov’, March 20, 1973, st. 1242-A, by Sigsbee dredge. The second series comprises only the anterior half of the animal due to the high amount of debris in the posterior part, which destroyed the sections of this region. Otherwise, the preservation of the specimens was very good. The section series (“I” with 8 slides, “II” with 6 slides), made already in 1995 by BR, are now deposited in the Malacological Section of the Zoologische Staatssammlung München (SNSB-ZSM Moll #20220130-31).
Teuthirostria cancellata Moskalev, 1976: a single section series of a paratype from off Northern Peru. Although stated by Moskalev (1976: 64) this is not the type locality, since Moskalev designated the holotype from another station (see below). Preservation of the specimen was very good. The section series (9 slides) is deposited in the Malacological Section of the Zoologische Staatssammlung München (SNSB-ZSM Moll #20220132).
Both species (and also Coccopigya viminensis (Rocchini 1990), see below) had been fixed and preserved in 70% ethanol; after dehydration they were embedded in Spurr's (1969) resin. Ribboned serial cross sections were prepared with Ralph knives according to Ruthensteiner (2008). The sections were stained with methylene blue-azure II (Richardson et al. 1960) and coverslips were mounted with araldite. Such series served as basis for the computer-aided 3D-graphical treatments of Teuthirostria and Fedikovella. The software AMIRA (version 6.4) was applied following the detailed protocol of Ruthensteiner (2008). Section series of the remaining species were directly examined to check certain details of organization. In addition, high-resolution microphotographs were prepared to show certain details of anatomy and histology.
Paracocculina laevis (Thiele, 1904) (two section series with 13 resp. 5 slides) and Coccocrater radiata (Thiele, 1904) (two section series with 6 resp. 3 slides) were loaned from the Museum für Naturkunde in Berlin (see Acknowledgements). These section series served as basis of Thiele's (1904b) anatomical account. The sections (paraffin-celloidin technique, transversal, about 10 μm thick) are very well done, the staining (probably haemalaun-eosin) has survived for longer than a century without significant bleaching. The sections show very well preserved histology.
Coccopigya viminensis (Rocchini 1990): four section series, one juvenile and three adult specimens. All specimens were collected alive from wood from the type locality (Marco Oliverio, pers. comm.). Ribboned serial sections were prepared (same protocol as for Teuthirostria and Fedikovella, see above). Cover slips were mounted with araldite. The 4 section series are deposited in the Malacological Section of the Zoologische Staatssammlung München (SNSB-ZSM Moll #20220133-36).
Cocculina craigsmithi McLean, 1992: one section series of a mature, hermaphroditic animal, the largest paratype (shell length 9.7 mm) from sample LACM 2392 (see McLean 1992). Ethanol preserved specimens were dehydrated and embedded in paraplast. Serial sections (7 μm thick) were stained with Heidenhain’s Azan and cover slips were mounted with EUKITT®. The section series will be deposited at Los Angeles County Museum (LACM).
Results
General remarks
The new, well-preserved material and better equipment than that available longer than 30 years ago enabled the application of the semithin (instead of paraffin) technique. The thinner (2 μm instead of 5–10 μm) sections enable much better optical observation in the microscope, and also the histological preservation of specimens was superior to those of the previous study on cocculinids by the senior author (Haszprunar 1987). Based on new insights from the new material also old section series of the species described in the previous study were critically re-examined. Necessary corrections mainly concern the alimentary tract as follows: (1) The paired “large glandular folds” (Haszprunar 1987: 312) are in fact the (more or less well developed) salivary glands. (2) The laterally situated “glandular fields” represent additional buccal glands of unclear homology. (3) In all species two midgut glands are present: Although the right one is usually quite small, the opening of the oesophagus into the stomach clearly lies between two different openings.
3D-anatomy of Fedikovella caymanensis Moskalev, 1976
Previous studies
Cocculinidae gen. sp. E Moskalev and Vilenkin (1975: 139); Fedikovella caymanensis Moskalev 1976: 62, pl. 1, Fig. 1 [radula], pl. 2, Figs. 1, 2 [SEM of shell and protoconch]; Wolff 1979: 121,126; Hickman 1983: 82, Fig. 27 [SEM of radula]; McLean and Harasewych 1995: 17–18 [shortened English translation of the original Russian description]; Leal and Harasewych 1999: 120, Figs. 2A–D, 3A–E [description and figures of shell, protoconch, radula and external morphology by means of SEM]; Strong et al. 2003 [cladistic analysis of Cocculinidae].
External morphology
The mouth opening is flanked by very large oral lappets and shows cuticular “hairs” at its dorsal lip. A single pair of smooth, cephalic tentacles (each about 290 µm long) is situated at the large head. Eyes or optical glands are lacking. From the basis of the right cephalic tentacle a ciliated rim leads along the right neck backwards to reach the right part of the mantle cavity.
The foot sole shows two different epithelia: a marginal, ciliary girdle surrounds the central, non-ciliated region. Solitary, large mucous cells can be found in the epipodial region alongside the whole length of the foot. The prominent pedal gland is located within the anterior part of the foot (Figs. 1A, 2B, D) and consists of large and round mucous cells. It opens at the anterior side via a horizontal slit.
A–F 3D-microanatomy of Fedikovella caymanensis (paratype). A Overview in left, anterior view, body wall transparent. B Buccal apparatus, again left anterior view. C Digestive system, again left anterior view. D Digestive system, dorsal view (see also Fig. 7 scheme gut). E Nervous system, dorsal view. F Coelomatic and reproductive organs, ventral view. au auricle of heart, B/B′ right/left buccal ganglion, bc buccal commissure, bm buccal muscles, bn branchial nerve, bs buccal sphincter, C/C′ right/left cerebral ganglion, ca cartilage (radula cushion), cc cerebral commissure, co copulatory (right) cephalic tentacle, ct (left) cephalic tentacle, dg digestive gland, eso epipodial sense organ (paired), gd gonoduct, gg gonad gland, gi gill, h heart, hg hypobranchial gland, i intestine, lk left kidney, ns nervous system, oe oesophagus, oep oesophageal pouch, Os osphradial ganglion, ov ovary, P/P′ right/left pedal ganglion, pc pericard, pco pedal commissure, pg pedal gland, ph pharynx, Pl/Pl′ right/left pleural ganglion, pn/pn′ right/left pedal nerve, ra radula, ras radula sheath, re rectum, rm radula membrane, rpd renopericardioduct, rs receptaculum seminis, sag salivary glands, sp supra-oesophageal ganglion, st stomach, sto statocysts, ve ventricle of heart; *left and right end of (undetected) visceral loop
Cross section in frontal view showing histological details of Fedikovella caymanensis (paratype). A Central portion of buccal apparatus. B Whole animal at line of posterior buccal apparatus. C Detail of D. D Cross section at line of posterior end of buccal cartilages and of statocysts. B/B′ right/left buccal ganglion, bm buccal muscles, ca cartilage (radula cushion), cec cerebropedal connective, clc cerebropleural connective, hg hypobranchial gland, i intestine, mc mantle cavity, oe oesophagus, oep oesophageal pouch, Os osphradial ganglion, P/P´ right/left pedal ganglion, pg pedal gland, ra radula, ras radula sheath, rp rectal papilla, spc start of supra-oesophageal connective, sl statolith, st statocyst
The mantle margin is smooth and does not display any tentacles. Whereas the outer side shows a very flat epithelium, the cells of the inner side are much higher and often glandular. The subpallial gland is prominent. A single, nearly fused pair of elongated (about 240 μm long) epipodial sense organs (for terminology see Haszprunar et al. 2017) is placed at the animal's posterior end (Fig. 1A, B, E).
Shell muscles
The smooth shell muscles form a horse-shoe shaped organ, which is divided into discrete bundles by the afferent mantle sinuses (Fig. 3A, D). Its muscle fibres reach into the foot region, where the inner fibres inter-cross each other. The musculature of the foot itself is well developed (Fig. 3A).
Cross sections in frontal view with histological details of Fedikovella caymanensis (paratype). A Cross section through central body. B Detail of a section slightly behind Fig. 2A. C Cross section at midline of stomach. D Cross section at line of ovary. au auricle of heart, dg digestive gland, gd gonoduct, gg genital gland, gs gastric shield, i intestine, lk left kidney, mc mantle cavity, oep oesphageal pouch, peg premature eggs in ovary, poe posterior oesophagus, ras radula sheath, re rectum, sm shell muscle, st stomach, ve ventricle of heart
In addition, there are two symmetrical, longitudinal, and again smooth muscles, the head retractors, which originate from the dorsal and lateral walls of the head. They run dorso-laterally backwards and insert at the shell immediately inwards of the anterior end of each shell muscle. These insertion zones are quite elongated.
