Abstract
The accessory neural arch is an oddly distributed character present in several non-acanthomorph teleostean taxa. Its homology was often implied but never satisfyingly tested. In this study, we attended this pending problem. We analyzed the morphology, development, and systematic distribution of the accessory neural arch in teleosts. Using a comprehensive taxon sampling of cleared and stained specimens, we evaluated if the accessory neural arch fulfils existing homology criteria. We then combined these data with recent genetic phylogenies and ancestral character state estimation to reconstruct the evolutionary history of the accessory neural arch. While its gross morphology and development fit homology criteria, results from ancestral character state estimations suggest multiple independent evolutions within teleosts. Although the accessory neural arch cannot be homologous between several teleostean taxa, the concept of parallelism may explain the presence of such a similar character in a variety of non-acanthomorph teleostean taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The accessory neural arch (ANA) is a paired, endochondral ossified structure between the occiput and the neural arch of the first vertebra positioned in the third myoseptum (Brühl 1956; Forey 1973; Fink and Weitzman 1982; Bemis and Forey 2001; Britz and Johnson 2010; Johnson and Britz 2010). Principally it is very much like a regular neural arch in its shape but has no affiliation with a vertebral centrum. Furthermore, it develops much similar as neural arches from a paired cartilaginous precursor (de Pinna and Grande 2003). This has provoked various ideas of vertebrae being ontogenetically fused into the occiput, leaving a ‘free’ neural arch (e.g., Forey 1973; Bemis and Forey 2001). Subsequent ontogenetic studies, however, could not confirm these hypotheses as neurocranial-vertebrae-fusions do not appear in taxa with ANA (Johnson et al. 2009; Britz and Johnson 2010; Johnson and Britz 2010). In several studies, the ANA was mentioned and described for non-acanthomorph teleosts (Fink and Fink 1981; Rosen 1985). The presence of an ANA in Otophysi was especially critically discussed, because of their modified anterior vertebral column, i.e., the presence of the Weberian apparatus (de Pinna and Grande 2003; Grande and de Pinna 2004; Britz and Hoffmann 2006). Patterson and Johnson (1995) and Johnson and Patterson (1996) summarized the distribution of the ANA in euteleosts (sensu Johnson and Patterson 1996). The remarkable distribution of the ANA puzzled many systematists, who investigated the homology of this structure. In phylogenetic hypotheses, frequent reductions (if homologues) or numerous independent origins (if convergent) had to be assumed (Patterson and Johnson 1995; Johnson and Patterson 1996). A call for a more robust cladogram of teleosts and a more extensive survey of the ANA has, therefore, been formulated more than 25 years ago (Patterson and Johnson 1995).
In recent years, more and more robust cladograms for teleosts have become available based on extensive phylogenomic studies (e.g., Betancur et al. 2013, 2017; Near et al. 2013; Hughes et al. 2018; Straube et al. 2018). Therefore, analyzing the homology of the ANA becomes a task again. To determine if a character such as the ANA is homologous, the ANA must first be re-evaluated in light of homology criteria as formulated by Remane (1952), i.e., sameness in position, development, and composition. These criteria are needed to ascertain that characters that are compared are actually the ‘same’. Afterwards the homology can be tested based on the continuous presence from extant taxa back to a common ancestor (e.g., Patterson 1982, 1988; Wagner 1989; de Pinna 1991; Rieppel 1992; Brower and Schawaroch 1996; Brigandt 2003; DiFrisco 2021).
While de Pinna (1991) suggested to test the continuous presence of a character by constructing phylogenies using the observed character and letting the most parsimonious phylogeny indicate if the criterion of continuity is fulfilled, mapping character distributions on already present phylogenies and performing ancestral character state estimations (ACE) were applied in recent years (e.g., Davis et al. 2016; Herrel et al. 2016; Zattara and Bely 2016; Sauquet et al. 2017). This procedure was partly met with criticism as the examined character has no influence on the respective phylogenies (e.g., Smith et al. 2005; Assis and Rieppel 2011; Assis and Santos 2014; Assis 2015). However, the independence of phylogeny and character is also a strength of this method.
In this study, we now combine recent phylogenies with a comprehensive survey on the distribution, development and morphology of ANAs in teleosts as well as an ancestral character state estimation to investigate the homology of this puzzling structure.
Material and methods
For examination of accessory neural arches, specimens from the ichthyological collection of the Deutsches Meeresmuseum have been used. The specimens were cleared and double-stained (cartilage in blue and bone in red) following the protocols of Dingerkus and Uhler (1977) and Taylor and Van Dyke (1985). In total 418 specimens of 117 species and 59 families were studied (listed in Supplement 1), including ontogenetic material from six species [Clupea harengus Linnaeus 1758, Coregonus maraena (Bloch 1779), Esox lucius Linnaeus 1758, Kneria stappersii Boulenger 1915, Osmerus eperlanus Linnaeus 1758, and Thymallus thymallus (Linnaeus 1758)]. Pictures were taken with a Canon EOS 80D camera system with a Canon MP-E 65 mm objective.
Character mapping as well as ancestral character state estimations were carried out in R using the packages APE (package version: 5.4-1) (Paradis et al. 2004), Phytools (package version: 0.7-70) (Revell 2012), and Parallel (package version: 4.0.4) (R Core Team 2021). The discrete character states were obtained from the material mentioned above as well as from literature (Weitzman 1974; Rosen 1985; Patterson and Johnson 1995; Baldwin and Johnson 1996; Fink and Fink 1996; Forey et al. 1996; Johnson and Patterson 1996; Grande and Bemis 1998; Sanford 2000; Harold 2002; Hilton 2002, 2003; Sato and Nakabo 2002; de Pinna and Grande 2003; Grande and de Pinna 2004; Britz and Johnson 2010; Grande 2010; Schnell et al. 2010; McDowall and Burridge 2011; Kanehira et al. 2012; listed in Supplement 2) and afterwards were mapped on the phylogenetic tree provided by Betancur et al. (2017). Non-teleost actinopterygian taxa were chosen as outgroups and reduced to their last common ancestor, i.e., Polypteriformes, Acipenseriformes, Amia, and Lepisosteiformes, respectively. Similarly, Myctophiformes and Acanthomorpha in which no accessory neural arch is present were reduced to their last common ancestor. Also, otophysan taxa were reduced to their last common ancestor respectively, i.e., Cypriniformes, Characiformes, Gymnotiformes, and Siluriformes, because the presence or absence of an ANA in these taxa is heavily disputed and it is not possible to document an ANA in adult specimen. However, due to the undisclosed state of the presence of an ANA in Otophysi, we tested two hypotheses: (1) the presence of the ANA in Otophysi based on the assumption that the claustrum of the Weberian apparatus is homologues to the ANA (Grande and de Pinna 2004), and (2) the absence of an ANA in Otophysi (Britz and Hoffmann 2006). For the ancestral character state estimation, different models (character state change on either equal rates or different rates and calculated model parameters, i.e., root probabilities [pi] and transition matrix [Q]) were first calculated for the underlying data using the fitMK-function provided by phytools (Revell 2012). Then the Akaike information criterion of all models were compared by calculating the Akaike weights (using the function aic.w in phytools), which represent the relative likelihood of a model, to find the most-fitting model. Afterwards ancestral character states were calculated based on 2000 simulated stochastic character maps using the make.simmap- and describe.simmap-functions in phytools.