Mantle cavity
As usual in cocculinidan limpets the mantle cavity is a flat and shallow. To the very left a distinct (higher, ciliated) osphradial epithelium overlies the osphradial ganglion. The anterior portion of the mantle roof shows 12–15 short, transversally orientated folds with flat epithelium and is devoid of distinct ciliary zones, whereas a typical cocculinid pseudoplicatid gill is entirely lacking (Fig. 2A–C). Behind the folds the hypobranchial gland does not form a pouch-like organ, but is a simple, high, glandular epithelium (Fig. 2B, C). More posteriorly the anterior portion of the pericardium with the heart occupies the left mantle roof, whereas more than 80% are situated viscerally, i.e. behind the posterior end of the mantle cavity. The single, left kidney (Figs. 1F, 3A, B) occupies considerable space of the central posterior mantle roof. The right mantle cavity shows from anterior to posterior the narrow opening of the left kidney, the distal part of the rectum with the anal opening, and a distinct area of a genital gland, which surrounds the genital opening (Figs. 1F, 3A).
Circulatory and excretory system
Several symmetrical sinuses run between the shell muscles and transport haemolymph from the body’s haemocoel to the mantle sinus, where oxygenation takes place like also in the anterior part of the mantle cavity. The oxygenated haemolymph and that from the efferent sinus from the kidney are collected in front of the heart. The single auricle is situated anteriorly right to the ventricle (Figs. 1F, 3A, B). An aortic vessel with two lateral, longitudinal muscles leads forwards, then the blood flows freely within the body’s haemocoel. A blood gland could not be detected, however. A short, ciliated renopericardial duct connects the pericardium to the single left kidney (Fig. 1F). A nephridial gland could not be detected.
Genital system
The gonad is a compact pouch, which occupies the posterior ventral part of the animal (Fig. 1F). It contains about 20 mature eggs and many others in all stages of development but no sperm. Mature eggs are very large (diameter up to 300 × 200 × 200 μm) with big nuclei (less than 60 μm) and many yolk granules, but lack a distinct vitelline layer. Premature eggs are much smaller (about 45 μm, nuclei about 20 µm), and their yolk granules are smaller and less numerous than those of mature eggs (Fig. 1D). The eggs are deformable to a high degree.
The gonoduct is a broad tube emerging from the anterior right side of the gonad (Figs. 1F, 3C). It runs forwards along the inner side of the right shell muscle. The epithelium of the gonoduct (about 200 μm long) is characterized by high cylindrical, but not tubular mucous cells. A prominent receptaculum seminis is present around the proximal region of the gonoduct close to the entrance of the gonad (Fig. 3A). It forms a vesicle filled with a mass of sperm orientated towards the wall. In front of the opening of the gonoduct into the right mantle cavity, the same kind of epithelium as found in the receptaculum builds up a “genital gland”, which covers the right posterior end of the mantle cavity. The genital opening forms a papilla. From there the seminal groove leads forwards along the right neck towards the basis and along the right cephalic tentacle.
Buccal apparatus
Flanked by prominent, non-ciliated oral lappets the mouth opening of Fedikovella caymanensis is located at the ventral side of the head and is equipped with a prominent sphincter muscle. Its outer dorsal lip shows cuticular hairs with a “ciliary” appearance (also in SEM; see Leal and Harasewych 1999: Fig. 3B). The inner part of the oral opening is equipped with a jaw. Whereas the dorsal part of the jaw is poorly developed, the lateral parts are covered by a homogeneous layer of very thick, pronounced cuticle. At the anterior wall of the buccal cavity there are very prominent pouches of a paired salivary gland (Fig. 1B), whereas the sublingual pouch lacks a glandular epithelium.
The radula teeth of Fedikovella are described in detail by Leal and Harasewych (1999: Fig. 3C–E). The total length of the radula is about 1.4 mm, equivalent to about 60% of the total body length of F. caymanensis I. The radula extends in between and dorsally of a single pair of large, elongated radular cartilages backwards (Fig. 2A–C). After two-thirds of its length the radula turns in a sharp, nearly 90° angle downwards (Fig. 1B) and ends with a bifid formation zone (Fig. 2A).
The buccal apparatus is associated with several thick and cross-striated muscles. Attached to the two radular cartilages are the fasciated buccal muscles in the form of two distinct bundles. Within the anterior region of the buccal cavity, the cartilages are ventrally linked by a horizontal muscle.
Two glandular folds, which are ciliated at their inside, are present at the central dorsal wall of the buccal cavity. This so-called dorsal food channel continues into the oesophagus (Fig. 2A–C). At the point of emergence the buccal ganglia with their short commissure are located (Fig. 2A).
Posterior alimentary tract
The anterior part of the dorso-ventrally depressed oesophagus still shows the dorsal food channel (Fig. 2B, C). Large oesophageal pouches are laterally positioned (Fig. 2A–C). The anterior oesophagus runs straight backwards until the pouches separate and form cul-de-sacs among the intestinal loops (Figs. 1C, 3A). At this point, the oesophagus describes an S-shape reflecting the torsion zone. The posterior oesophagus is a tube which is oval to triangular in cross section (Fig. 3C, D). More or less in the median line it first slightly shifts to the left, then slightly crosses to the right (Fig. 3D), where it opens from the ventral side into the posterior end of the stomach, in between the two openings of the midgut glands.
The stomach is located in the posterior part of the animal. It is surrounded by the lobes of the digestive glands (Fig. 1C, D). The dorsal wall is occupied by a thick, cuticularized gastric shield (Fig. 3C), the ventral wall shows cylindrical, ciliated mucus cells. The posterior stomach and the first loop of the intestine show a longitudinal groove, a typhlosole. The content of the stomach and the intestine consists of decayed rests of plant material (Fig. 3B–D).
The paired midgut glands are characterized by many lobes and open into the posterior part of the stomach (Fig. 3.5.2–2 C, D, Fig. 3.5.2–2 D), dorsal and ventral to the opening of the oesophagus. The midgut lobes envelope the ventral and lateral side of the stomach and occupy the whole volume of the apex as well as the free space between the intestinal loops (Fig. 3.5.2–2A).
The intestine emerges from the anterior wall of the stomach (Fig. 3.5.2–1 D, Fig. 3.5.2–2D), flanking the entering oesophagus. The several quite complex and narrow intestinal loops occupy the ventral centre of the animal below the stomach (Figs. 1A, C, D, 3C, D). The last intestinal loop leads forwards along the left side and crosses over the stomach from left to right (Fig. 1C). On its course it passes the pericardium to the right and continues beneath the posterior end and to the right of the kidney (Figs. 1F, 3A, B). Finally, the rectum opens into the anterior, right side of the mantle cavity by a short papilla (Fig. 1B).
Nervous system
The central nervous system of Fedikovella caymanensis is hypoathroid-dystenoid and streptoneurous. The wide cerebropedal nerve ring and the visceral loop both surround the buccal apparatus (Figs. 1A, E).
The cerebral ganglia are situated laterally at the bases of the tentacles and are interconnected via a thick, elongated commissure, which extends between the oral sphincter and the anterior buccal muscles (Fig. 1E). From the cerebral ganglion, several fibres detour towards the mouth region. The tentacular nerve is the most prominent one originating laterally from each cerebral ganglion and supplying the cephalic tentacle. An optic nerve could not be detected. Starting from the ventral side of each cerebral ganglion two parallel connectives lead to the pleuropedal complex (Figs. 1E, 2B).
The buccal ganglia are positioned at the dorsal emergence of the oesophagus (Figs. 1E, 2A) and supply the buccal musculature. The short buccal commissure interconnects their posterior ends.
Whereas the right pleural ganglion shows more or less equal distances to the cerebral and pedal ganglia (dystenoid condition), the left pleural ganglion is closer to the pedal one (hypoathroid condition) (Fig. 1E). Both pleural ganglia are somewhat higher positioned than the pedal ones, with which they are joined via a short connective. They give raise to thick mantle nerves (omitted in Fig. 1E), which both lead laterally upwards, penetrate the shell muscles and enter the mantle border, where they form a nerve ring surrounding the whole body.
The very large pedal ganglia (Figs. 1E, 2D) lie below and behind the statocysts, the single pedal commissure is thick and short. The pedal nerves branch into a net of nerve fibres supplying the pedal sole (Fig. 1E). One thick pedal nerve/cords (a few nuclei of nerve cells are present) runs on each side from the pedal ganglion backwards in between the ventral portions of the shell muscles, whereas the nerves near the pedal ganglia run forwards.
The visceral loop of Fedikovella caymanensis resembles the situation in Coccopigya hispida (cf. Haszprunar 1987). The right branch of the streptoneurous visceral loop emerges from the right pleural ganglion and leads steeply upwards. Then it crosses over the whole buccal apparatus to the left side of the body and reaches the supraoesophageal ganglion. From there a connective enters the left mantle roof and forms there the small osphradial ganglion. The branchial nerve emerges from the osphradial ganglion and crosses to the right side again, supplying the whole anterior mantle roof (Fig. 1A, E).
From the left pleural ganglion the left branch of the visceral loop leads below the buccal apparatus to the right. After a short distance the suboesophageal ganglion is featured as a small thickening. Unfortunately the visceral ganglion could not be detected.
Sensory organs
The cerebral tentacles and the mantle margin are smooth, eyes are entirely lacking. Whereas the stalk of the basally fused Epipodial Sensory Organs (see Haszprunar et al. 2017 for naming) is composed by cuboidal cells, the epithelium of the blunt tip consists of highly cylindrical cells.
The osphradial epithelium is very simple, with no trace of zonation. The epithelium is more than twice as high (about 45 μm) than the mantle epithelia of the adjacent areas (about 23 μm, Fig. 3.7.2–4C) and is composed of ciliated cells with interspersed mucous cells. Fine nerve fibres emerge from the underlying osphradial nerve and reach the surface of the epithelium.
The two statocysts lie at the anteriodorsal side of the short pedal commissure and are close to each other (Fig. 2D). They are small (ca. 70 μm in diameter). Each contains a single, nearly circular statolith (diameter ca. 25 μm).
Remarks
The clear differences in protoconch sculpture (no honeycomb-pattern as in typical cocculinids; see Leal and Harasewych 1999: Figs. 2B–D), copulatory organ (not distinctly specialized and of equal size to the left cephalic tentacle), gill-type (many transversal leaflets versus a single (left) pseudoplicatid gill), the lack of a pouch-like hypobranchial gland and of a blood gland, the somewhat modified radula (see Leal and Harasewych 1999), the mid-right position of the straight (vs. bended) posterior oesophagus with ventral (versus dorsal) opening into the stomach, and the fused bases of the Epipodial Sense Organs provide sufficient characters to confirm for generic separation of Fedikovella caymanensis from all other cocculinids suggested by Moskalev (1976), for higher systematics see below.
According to Moskalev (1976) and McLean and Harasewych (1995) a sample off Chateau Belair Bay, St. Vicent, Lesser Antilles, which was tentatively identified as Cocculina beanii Dall, 1882, should be included into Fedikovella. This inclusion is supported by the concentric sculpture of the protoconch and the equally developed cephalic tentacles, the lack of the gill (the specimens were poorly preserved, thus the small horizontal leaflets probably were not visible in external view). However, the reported prominent gill (Dall 1882: 404), the separated copulatory organ (Marshall in McLean and Harasewych 1995), and certain differences in the shell and radula of Cocculina beanii contradict the (tentative) identification of the new material by McLean and Harasewych (1995), which has been doubted also by Leal and Harasewych (1999: 120ff). As long as no detailed anatomical or molecular data are available for the true Cocculina beanii, it seems best to retain this species formally within Cocculina and to name the new material of McLean and Harasewych (1995: 20: “Chateau Belair Bay, St. Vincent, Lesser Antilles (13° 10.5′ N, 61° 15.5′ W), 421 m, on wood, with Coccopigya mikkelsenae, new species. Eight specimens collected by deep-submersible Johnson-Sea-Link II, dive 1742, 23 April 1989. Material: 3 specimens USNM 860358, 3 specimens HBOM 065:03787, 2 specimens LACM 151188”) as a different species. The formal naming should be done with designation of a holotype and is beyond the scope of this anatomical paper.
3D-anatomy of Teuthirostria cancellata Moskalev, 1976
Previous studies
Teuthirostria cancellata Moskalev 1976: 64, pl. 1, Fig. 2 [drawing of radula], pl. 2, Figs. 3, 4 [SEM of shell]; Marshall 1996: 256, Figs. 24, 25 [SEM of radula]; Strong et al. 2003 [cladistic analysis].
Moskalev’s description was translated by G.V. Shkurkin in a privately circulated “reprint” dated December 1978. That translation is repeated here, altered towards a more telegraphic style and with updated terminology:
“Material and finding locality: Northern Peru, SRV “Akademic Kurchatov”, cruise 4, 31st Oct. 1968, st. 293, Sigsbee Dredge, 8° 26′ 2S, 80° 54′ 0 W, depth 5540–5200 m (type locality), on a beak of a squid, together with two specimens of Bathypelta pacifica Dall [footnote: the deepest finding of this species], 6 specimens, shell length from 1.05 to 2.75 mm. Holotype: specimen with inventory number 1 (the largest specimen with good preservation of the soft parts). Northern Peru, SRV “Akademik Kurchatov”, cruise 4, 4th Oct. 1968, stn. 301, 5° 51′ 7S, 81° 48′ 8 W, depth 5300–5320 (this sample contained Neopilina sp.), 1 specimen, shell length 3.63 mm.
Description of the shell: Shell small and thin, apex is posteriorly placed (80% of shell length), the preserved protoconch lacks a sculpture [possibly eroded?]. Anterior slope of shell convex, posterior slope straight. Shell aperture ovoid, narrowing toward the posterior end. Colour cream, shell semitransparent, contours of soft part are visible through shell. External sculpture latticed of similar, crossed radial and concentric ribbons, forming quadrangles with the long side along the radial ribs. Shell inside yellowish-white.
Radula and soft parts: The central plate is in firm contact with the basal membrane, contours are not observable. Anterior edge slightly wavy, anteriorly separating from the basal membrane and ending over posteriorly. No subcentral [1st lateral] teeth. Lateral teeth of different sizes, 3rd lateral smallest, 1st and 2nd ones with denticles, 3rd one lacks denticles, 4th one is the largest with three denticles and creases. Right [cephalic] tentacle significantly larger than left and with distinct groove. Two epipodial tentacles at posterior end of body.”
Based on SEM-investigations Marshall (1996: Figs. 24, 25) added several details of the radula: though of a general cocculinid type, the radula of Teuthirostria cancellata is markedly asymmetrical and there are substantially fewer marginal teeth per transverse row than in any other known cocculinid species.
External morphology
The specimen investigated has a body length of 1.53 mm, a maximal width of 1.3 mm, and a height of 1.12 mm. There is slight damage to the anterior foot, otherwise the specimen is intact. Like in Fedikovella caymanensis the foot sole is divided into a non-ciliated central and a surrounding, densely ciliated marginal zone (Fig. 5A). A stripe of densely arranged mucous cells is situated along the epipodial side of the lateral foot (Fig. 5A). The pedal gland occupies the anterior foot mass.
The broad mouth opening is flanked by large, non-ciliated oral lappets (Fig. 5D). The cephalic tentacles are both smooth, but differ in size and structure (Fig. 5C, D): the left one is quite short (230 µm) and smooth, the right one is much longer (630 µm) and thicker and shows a ventral ciliated seminal rim. This causes a somewhat asymmetrical placement of the head within the anterior part of the shell, at least in the contracted condition. We confirm the presence of a single pair of short (about 110 µm) Epipodial Sense Organs (ESOs) in the posterior subpallial cavity (Fig. 4A, F).
3D-microanatomy of Teuthirostria cancellata (paratype). A Overview, left side view, body wall transparent. B Buccal apparatus, left side view. C Digestive system, left side view. D Digestive system, frontal view (see also Fig. 7). E Coelomatic and reproductive organs, ventral view. F Nervous system, dorsal view. B/B′ right/left buccal ganglion, bc buccal commissure, bm bucal muscles, bn´ branchial nerve, bs buccal sphincter, C/C′ right/left cerebral ganglion, ca cartilage (radula cushion), cc cerebral commissure, co copulatory (right) cephalic tentacle, ct (left) cephalic tentacle, dg digestive gland, eso epipodial sense organ (paired), gd gonoduct, gg genital gland, gi gill, h heart, i intestine, lk left kidney, ns nervous system, oep oesophagus pouch, Os osphradial ganglion, ov ovary part of the hermaphroditic gland, P/P′ right/left pedal ganglion, p/p′right/left pedal nerve, pc pericard, pg pedal gland, Pl/Pl′ right/left pleural ganglion, ra radula, ras radula sheath, re rectum, rm radula membrane, rpd reno-pericardioduct, rs receptaculum seminis, Sb suboesophageal ganglion, sg salivary glands, Sp supra-oesophageal ganglion, spc supraoesophageal connective, st stomach, sto statocysts, t/t′ right/left tentacle nerve, vl visceral loop
The mantle margin of Teuthirostria cancellata is smooth and lacks papillae, but shows the same type of mucous cells as at the epipodial stripe. However, contrary to other cocculinids (Haszprunar 1987; Strong et al. 2003) a true subpallial gland is lacking. The inner mantle is occupied by large haemolymph sinuses.
As typical for cocculinids the paired shell muscle is U-shaped and is divided into many distinct bundles, which are separated by narrow blood sinuses. The inner anterior ends of the insertion areas form distinct hooks, which are the attachment areas of the head retractors. The fibres of both muscle systems are of the smooth type.
Mantle cavity
The very shallow mantle cavity lacks a true gill, instead a broad ciliary band and a small, non-ciliated fold (Fig. 5B) is present at the respective position. Contrary to many other cocculinids (Haszprunar 1987) a pouch-like hypobranchial gland (“brood pouch”) is absent, also an osphradium is lacking. The left posterior corner of the mantle cavity forms the opening of a short but distinct receptaculum (Insert of Fig. 5C). The pericardium is situated slightly to the left, its posterior portion lies behind the mantle cavity (Figs. 4A, E, 6C). The single, left kidney occupies a central position of the mantle roof (Fig. 4A, E), to the right the elongated anal papilla reaches into the right subpallial cavity (Fig. 4C, D). The glandular genital opening forms a broad funnel at the very right end of the mantle cavity and is anteriorly continued by the genital gland (Figs. 4E, 6C).