Results
Systematic distribution of the ANA
The accessory neural arch is present in many species of several non-acanthmorph teleost taxa, i.e., Elopidae, Clupeoidei, Alepocephaliformes, some Galaxiidae, Esocidae, Salmoniformes, most Osmeriformes, Gonostomatidae, and some Aulopiformes (Fig. 1). Of the Elopomorpha, the earliest branching taxon within the Teleostei, only in a single genus, Elops, an ANA is present (Figs. 1, 2a). Its sister taxon, Megalops, does not show any traces of an ANA, which is the same for all other investigated elopomorphs. None of the examined Osteoglossomorpha have an accessory neural arch either. Within the Otomorpha, which comprise Clupeiformes, Alepocephaliformes, Gonorynchiformes, and the Otophysi, a distinct ANA was observed in two taxa, i.e., Clupeoidei (Fig. 2b, c) and Alepocephaliformes (Fig. 2d, e). While the earliest branching clupeiform, Denticeps clupeoides, does not have an ANA, it is present in all examined families within its sister taxon, Clupeoidei. Only in few examined clupeid species, i.e., Hyperlophus vittatus, Laeviscutella dekimpei, and Pellonula leonensis, no ANA was observed. In Clupeichthys aesarnensis, we observed an unpaired, ossified structure anterior to the first neural arch that could be the remnants of an accessory neural arch. Within the Alepocephaliformes, we observed an ANA in Alepocephalidae and Platytroctidae, and only few genera do not exhibit an accessory neural arch, i.e., Maulisia, and Xenodermichthys. In some specimens of Alepocephalus bicolor, we only found one half of the ANA, either the right or the left one (e.g., A. bicolor DMM IE/13719, SL = 106.1 mm; Fig. 2d). Within the Gonorynchiformes, no ANA was detected. However, some larvae of Kneria stappersii showed a structure similar to an ANA (see below).
Phylogenetic relationships of non-acanthomorph actinopterygian fishes based on Betancur et al. (2017) with the mapped absence (blue) and presence (red) of the accessory neural arch. The absence of the ANA in Otophysi is hypothesized (Britz and Hoffmann 2006) and the estimated likelihoods of absence and presence are plotted for each node, which are based on Model 1 (Table 2)
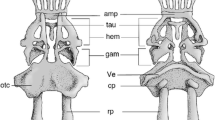
Lateral view of occipital–vertebral region of cleared and stained specimen of a Elops senegalensis (DMM IE/11008, SL = 59.1 mm); b Anchoviella cayennensis (DMM IE/14935, SL = 64.5 mm); c Clupea harengus (DMM IE/12064, SL = 62.2); d Alepocephalus bicolor (DMM IE/13719, SL = 110.0 mm); e Normichthys operosus (DMM IE/13808, SL = 67.0 mm); f Esox lucius (DMM IE/14327, SL = 46.0 mm); g Coregonus maraena (DMM IE/13723, SL = 37.0 mm); h Triplophos hemingi (DMM IE/12621, SL = 165.0 mm); i Osmerus eperlanus (DMM IE/11742, SL = 48.8 mm); j Saurida gracilis (DMM IE/14949, SL = 67.5 mm). ANA accessory neural arch, BO basioccipital, NA neural arch, NS neural spine, V1 first vertebral centrum. Scale bar = 1 mm
Within the Euteleostei, no accessory neural arch is found in the earliest branching taxon, i.e., Lepidogalaxias. In the Protacanthopterygii (sensu Betancur et al. 2017), we found no ANA in the galaxiiform genus Galaxias, while an ANA is present in the family Esocidae (Fig. 2f) within the Esociformes, and all examined Salmoniformes (Fig. 2g). Within the Stomiatii, only the earliest branching taxon of the Stomiatiiformes, i.e., Gonostomatidae (Fig. 2h), and the Osmeriformes (Fig. 2i), except their earliest branching taxon, i.e., Retropinnidae, exhibit an accessory neural arch. None of the examined Ateleopodiformes have an ANA. Within the Aulopiformes, we found an ANA only in three of the examined families, i.e., Aulopidae, Scopelarchidae, and Synodontidae (Fig. 2j). In none of the investigated Myctophiformes and Acanthomorpha an accessory neural arch was observed.
Morphology of the ANA
In general, the accessory neural arch has a similar morphology like the first neural arch. It consists of two ossified halves which together form an arch and that are positioned dorsal to the occipital condyle of the basioccipital or dorsal to the unossified gap between basioccipital and the first vertebra. While only in few taxa the shape of the ANA differs significantly from the respective first neural arch, the shapes of the ANA can significantly vary between taxa.