Cross sections in frontal view with histological details of Teuthirostria cancellata (paratype). A Lateral margin of foot. B Pallial cavity with vestigial gill rim. C Copulatory tentacle and anterior buccal apparatus. Inset: Receptaculum seminis (longer diameter 80 µm). D Posterior end of buccal apparatus. bc buccal cavity, bg buccal gland, bn branchial nerve, bs buccal sphincter, ci cilia, co (right) copulatory tentacle, ct (left) cephalic tentacle, dfc dorsal food channel, gi gill, i intestine, mc mantle cavity, mu mucus cells, ol oral lappet, ras radula sheath, sg salivary glands, spg sperm groove. Asteriscs jaws
Cross sections in frontal view with histological details of Teuthirostria cancellata (paratype). A Cross section through the central body with digestive organs. B Heart and left kidney. C Buccal apparatus. D Prominent salivary glands and dorsal food channel of anterior oesophagus. bm buccal muscles, ca cartilage (radula cushion), dfc dorsal food channel, dg digestive gland, gd gonoduct, h heart, i intestine, lk left kidney, mc mantle cavity, oe oesophagus, oep oesphagus pouch, pc pericard, poe posterior oesophagus, ra radula, re rectum, rpd renopericardioduct, sg salivary glands, st stomach
Circulatory and excretory system
The visceral haemocoel is connected to the mantle margin by several sinuses which run between the portions of the shell muscles. Blood from the mantle roof and the mantle margin is collected by prominent sinuses, which are united immediately in front of the heart auricle together with a second stream of blood, which comes from the anterior edge of the kidney. The heart itself is monotocardian, the auricle is situated in front of the ventricle. The pericardium is connected via a ventrally situated, narrow and ciliated tube with the single, left kidney (Fig. 6B). The latter is placed in the pallial roof to the right of the pericardium and has its opening close to the rectum (Figs. 4A, E, 6B, C). The typical cocculinid aorta (see Haszprunar 1987 for details) with its two longitudinal muscle fibres is present, whereas a blood gland is entirely lacking.
Genital system
In the single specimen investigated only the testis is fully developed, but also a small area of a pre-mature, ovarian epithelium is found ventrally at the distal part of the gonad (Figs. 12B, 12E). The whole gonad occupies the ventral, posterior part of the animal and is divided into two large lobes at the posterior end (Fig. 4E). The proximal gonoduct reaches a short distance into the gonad´s lumen and shows cuboidal mucous cells, whereas the epithelium of the distal gonoduct and of the pallial genital gland (Fig. 6C) consists of highly cylindrical glandular cells.
A short receptaculum seminis can be found to the very left. It opens via a distinct duct into the left mantle cavity and does not have any direct connection to the remaining genital system. The lumen shows decaying sperm cells (insert of Fig. 5C).
Buccal apparatus
As a whole the anterior alimentary tract is very prominent and large, whereas the posterior gut appears relatively smaller and densely packed. The somewhat tapered snout shows two prominent, non-ciliated oral lappets (Fig. 5D). The large mouth opening is ventrally situated and equipped with a thick sphincter muscle (Figs. 4B, 5C, D), its broad, anterior lip shows cuticular hairs. The epithelium of the mouth opening consists of up to 25 µm high, cylindrical cells and continues into the small sublingual cavity, where it becomes subsequently lower and lacks the hairy extensions.
There is a single, massive (i.e. no teeth-like elements) jaw, which consists of two lateral thick portions being interconnected by a thinner central one (asteriscs in Fig. 5D). Two large, tubular salivary glands, which are bent inwards, open laterally into the anterior buccal cavity (Figs. 4B, 5C, 6D). Subradular organ and sublingual glands are entirely lacking.
The details of the rhipidoglossate radula have been described by Moskalev (1976; see above). Its radular sheath shows a short caecum, leads straight backwards, then bends downwards and forwards and ends in a bifid structure (Fig. 4B). The cross-striated buccal muscles form many distinct bundles and connect the two radular cartilages with the radular membrane (Figs. 4B, 6A). The anterior, ventral sides of the cartilages are interconnected by a horizontal muscle (Fig. 6C).
The dorsal portion of the buccal cavity has two glandular folds, which are ciliated at their inner side (Fig. 5C, D). These folds form the dorsal food channel, which directly continues into the anterior oesophagus (Fig. 6C, D).
Posterior alimentary tract
The dorsoventrally depressed anterior oesophagus is slightly asymmetrical and has very large lateral glandular pouches, which run backwards for three quarters of the animal and lie between the intestinal loops (Fig. 6A). The posterior oesophagus is oval to triangular in cross sections (Fig. 6A). It leads backwards starting from a median position and forming an elongated S, finally entering the ventral side of the posterior stomach between the openings of the midgut glands. The epithelium of the posterior oesophagus is up to 50 µm high and densely ciliated, many mucous cells are interspersed.
The stomach forms a large sac in an upper dorsal position and is surrounded by the two midgut glands (Figs. 4A, C, E, 6A). It is provided with a prominent gastric shield with tooth in dorsal position, whereas a caecum is lacking. The ventral epithelium consists of cylindrical ciliated und mucous cells.
There are two wide and distinct openings to the midgut glands in mid-ventral position, left and right to the entrance of the oesophagus. The many lobes of the digestive glands occupy the whole apical area of the animal and fill the free space between the intestinal loops (Fig. 6A).
The intestine emerges from the anterior ventral portion of the stomach (Fig. 7A). First, its three narrow loops are situated below the stomach showing first a clockwise then an anticlockwise course (Fig. 4D). This is continued by a cork-shrew-like tube, which passes between the gonad, gonoduct and anterior stomach. Passing also the pericardium to the right the rectum, now provided with a distinct, ciliated epithelium, leads to the very right and forms an elongated papilla, which reaches into the anterior right subpallial cavity just to the left of the genital gland (Fig. 6C).
The gut content varies: the stomach lumen includes various granules and fungal hyphens. In the intestine the granules decrease subsequently in number so that the cork-screw portion only shows fungal hyphens. The lumen of the rectum lack any content in the specimen studied (Fig. 6C).
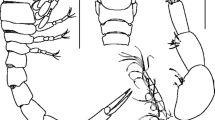
Schema of gut courses in certain cocculiniforms. A Teuthirostria cancellata. B Cocculina ovata (modified after Haszprunar 1987). C Fedikovella caymanensis. D Mcleaniella moskalevi (modified after Strong and Harasewych 1999). Blue course of oesophagus; violet openings of the midgut glands; pink contour of stomach; red inner course of stomach; green course of intestine and rectum
Nervous system (Fig. 4F)
The cerebral ganglia are located below the base of the cephalic tentacles. Their short, thick commissure is placed immediately above the oral sphincter muscle. Several thin cerebral nerves supply the oral region, the right tentacular (copulatory) nerve is somewhat thicker than the left one.
The thin buccal connectives emerge from the ventral side of each cerebral ganglion. The buccal ganglia are located at the dorsal emergence line of the anterior oesophagus and are interconnected by a short commissure.
The anterior nerve ring shows a hypathroid–dystenoid condition. The left pleural ganglion is adjacent to the pedal one (hypathroid), whereas the right pleural ganglion is clearly separated (dystenoid). The large pedal ganglia are close to each other, thus the pedal commissure is short. There is one major pedal nerve of each pedal ganglion leading backwards.
The streptoneurous visceral loop is very wide and shows small and inconspicuous ganglia. Starting from the left pleural ganglion the quite thin visceral loop leads below the buccal apparatus to the right. The suboesophageal ganglion is situated immediately above the right pleural ganglion. The visceral loop continues further to the right and then upwards, but the posterior course could not be traced in the sections. From the right pleural ganglion the visceral loop leads upwards and crosses above the buccal apparatus to the very left side. From there the branchial nerve enters the left mantle roof, forms a very small osphradial ganglion and then continues to the right side supplying the anterior mantle roof (Fig. 5B). Again the connective from the supraoesophageal to the visceral ganglion could not be detected.
Sensory organs
The cephalic tentacles are non-papillate, the ESOs show a blunt tip with high cylindrical cells. A small osphradial ganglion is found in front of the anterior end of left shell muscle, but contrary to the statement in Strong et al. (2003) a distinct osphradial epithelium could not be detected. Eyes or optical nerves are entirely lacking. Each of the very small (diameter 50 μm) and mid-ventrally situated statocysts contains a single statolith.
Remarks
Comparing the size of the specimen investigated (shell length 1.95 mm) with the maximum reported for the species (3.63 mm) it is likely that the investigated specimen represents a protandric juvenile of an overall hermaphroditic condition, the latter is indicated by the presence of a receptaculum and the earliest traces of an ovary. However, it is unlikely that the conditions of fully mature specimens are significantly different from the present specimen. The isolated position of the receptaculum suggests external fertilization in the mantle cavity, i.e. entaquatic conditions.