In Elops senegalensis, the accessory neural arch is larger than the neural arches, because its dorsal portion is extended antero-posteriorly (Fig. 2a). We did not witness a similar ANA in any other taxon. In clupeoids the shape of the ANA is much like the shape of the first neural arch. In general, the ANA in clupeoids can be described as rectangular or trapezoid with a smaller proximal and a broader distal portion (Fig. 2b). Depending on the size of the first neural arch, the ANA may be larger or smaller. In Clupeidae, the accessory neural arch is rather narrow and more elongated, which can be summarized as rod-like, than in other clupeoid families (Fig. 2c). In alepocephaliforms two shapes of the ANA can be distinguished: In Searsia and Alepocephalus the accessory neural arch is drop-shaped and much smaller than the first neural arch (Fig. 2d), while in Normichthys and Holtbyrnia the ANA is much like the first neural arch in shape (trapezoid) and size (Fig. 2e).
The ANA of Esox principally equals the first neural arch (Fig. 2f). Like in Esox, the accessory neural arch in salmoniforms does resemble the first neural arch, although it is generally broader dorsally. The ANA of Coregonus is narrower than the first neural arch (Fig. 2g). Within the Stomiatiiformes, the accessory neural arch of the Gonostomatidae is narrow and trapezoid (Fig. 2h). In osmeriforms, the shape of the ANA varies between the two families Osmeridae and Salangidae. In osmerids the ANA is rectangular, which is very similar to their first neural arch (Fig. 2i), while in salangids it is short and stout, which, again, is very similar to their first neural arch. The shape of the ANA in aulopiforms in general is almost rectangular, but much narrower than the first neural arch (Fig. 2j).
Development of the ANA
There is a high resemblance in the development of the accessory neural arch in the different taxa. In the studied species of clupeoids (Clupea harengus), esocids (Esox lucius), salmoniforms (Coregonus maraena, Thymallus thymallus), and osmerids (Osmerus eperlanus) the ANA has a cartilaginous precursor that develops later than the anterior neural arches (Fig. 3a–i). Both, the left and right half, are of the same size when they first emerge. While they are very narrow in Clupea (Fig. 3a–c), they are stouter in Osmerus. In Coregonus and Thymallus both halves are very small and of a more plate-like shape. In some of the larval specimens of Coregonus we observed only one half of the ANA. While in clupeoids the development timing of the ANA is postponed compared to the neural arches, the ANA develops at the same time as the following neural arches in Esox and Thymallus (Fig. 3d–i).
Cleared and stained ontogenetic stages showing the development of the accessory neural arch in (a–c) the clupeid Clupea harengus (a DMM IE/11793, SL = 28.5 mm; b DMM IE/13447, SL = 22.5; c DMM IE/12076, SL = 28.2 mm), d–f the esocid Esox lucius (d DMM IE/11789; SL = 17,7 mm, e DMM IE/11789, SL = 20.8 mm; f DMM IE/15718, SL = 24.5 mm), g–h the salmonid Thymallus thymallus (DMM IE/11786, g SL = 15.5 mm; h SL = 26.4 mm), and i the osmerids Osmerus eperlanus (DMM IE/13826, SL = 23.2 mm). Ontogenetic stages of Kneria stappersii (DMM IE/16879, j SL = 8.5 mm; k SL = 13.2 mm; l SL = 15.7 mm) show individuals with vertebrae 1 fused to the basioccipital (j–k) and without such a fusion (l). ANA accessory neural arch, BO basioccipital, NA neural arch, NS neural spine, V1 first vertebral centrum, V2 second vertebral centrum. Scale bar = 200 µm
In few larval specimens of Kneria stappersii, we witnessed an ossified neural arch above the posterior protrusion of the basioccipital (Fig. 3j). It resembles much the first neural arch in its shape and in terms of the developmental progress, however, does not attach to a vertebral centrum and, therefore, meets the criteria of an ANA. In other examined specimens of Kneria stappersii, the two anterior-most neural arches differ in shape from the following neural arches (Fig. 3k). In specimen with the presumable ANA, only one of these two neural arches is present, though, the presumable ANA resembles the ‘missing’ neural arch (Fig. 3k, l). Furthermore, the first vertebral centrum in Kneria stappersii has a heterocoelous articulation with the occipital condyle, while all vertebral centra articulate amphicoelously. In the specimens with a presumable ANA, the first vertebral centrum and the occipital condyle share an amphicoelous articulation. It seems like in these specimens the true first vertebral centrum is fused to the basioccipital. As a result of this malformation the first neural arch may be perceived as ANA.