The somewhat juvenile condition does not explain the lack of gill, however, since even much smaller true cocculinids and bathysciadiids show a pseudoplicatid gill (Haszprunar 1998; Hartmann et al. 2011). It is, therefore, very probable that the conditions observed in the present specimen represent the adult stage. For example, the whole alimentary tract shows the same number of intestinal loops like in much larger cocculinid species.
Features of the protoconch (smooth?), radula (substantially fewer marginal teeth than other cocculinids), mantle cavity (no true gill, no pouch-like hypobranchial gland), and alimentary tract (massive jaw, tubular salivary glands, median posterior oesophagus with ventral opening into stomach) clearly justify separate generic status of this species, for higher systematics see below.
Anatomical and systematic notes on Paracocculina laevis (Thiele, 1904)
Dating
According to Bieler and Boss (1989: 12) the actual date of Thiele´s (1904a) publication was the second half of January 1904, although the title page says “1903”.
Previous studies
Cocculina laevis Thiele 1904a: 127, pl. 5, Figs. 11, 12 [shell]; Thiele 1904b: 149, pl. 6, Figs. 14–26 [anatomy, radula]; Thiele 1909: pl. 1, Fig. 6 [radula], pl. 2, Figs. 16, 17 [shell]. Paracocculina laevis Haszprunar 1987: 321 [Table 1: diagnosis].
Anatomy
As described and figured by Thiele (1904b) Paracocculina laevis has two posteriorly placed Epipodial Sense Organs (ESOs), a well-developed subpallial gland, and its hypobranchial gland forms a large pocket, a pouch-like hypobranchial gland is present. Also the blood gland is well developed. The osphradial ganglion lies at the left side of the mantle roof. In contrast to earlier assumptions (Haszprunar 1987), which were based on the text description by Thiele (1904b), the copulatory organ is found at the posterior end of the right oral lappet and is cerebrally innervated. As a whole the conditions of the copulatory organ strongly resemble those of Cocculina rathbuni Dall, 1882 (see McLean 1992 for SEM-photos). We confirm the presence of a single receptaculum at the left side, and of a gonoduct with thick, glandular epithelium. The eye is modified to a basitentacular gland with a broad opening.
Remarks
The conditions of the copulatory organ, which are nearly identical to those of Cocculina rathbuni, eliminates one main reason of generic separation. Moreover, the formation of a copulatory organ from the right oral lappet probably is a synapomorphic condition among cocculinids, suggesting a clade consisting of Paracocculina laevis and all remaining Cocculina species (cf. McLean 1987, 1992; McLean and Harasewych 1995; Strong and Harasewych 1999). On the other hand, a clear difference between Paracocculina laevis and Cocculina species remains in that the latter show a fusion of the supraesophageal and visceral ganglion and a distinct shift of the osphradial ganglion to a central instead of left position in the mantle roof. Therefore, Paracocculina still remains generic status.
Anatomical and systematic notes on Pedococculina cervae (Fleming, 1948)
Previous studies
Cocculina cervae Fleming 1948: 88, 92: textfig. 1a-d [shell]; Marshall 1986: 508, Fig. 2A [copulatory organ], 3A-C [SEM shell and protoconch], 12A [SEM radula], Table 3 [shell measurements]; Tecticrater cervae Dell 1956: 60; Powell 1979: 81, Fig. 10:9 [shell]; Paracocculina cervae Haszprunar 1987: 321 [Table 1: diagnosis].
Remarks
Cocculina cervae Fleming, 1948, which was also placed in Paracocculina by Haszprunar (1987), differs significantly from Paracocculina laevis and all other cocculinid species investigated in having a pedally situated and innervated copulatory organ with a flagellar structure at its tip (Marshall 1986: Fig. 2A). Also the cladistic analysis by Strong and Harasewych (1999) place Cocculina cervae quite distantly from Paracocculina. Therefore Cocculina cervae Fleming, 1948 is here designated as type species of Pedococculina gen. nov., which is characterized as follows:
Cocculinid limpets with high, posterior shell apex and fine radials. A single pair of epipodial tentacles (Epipodial Sense Organs, cf. Haszprunar et al. 2017). A pair of regular cephalic tentacles plus an additional copulatory organ at right side of foot being pedally innervated (diagnostic). Subpallial glands well developed, the hypobranchial gland forms a large pocket. Gonoduct shows massive glands, a single receptaculum is present at the left side of the mantle cavity.
Anatomical and systematic notes on Coccocrater radiata (Thiele, 1904)
Previous studies
Cocculina radiata Thiele 1904a: 128, pl. 5, Fig. 13 [shell]; Thiele 1904b: 150, pl. 6, Figs. 1–13–13 [anatomy, radula], Thiele 1909: pl. 1, Fig. 1 [animal], pl. 2, Figs. 18–19 [shell]. Coccocrater radiata Haszprunar 1987: 321 [Table 1: diagnosis].
Anatomy
The presence of a single pair of Epipodial Sense Organs (ESOs), the lack of a subpallial gland, the small hypobranchial gland, the papillate anus, and the single osphradial ganglion at the left side are confirmed. The blood gland is weakly developed. The copulatory organ, which is situated on the right cephalic tentacle, is cerebrally innervated. According to Thiele’s (1904b) description, which could be confirmed, the single receptaculum occupies a central position in the animal's body. The gonoduct is built up by prominent glands. The genital opening is not situated at the right side as usual in the Cocculinidae, but is centrally placed, where a broad fold is formed. The nervous system resembles that of Coccopigya hispida (cf. Haszprunar 1987: Fig. 6), whereas the eyes of Coccocrater radiata are completely reduced.
Remarks
The present investigation confirmed most of Thiele’s (1904b) description. In particular the central position of the genital opening, the type of copulatory organ, which is formed by the right cephalic tentacle, and the neural conditions (see above) clearly separate this species, which has been originally designated by Haszprunar (1987) as type species of Coccocrater, from Cocculina, although the cladistic analysis (Strong et al. 2003) indicate a sistergroup relationship between both genera. The non-spinose periostracum and the presence of epipodial tentacles provide clear diagnostic differences also to Coccopigya. The systematic significance of the centrally placed gonopore and receptaculum is not clear at present.
Anatomical and systematic notes on Coccopigya viminensis (Rocchini, 1990)
Previous studies
Cocculina viminensis Rocchini 1990: 48, pl. 1, Figs. 1, 2. [shell]. Coccopigya viminensis Dantart and Luque 1994: 280, Figs. 7–13–13 [SEM of shell, protoconch, periostracum, radula], Figs. 14, 17, 19 [SEM of soft parts];
Anatomy
In general, the anatomy of this species is typically cocculinid (cf. Haszprunar 1987 and Strong et al. 2003 for detailed outlines). Accordingly, the following description is restricted to specific and generic characters. The species entirely lacks epipodial appendages, whereas the subpallial glands are very large, although only present in the anterior half of the body. The distal rim of the oral lappets show a densely ciliated epithelium. The gill is small and less folded than in other cocculinids, the hypobranchial gland forms a small pocket, the anus is papillate, a typical pouch-like hypobrnachial gland is present with some widening to the left side. The single osphradial ganglion is situated at the left mantle roof. Whereas the anterior region of the foot is highly glandular, the blood gland is weakly developed. The copulatory organ is very similar to that of Coccopigya hispida (Marshall 1986: Fig. 2D; see also Dantart and Luque 1994: figs. 17–19). A single receptaculum is situated at the very left in the animal's body, its opening is close to the broad opening of the pouch-like hypobranchial gland. The gonoduct is built up by massive glands and opens to the very right. The alimentary tract is typical for cocculinid limpets: cuticular hairs at the dorsolateral mouth opening, pouch-like salivary glands (see above), glandular sublingual pouches, and broad oesophageal pouches. As usual the oesophagus runs backwards along the left side of the body, then bends and opens into the stomach from the dorsal right side between the openings of the two midgut glands. The posterior stomach and the first loop of the intestine show a longitudinal groove, the anus is papillate. The nervous system is identical to Coccopigya hispida Marshall, 1986 (cf. Haszprunar 1987: Fig. 6). The eyes are modified to basitentacular glands but lack pigments.
Remarks
Based on SEM results Dantart and Luque (1994) transferred Cocculina viminensis into the genus Coccopigya. This is clearly supported by the anatomical data provided herein. Coccopigya viminensis shows that a tube-like anus is not diagnostic for all Coccopigya species, this character might enable a further subdivision of this genus in future.
Anatomical and systematic notes on Cocculina craigsmithi McLean, 1992
Previous studies
Cocculina craigsmithi McLean 1992: 402 ff, Figs. 1–8–8 [shell, animal, SEM radula], Table 1 [shell measurements]; Bennett et al. 1994, Figs. 3B, E [animals in situ]; Deming et al. 1997 [enzyme assays].
Anatomy
The anatomy of this species is typically cocculinid (cf. Haszprunar 1987 for detailed outline) in most characteristics. Therefore, the following description is restricted to specific and generic characters. Cocculina craigsmithi has a single pair of ESOs, whereas subpallial glands are lacking, a distinct pedal gland is present but not very prominent. The oral lappets are quite small as is the “hairy” cuticularized area around the mouth opening. The prominent gill is seemingly bipectinate (McLean 1992: Fig. 5), however, the sections show the typical pseudoplicatid structure. The hypobranchial gland forms a small pocket, the anus is non-papillate, and a typical pouch-like hypobrachial gland is lacking. The single osphradial ganglion is positioned in the centre of the mantle roof. A blood gland is absent.
The prominent copulatory organ is formed by the posterior end of the right oral lappet (see also McLean 1992: Fig. 5). A single, prominent receptaculum extends from the very left to the center of the animal's body and opens towards the right side in close association with the opening of the gonoduct. The distal part of the gonoduct is built up by tubular glands similar to Cocculina baxteri McLean, 1987 (see Haszprunar 1987: Fig. 9), the hermaphroditic gonad is highly lobate and shows spermiogenesis in the outer portions, whereas oogenesis is found in the inner portion.
The inner alimentary tract is typical for cocculinid limpets in most respects: small, pouch-like salivary glands (see above), glandular sublingual pouches, and very broad oesophageal pouches. As usual the posterior oesophagus runs backwards along the left side of the body, then bends and opens into the stomach from the dorsal left side between the openings of the two midgut glands. The midgut glands, however, are unique in forming labyrinth-like organs, the epithelium of which shows high similarity with those of molluscan gills with endosymbiotic bacteria. The posterior stomach and the first loop of the intestine show a longitudinal groove, the anus is non-papillate.
The nervous system is identical to Cocculina ovata Schepman, 1908 (cf. Haszprunar 1987), but eyes are entirely lacking. The tips of the Epipodial Sense Organs are unusual in being highly glandular.
Remarks
Although never proposed by any direct investigator Kiel et al. (2020: 123) placed Cocculina craigsmithi in the lepetelloid genus Pyropelta because of the similar habitat. This needs to be corrected in favour of the original placement among the Cocculinidae based on conditions of shell, radula, and anatomy.
The generic status of Cocculina craigsmithi has been originally established on the basis of shell and radula characters and by the type of the copulatory organ. This can be confirmed by additional characters such as the absence of the subpallial gland and the central position of the osphradial ganglion, the latter is diagnostic for the genus Cocculina (Haszprunar 1987). The conditions of the receptaculum (single, left side), a simple seminal groove, and the structure of the distal gonoduct (tubular glands) place Cocculina craigsmithi closest to Cocculina baxteri McLean, 1987 and Cocculina cowani McLean, 1987.
Unique characters of Cocculina craigsmithi are the ESOs with their glandular tips, but the specific function of this character remains unclear. Presence of symbiotic bacteria in the modified (labyrinth-like) midgut gland is likely due to the environment on decaying whale bone or hydrothermal vents respectively, although Deming et al. (1997: 165) failed to detect any Rubisco or APS-reductase activity as typical for mussels with endosymbiotic bacteria in their gills. Osteopelta species (Vetigastropoda—Lepetelloidea) share the feeding biology on whale bone (Marshall 1987, 1994) and although they have a somewhat modified alimentary tract, there is no (light microscopical) trace of endosymbiotic bacteria (Haszprunar 1988a). The overall cocculinid anatomy of Cocculina craigsmithi and the close similarity to Cocculina baxteri and Cocculina cowani suggests that the ecological shift from decayed wood to whale cadaver or hydrothermal vent habitat in a poorly to non-oxygenated environment with chemoautotrophic primary production (Smith et al. 1988; Bennett et al. 1994; Smith and Baco 1998, 2003) occurred quite recently. On the other hand, the occurrence of conspecific animals at decayed whale cadaver and hydrothermal vent habitat is shared with the lepetelloid limpets Pyropelta musaica McLean and Haszprunar, 1987 and Pyropelta corymba McLean and Haszprunar, 1987 as well as with certain other invertebrate taxa (Smith et al. 1988; Bennett et al. 1994; Smith and Baco 1998, 2003).
General discussion
Feeding biology and related characters
In general cocculiniform limpets live and feed on biogenic substrates with reduced oxygen supply, mainly decaying wood, but occasionally also on cephalopod beaks or on whale bone, or are inhabitants of hydrothermal vent areas (Lesicki 1998; Chen and Linse 2020). However, it remains to be solved, whether the substrate itself or the actively decaying fungi or bacteria provides the basis of nourishment and whether or not digestion is accomplished by the animals themselves or additionally or exclusively by endosymbiotic bacteria.
Because the type of nourishment on decaying cephalopod beaks is shared with the closely related Bathysciadiidae, the systematic placement of Teuthirostria cancellata is of particular interest. Indeed, the very low number of marginal teeth probably represents a first, minimal step of specialization of the alimentary tract, which is much more pronounced in the Bathysciadiidae (Haszprunar, 1988b, 1998; Hartmann et al. 2011). On the other hand, the hot-vent inhabitant Cocculina enigmadonta shows an increased number of marginal teeth compared to other cocculinids suggesting feeding on bacterial films (Chen and Linse 2020).
Teuthirostriidae, fam. nov.
Both, Fedikovella caymanensis and Teuthirostria cancellata clearly differ in several anatomical characters from all remaining cocculinids described anatomically up to now (Haszprunar 1987, 1988b; Strong et al. 2003; Chen and Linse 2020; herein). Moreover, these genera (Cocculina, Paracocculina, Coccocrater, Coccopigya, Macleaniella, Pedococculina) share certain similarities with the Bathysciadiidae (see Hartmann et al. 2011 for review of the latter family), namely the pseudoplicatid gill and the reticulate protoconch pattern. Accepting these similarities as synapomorphies, Cocculinidae in the traditional sense (including genera Fedikovella and Teuthirostria) would become paraphyletic towards Bathysciadiidae, with the cocculinid type of radula as the main symplesiomorphy. Therefore, a new family, Teuthirostriidae is erected for Fedikovella and Teuthirostria. The remaining Cocculinidae (sensu stricto) share the unusual left-side position of the posterior oesophagus, which is considered as a synapomorphy of a monophyletic clade.
Diagnosis of Teuthirostriidae fam. nov.: Cocculiniform limpets with protoconch lacking the typical cocculinid-bathysciadiid reticulate sculpture. Right cephalic tentacle serves as copulatory organ, a single pair of posterior ESOs. Shallow mantle cavity lacking both a pouch-like hypobranchial gland and a pseudoplicatid gill. Rhipidoglossate radula cocculinid-like, in Teuthirostria with reduced number of marginals. Prominent salivary glands, posterior esophagus in midventral position (left in Cocculinidae) enters the stomach from below (instead from left dorsal in true cocculinids).
Higher affinities of the Cocculiniformia
A detailed phylogenetic (cladistic) analysis of the Cocculinidae was provided by Strong et al. (2003). Herein we focus on the discussion of the affinities of the Cocculiniformia (Teuthirostriidae, Bathysciadiidae, Cocculinidae) as a whole.
Haszprunar (1988b, c, 1998) proposed a clade Cocculiniformia with two superfamilies, Cocculinoidea and Lepetelloidea. However, later cladistic studies of phenotypic and genotypic characters showed that this hypothesis cannot be upheld. Shared similarities of both taxa are better interpreted either as symplesiomorphies or as homoplasies; Lepetelloidea are to be placed within Vetigastropoda (Ponder and Lindberg 1997, 2015; Aktipis and Giribet 2012; Zapata et al. 2014; Cunha and Giribet 2019), whereas the Cocculiniformia represent a clade proper. This separation has been supported by all recent molecular analyses.
Ponder and Lindberg (1997: 221) proposed (with some hesitation) a sistergroup relationship of Cocculinida with Neritimorpha. This was based on three assumed synapomorphies, namely the lack of jaws, the lack of salivary glands, and the presence of sublingual glands. However, these assumptions are now contradicted by the conditions in Teuthirostria (strong jaw, prominent salivary glands) and by the presence of sublingual glands in the docoglossate Pectinodonta (Sasaki 1998: Fig. 16d).
Sasaki (1998) proposed a “rhipidoglossate clade” {Neritimorpha [Neomphalus (Cocculina and Vetigastropoda)]}. However, also this hypothesis is doubtful: (1) according to Strong and Harasewych (1999), Strong et al. (2003) and the data presented herein, the genus Cocculina is one of the most advanced genera of the Cocculinidae and thus a problematic representative for higher phylogenetic studies. (2) Many characters of Haszprunar’s (1988c) and Ponder and Lindberg’s (1997) studies were not included in the data matrix. (3) The study only included two caenogastropods (Ampullaria, Biwamelania) and none of Heterobranchia. (4) Coding of certain characters is problematic: e.g. #15: a horseshoe-shaped shell muscle equals a paired (not unpaired) condition (see Haszprunar 1985; Wanninger et al. 1999); #61: Cocculina and Lepetodrilus have salivary glands (visible only in sections; see above). (5) The results imply a re-establishment of the right one of all paired organs (auricles, excretory organs, ctenidia, osphradia) in the Vetigastropoda; this is quite improbable in particular concerning the right excretory organ and its urinogenital function. Indeed, the statistic support for the above mentioned clade is poor. On the other hand, the inclusion of detailed comparative data on the buccal musculature is of great potential value, but still needs verification by more taxa studied.