Ancestral character state estimation
The evolution of the ANA was analyzed based on two different hypotheses: (1) presence of an ANA in otophysan taxa, and (2) absence of the ANA in otophysan taxa. In the following, we describe the three models with the highest AIC-weights in comparison to each other for both hypotheses in descending order (Tables 1, 2). For hypothesis (1) the following models were retrieved: Model 1 (Table 1), the most-preferable model (AIC weight = 0.292), suggested the evolution of the ANA in a common ancestor to all actinopterygians and subsequent reductions in non-teleost actinopterygians as well as multiple reductions within teleosts (Fig. 4a). Results from model 2 (Table 1, AIC weight = 0.204) are ambiguous as estimated node probabilities for presence and absence are often equal. However, the ANA under this model evolved multiple (at least nine) times anew (Fig. 4b). Model 3 (Table 1), the least preferred of the herein described models (AIC weight = 0.155) suggested the evolution of the ANA once in Elops and in a common ancestor to all Clupeocephala and with subsequent reductions in multiple taxa and a reversal in Aulopiforms (Fig. 4c).
Phylogenetic relationships of non-acanthomorph actinopterygian fishes based on Betancur et al. (2017) with the mapped absence (blue) and presence (red) of the accessory neural arch. The presence of the ANA in Otophysi is hypothesized (Grande and de Pinna 2004) and the estimated likelihoods of absence and presence are plotted for each node for three different models (a-c) described in Table 1. Numbers indicate taxa: 1, Actinopterygii; 2, Teleostei; 3, Clupeocephala; 4, Clupeiformes; 5, Alepocephaliformes; 6, Gonorynchiformes; 7, Otophysi; 8, Argentiniformes; 9, Galaxiiformes; 10, Esociformes; 11, Salmoniformes; 12, Osmeriformes; 13, Stomiatiiformes; 14, Aulopiformes
Under the assumption that the ANA is absent in Otophysi, all three models shown in Table 2 resulted in very similar estimated ancestral character states (Fig. 1)The accessory neural arch evolved at least eleven times independently within the Teleostei independent of model properties (Fig. 1, Table 2): (1) within the Elopomorpha in the genus Elops; (2) in the Clupeoidei, where it was reduced few times afterwards; (3) at the base of the Alepocephaliformes, where it seemingly was reduced once within the family Alepocephalidae; (4) within the Galaxiiformes in the genus Aplochiton; (5) within the Esociformes in the genus Esox; (6) at the base of the Salmoniformes; (7) in the Osmeriformes at the base of the Osmeroidei, comprising Osmeridae, Plecoglossidae and Salangidae; (8) within the Stomiatiiformes at the base of the Gonostomatidae; (9–11) three times within the Aulopiformes including twice in the paraphyletic Synodontidae (sensu Betancur et al. 2017) and once in the Aulopidae.