Also the various analyses of molecular data have not yet fully resolved the situation, since all molecular analyses suffer from a “long branch” of Cocculiniformia. Based on the “5 gene approach” the study by Aktipis and Giribet (2012) revealed a sistergroup relationship of Cocculiniformia and Neomphalida. Also Kano and Warén (2013) mentioned that a sistergroup relationship of Cocculiniformia and Neomphalida is “supported solely but strongly by 18S sequences”. Accordingly, this “Neomphaliones”-hypothesis has been accepted by Bouchet et al. (2017). Most recently, Lee et al. (2019) again provided support of a cocculiniform-neomphalidan clade by ML-analysis of mitogenomes plus nuclear genes (18S, 28S, H3), but omitted the Patellogastropoda from their analysis. The same shortcoming concerns the “Neomphaliones”-hypothesis provided by Chen and Linse (2020) based on COI-sequences, which are known to be of minor significance for deep phylogenies. However, the analysis of mitochondrial protein genes among basal gastropod groups by Sun et al. (2019) includes also Patellogastropoda and again supports Neomphaliones. Unfortunately, both Neomphalida and Cocculiniformia have not been represented in the recent phylogenomic analyses on deep gastropod relationships (e.g. Zapata et al. 2014, 2015; Cunha and Giribet 2019).
Indeed, in support of the “Neomphaliones”-hypothesis both clades, Cocculiniformia and Neomphalida, share the entire loss of the right kidney and a glandular gonoduct. On the other hand, such conditions are also present in Neritimorpha, many Caenogastropoda, and Heterobranchia, thus may be a synapomorphy of all these groups or due to parallel evolutionary traits. An additional, shared character of Cocculiniformia and Neomphalida is the presence of statocysts with single, concentrically structured statoliths in the adult condition versus many small statoconia in Patellogastropoda, Vetigastropoda, and Neritimorpha. However, concentrically structured statoliths are also found in several clades of Caenogastropoda and Heterobranchia, thus homoplasy cannot be excluded.
Summing up, currently there is growing but not yet sufficient geno- and phenotypical evidence for a sistergroup relationships of Cocculiniformia and Neomphalida, namely in favour of the “Neomphaliones”-hypothesis. According to our morphological data on Fedikovella and Teuthirostria, which are probably more plesiomorphic than the remaining Cocculinda, we recommend to include molecular data of these taxa for a better und more robust understanding of cocculiniform relationships.
References
Aktipis SW, Giribet G (2012) Testing relationships among the vetigastropod taxa: a molecular approach. J Molluscan Stud 78:12–27. https://doi.org/10.1093/mollus/eyr023
Ardila NE, Harasewych MG (2005) Cocculinid and pseudococculinid limpets (Gastropoda: Cocculiniformia) from off the Caribbean coast of Colombia. Proc Biol Soc Wash 118:344–366. https://doi.org/10.2988/0006-324X(2005)118[344:CAPLGC]2.0.CO;2
Bennett BA, Smith CR, Glaser B, Maybaum HL (1994) Faunal community structure of a chemoautotrophic assemblage on whale bones in the deep Northeast Pacific Ocean. Mar Ecol Prog Ser 108:205–223. https://doi.org/10.3354/meps108205
Bieler R, Boss KJ (1989) Johannes Thiele and his contributions to Zoology. Part 1. Biography and bibliography. Nemouria (Occ Papers Delaware Mus Nat Hist) 34:1–30. https://www.biodiversitylibrary.org/page/45973931#page/61/mode/1up
Bouchet P, Rocroi J-P, Hausdorf B, Kaim A, Kano Y, Nützel A, Parkhaev P, Schrödl M, Strong EE (2017) Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61:1–516. https://doi.org/10.4002/040.061.0201
Chen C, Linse K (2020) From wood to vent: first cocculinid limpet associated with hydrothermal activity discovered in the Weddell Sea. Antarct Sci 32:354–366. https://doi.org/10.1017/S095410202000022X
Cunha TJ, Giribet G (2019) A congruent topology for deep gastropod relationships. Proc R Soc Lond B 286(20182776):1–8. https://doi.org/10.1098/rspb.2018.2776
Dall WH (1882) On certain limpets and chitons from the deep waters off the eastern coast of the United States. Proc US Natl Mus 81(26):400–426. https://doi.org/10.5479/si.00963801.4-246.400
Dantart L, Luque A (1994) Cocculiniformia and Lepetidae (Gastropoda: Archaeogastropoda) from the Iberian coasts. J Moll Stud 60:277–313. https://doi.org/10.1093/mollus/60.3.277
Deming JW, Reysenbach AL, Macko SA, Smith CR (1997) Evidence for the microbial basis of a chemoautotrophic community on a deep-sea whale skeleton: bone colonizing and animal-associated symbionts. Microsc Res Technol 37:162–170. https://doi.org/10.1002/(SICI)1097-0029(19970415)37:2%3c162::AID-JEMT4%3e3.0.CO;2-Q
Fleming CA (1948) New species and genera of marine Mollusca from the Southland Fiords. Trans & Proc R Soc NZ 77:72–92, plates 4–8. https://archive.org/details/transactions-and-proceedings-royal-society-new-zealand-77-072-092/mode/2up
Hartmann H, Heß M, Haszprunar G (2011) Interactive 3D anatomy and affinities of Bathysciadiidae (Gastropoda, Cocculinoidea): deep-sea limpets feeding on decaying cephalopod beaks. J Morphol 272:259–279. https://doi.org/10.1002/jmor.10910
Haszprunar G (1985) On the innervation of gastropod shell muscles. J Molluscan Stud 51:309–314. https://doi.org/10.1093/oxfordjournals.mollus.a065921
Haszprunar G (1987) Anatomy and affinities of cocculinid limpets (Mollusca, Archaeogastropoda). Zool Scr 16:305–324. https://doi.org/10.1111/j.1463-6409.1987.tb00077.x
Haszprunar G (1988a) Anatomy and relationships of the bone-feeding limpets Cocculinella minutissima (Smith) and Osteopelta mirabilis Marshall (Archaeogastropoda). J Molluscan Stud 54:1–20. https://doi.org/10.1093/mollus/54.1.1
Haszprunar G (1988b) Comparative anatomy of cocculiniform gastropods and its bearing on archaeogastropod systematics. In: Ponder WF (ed) Prosobranch phylogeny. Malac Rev Suppl 4, pp 64–84
Haszprunar G (1988c) On the origin and evolution of major gastropod groups with special reference to the Streptoneura. J Molluscan Stud 54:367–441. https://doi.org/10.1093/mollus/54.4.367
Haszprunar G (1998) Superorder cocculiniformia. In: Beesley PL, Ross GJB, Wells A (eds) Mollusca: the Southern Synthesis Fauna of Australia, vol 5B. CSIRO Publishing, Melbourne, pp 653–664
Haszprunar G, Kunze T, Warén A, Heß M (2017) A reconsideration of epipodial and cephalic appendages in basal gastropods: homologies, modules and evolutionary scenarios. J Molluscan Stud 83:363–383. https://doi.org/10.1093/mollus/eyx032
Hickman CS (1983) Radular patterns, systematics, diversity, and ecology of deep-sea limpets. Veliger 26:73–92. https://www.biodiversitylibrary.org/item/134166#page/91/mode/1up
Kano Y (2008) Vetigastropod phylogeny and a new concept of Seguenzioidea: independent evolution of copulatory organs in the deep-sea habitats. Zool Scr 37:1–21. https://doi.org/10.1111/J.1463-6409.2007.00316.X
Kano Y, Warén A (2013) Evolution and radiation of neomphaline gastropods: true antiquity at hydrothermal vents. Abstr WCM2013, Acoreana Suppl 8:70–71. https://www.researchgate.net/publication/256353403_Book_of_Abstracts_of_the_World_Congress_of_Malacology_2013
Kano Y, Takano T, Schwabe E, Warén A (2016) Phylogenetic position and systematics of the wood-associate limpet genus Caymanabyssia and implications for ecological radiation into deep-sea organic substrates by lepetelloid gastropods. Mar Ecol 37:1116–1130. https://doi.org/10.1111/maec.12376
Leal JH, Harasewych MG (1999) Deepest Atlantic mollusks: hadal limpets (Mollusca: Gastropoda: Cocculiniformia) from the northern boundary of the Caribbean Plate. Invertebr Biol 118:116–136. https://doi.org/10.2307/3227054
Lee H, Chen W-J & Samadi S (2018) Biodiversity exploration and phylogeny of Cocculinidae (Gastropoda: Cocculiniformia). Abstr Deep-Sea Biology Symp 2018:4. http://dsbsoc.org/wp-content/uploads/2014/12/15thDSBS_Abstracts.pdf
Lee H, Chen W-J, Puillandre N, Aznar-Cormanoa L, Tsaic M-H, Samedi S (2019) Incorporation of deep-sea and small-sized species provides new insights into gastropods phylogeny. Mol Phylogenet Evol 135:136–147. https://doi.org/10.1016/j.ympev.2019.03.003
Lesicki A (1998) Checklist of gastropod species referred to the order Cocculiniformia Haszprunar, 1987 (Gastropoda: Cocculinoidea et Lepetelloidea) with some remarks on their food preferences. Folia Malacol (krakow) 6:47–62. https://doi.org/10.12657/folmal.006.007
Lindberg DR (2008) Patellogastropoda, neritimorpha, and cocculinoidea. In: Ponder WF, Lindberg DR (eds) Phylogeny and evolution of the mollusca. University California Press, Berkeley, pp 269–294
Marshall BA (1986) Recent and tertiary cocculinidae and pseudococculinidae (Mollusca: Gastropoda) from New Zealand and New South Wales. NZ J Zool 12:505–546. https://doi.org/10.1080/03014223.1985.10428301
Marshall BA (1987) Osteopeltidae (Mollusca: Gastropoda): a new family of limpets associated with whale bone in the deep-sea. J Moll Stud 53:121–127. https://doi.org/10.1093/mollus/53.2.121
Marshall BA (1994) Deep-sea gastropods from the New-Zealand region associated with recent whale bones and an Eocene turtle. The Nautilus 108(1):1–8. https://www.biodiversitylibrary.org/page/8273231
Marshall BA (1996) A new subfamily of the Addisoniidae associated with cephalopod beaks from the tropical southwest Pacific, and a new pseudococculinid associated with chondrichthyan egg cases from New Zealand (Mollusca: Lepetelloidea). Veliger 39:250–259. https://www.biodiversitylibrary.org/page/42501381#page/264/mode/1up
McLean JH (1987) Taxonomic description of cocculinid limpets. Three new species and two rediscovered species. Zool Scr 16:325–333. https://doi.org/10.1111/j.1463-6409.1987.tb00078.x
McLean JH (1992) Cocculiniform limpets (Cocculinidae and Pyropeltidae) living on whale bone in the deep sea off California. J Molluscan Stud 58:401–414. https://doi.org/10.1093/mollus/58.4.401
McLean JH, Harasewych MG (1995) Review of Western Atlantic species of cocculinid and pseudococculinid limpets, with descriptions of new species. LA County Mus Contrib Sci 453:1–33. https://www.biodiversitylibrary.org/page/52113089#page/77/mode/1up
Moskalev LI (1976) Concerning the generic diagnosis of the Cocculinidae (Gastropoda, Prosobranchia). Trudy Instituta Okeanologii P.P. Shirshov, 99:59–70 (English translation by G.V.Shkurkin)
Ponder WF, Lindberg DR (1997) Towards a phylogeny of gastropod mollusks—an analysis using morphological characters. Zool J Linn Soc 119:83–265. https://doi.org/10.1006/zjls.1996.0066
Ponder WF, Lindberg DR, Ponder JM (2020) Biology and evolution of the Mollusca. vol 2. CRC Press, Taylor & Francis Group, Boca Raton, FL, p 870. ISBN-13: 978-0-8153-6184-8
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323. https://doi.org/10.3109/10520296009114754
Rocchini R (1990) Cocculina viminensis n.sp. Boll Malacol 26:47–52. http://www.societaitalianadimalacologia.it/Bollettino/Bollettino%201990/Boll.%20Mal.%201990%2047-52.pdf
Ruthensteiner B (2008) Soft Part 3D visualization by serial sectioning and computer reconstruction. In: Geiger D, Ruthensteiner B (eds) Micromolluscs: methodological challenges—exiting results. Zoosymposia 1, pp 63–100. https://doi.org/10.11646/zoosymposia.1.1.8
Sasaki T (1998) Comparative anatomy and phylogeny of the recent Archaeogastropoda (Mollusca: Gastropoda). Univ Mus Tokyo Bull 38:1–224. download order via: https://dl.ndl.go.jp/info:ndljp/pid/3140015
Schepman MM (1908) The Prosobranchia of the Siboga-Expedition. Part 1: Rhipidoglossa and Docoglossa. Siboga Exped Monogr 49a:1–98, plates 1–9. https://www.biodiversitylibrary.org/item/106906#page/11/mode/1up
Smith CR, Baco AR (1998) Phylogenetic and functional affinities between whale-fall, seep and vent chemoautotrophic communities. Biol Mar 39:345–346. https://doi.org/10.21411/CBM.A.4D29A602
Smith CR, Kukert H, Wheatcroft RA, Jumars PA, Deming JW (1988) Vent fauna on whale remains. Nature 341(6237):27–28. https://doi.org/10.1038/341027a0
Smith CR, Baco AR (2003) Ecology of whale falls at the deep-sea floor. Oceanogr Mar Biol Annl Rev 41:311–354. https://www.soest.hawaii.edu/oceanography/faculty/csmith/Files/Smith%20and%20Baco%202003.pdf
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43. https://doi.org/10.1016/S0022-5320(69)90033-1
Strong EE, Harasewych MG (1999) The anatomy of the hadal limpet Mcleaniella moskalevi (Gastropoda: Cocculinoidea). Invertebr Biol 118:137–148. https://doi.org/10.2307/3227054
Strong EE, Harasewych MG, Haszprunar G (2003) Phylogeny of the Cocculinoidea (Mollusca, Gastropoda). Invertebr Biol 122:114–125. https://doi.org/10.1111/j.1744-7410.2003.tb00077.x
Sun J, Liu Y, Xu T, Zhang Y, Chen C, Qiu JW, Qian PY (2019) The mitochondrial genome of the deep-sea limpet Bathyacmaea nipponica (Patellogastropoda: Pectinodontidae). Mitochondrial DNA B 4(2):3175–3176. https://doi.org/10.1080/23802359.2019.1668732
Thiele J (1904a) [Artbeschreibungen in] Martens E, Thiele J (eds) Die beschalten Gastropoden der Deutschen Tiefsee-Expedition 1898–1899. Wiss Erg Dtsch Tiefsee- Exp “Valdivia” 7:1–146, plates 1–5. https://www.biodiversitylibrary.org/item/43022#page/7/mode/1up
Thiele J (1904b) Die Anatomie und systematische Stellung der Gattung Cocculina. Wiss Erg Dtsch Tiefsee-Exp “Valdivia” 7:147–179, plates 6–7. https://www.biodiversitylibrary.org/item/43022#page/157/mode/1up
Thiele J (1909) Cocculinoidea und die Gattungen Phenacolepas und Titiscania. Syst Conchylien Cabinett Martini & Chemnitz 2(11a):1–48, plates 1–6. https://www.biodiversitylibrary.org/item/217195#page/9/mode/1up
Wanninger A, Ruthensteiner B, Lobenwein S, Salvenmoser W, Dictus WJAG, Haszprunar G (1999) Development of the musculature in the limpet Patella (Mollusca, Patellogastropoda). Dev Genes Evol 209:226–238. https://doi.org/10.1007/s004270050247
Wolff T (1979) Macrofaunal utilization of plant remains in the deep-sea. Sarsia 64(1–2):117–136. https://doi.org/10.1080/00364827.1979.10411373 (plates 1–5)
Zapata F, Wilson NG, Howison M, Andrade SCS, Jörger KM, Schrödl M, Goetz FE, Giribet G, Dunn CW (2014) Phylogenomic analyses of deep gastropod relationships reject Orthogastropoda. Proc R Soc Lond B281(1794):20141739. https://doi.org/10.1098/rspb.2014.1739
Zapata F, Wilson NG, Howison M, Andrade SCS, Jörger KM, Schrödl M, Goetz FE, Giribet G, Dunn CW (2015) Correction to: Phylogenomic analyses of deep gastropod relationships reject Orthogastropoda. Proc R Soc Lond (1802) B282(20142941):1–3. https://doi.org/10.1098/rspb.2014.2941
Zhang S, Zhang S (2018) Cocculina delphinicula sp. nov., a new cocculinid species from whale bone in the East China Sea (Gastropoda: Cocculiniformia). Zootaxa 4455:189–195. https://doi.org/10.11646/zootaxa.4455.1.9
Acknowledgements
We are indebted to Dimitri Ivanov (Moscow Museum of Natural History), who made the paratypes of Fedikovella caymanensis and Teuthirostria cancellata available to anatomical studies. We thank Marco Oliverio (University of Rome) to arrange the loan of specimens of Coccopigya viminensis from Rocchini (CISMA, Rome) as well as Luis Dantart (University of Barcelona) for providing further specimens. I am particularly grateful to the late Rudolf Kilias (Humboldt-Museum, Berlin) for the loan of the section series of Thiele's collection from the Museum für Naturkunde Berlin. The late James H. McLean (Los Angeles County Museum Natural History, CA) provided the specimens of Cocculina craigsmithi for serial sectioning. Bernhard Ruthensteiner and Willibald Salvenmoser (University of Innsbruck) prepared the serial sections.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Nagoya protocol
The content of the paper is not affected by the Nagoya Protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haszprunar, G., Wendler, S.Y.C., Jöst, A.B. et al. 3D-anatomy and systematics of cocculinid-like limpets (Gastropoda: Cocculiniformia): more data, some corrections, but still an enigma. Zoomorphology 141, 151–171 (2022). https://doi.org/10.1007/s00435-022-00556-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00556-6