Discussion
Systematic distribution
In non-teleost actinopterygians, i.e., Polypteriformes, Acipenseriformes, and Holostei, no accessory neural arch is present (Grande and Bemis 1998; Britz and Johnson 2010; Grande 2010), however, it is widely distributed within non-acanthomorph teleosts (Fig. 1, Suppl. 1–2). Within the two earliest branching teleostean taxa, Elopomorpha and Osteoglossomorpha, the ANA can only be found in one genus, i.e., Elops (Patterson and Johnson 1995; Forey et al. 1996; Johnson and Patterson 1996; Hilton 2002, 2003; Kanehira et al. 2012). Bemis and Forey (2001) hypothesized that the ANA in Elops is the remnant of a vertebra which is fused into the occiput. However, subsequent studies showed the neurocranial-vertebrae-fusion does not occur in taxa with an ANA (Britz and Johnson 2010). In Otomorpha, the ANA is more common and is present in Clupeiformes and Alepocephaliformes. Within the Clupeiformes, the earliest branching taxon, Denticeps clupeoides, has no ANA. Otherwise the ANA is present in all clupeoid family (Patterson and Johnson 1995; Johnson and Patterson 1996), and only absent in few genera, i.e., Jenkinsia, Hyperlophus, Laeviscutella, and Pellonula (Patterson and Johnson 1995; de Pinna and Grande 2003). While we found a possible remnant of an ANA in Clupeichthys, Patterson and Johnson (1995) reported no ANA for this genus. In Alepocephaliformes, the ANA is present in species of all three families (Johnson and Patterson 1996). However, several species and genera miss the ANA in the families Alepocephalidae, i.e., Bajacalifornia burragei, Leptochilichthys agassizi, Leptoderma macrops, Photostylus, Rinoctes, Rouleina, and Xenodermichthys, and Platytroctidae, i.e., Maulisia (Johnson and Patterson 1996). No ANA was found or reported for any gonorynchiform species (Patterson and Johnson 1995). De Pinna and Grande (2003), Grande and Young (2004), and Grande and de Pinna (2004) hypothesized that in Otophysi, the claustrum of the Weberian apparatus is homologous to the ANA, based on developmental data, i.e., their arch-like structure, topographical relationships, derivation from paired cartilages, and delayed formation relative to neural arches. Other extensive studies on the morphology and development of the Weberian apparatus in Otophysi, however, led to different hypotheses about the homology of the claustrum (e.g., Fink and Fink 1981, 1996; Coburn and Futey 1996; Bird and Mabee 2003; Grande and Young 2004; Britz and Hoffmann 2006; Hoffmann and Britz 2006; Bird and Hernandez 2007). This topic is still highly disputed, and no consensus has been reached yet (see Britz and Hoffmann 2006 for discussion). In Euteleostei, an ANA can only be found in Galaxiiformes, Salmoniformes, Esociformes, Stomiatiiformes, Osmeriformes, and Aulopiformes (Weitzman 1974; Patterson and Johnson 1995; Baldwin and Johnson 1996; Johnson and Patterson 1996; Sanford 2000; Harold 2002; Sato and Nakabo 2002; Schnell et al. 2010; McDowall and Burridge 2011). For Galaxiiformes, the ANA is only reported for the genus Aplochiton (Johnson and Patterson 1996; McDowall and Burridge 2011), just like the ANA is only present in one genus of the Esociformes, i.e., Esox (Patterson and Johnson 1995; Johnson and Patterson 1996). In contrast, an ANA is present in all Salmoniformes (Johnson and Patterson 1996; Sanford 2000). A drawing from Johnson and Patterson (1996) of Oncorhynchus clarkii does not depict an ANA, however, Sanford (2000), who also examined O. clarkii, did not mention the absence of the ANA in this species. In Osmeriformes, only the Retropinnidae are lacking an ANA (Patterson and Johnson 1995; Johnson and Patterson 1996). While in the Stomiatiiformes an accessory neural arch is only present in one family, the Gonostomatidae (Weitzman 1974; Patterson and Johnson 1995; Harold 2002; Schnell et al. 2010). Within the Aulopiformes an ANA is present in Aulopidae, Scopelarchidae, and Synodontidae (Patterson and Johnson 1995; Baldwin and Johnson 1996; Sato and Nakabo 2002). However, Rosenblattichthys seemingly is the only scopelarchid genus in which an accessory neural arch is found (Baldwin and Johnson 1996).
Morphology and development
The shape of the accessory neural arch varies greatly between some taxa. Yet, within each species, the ANA is usually very similar to the anterior-most neural arch. It seems likely that the ANA is a serial-homologue to neural arches, therefore, it is to be expected that its shape changes similar to the neural arches. Only few taxa show differences in shape between these two structures. For example, in Elops the ANA is dorsally broadened, however, a resemblance to the first neural arch is visible and the ANA could also be described as an anteriorly extended neural arch. The biggest difference may be found in some Alepocephaliformes. While the first neural arch in alepocephaliforms is generally trapezoid, the ANA of Alepocephalus and Searsia are drop-shaped and much smaller. In the salmoniform Coregonus the ANA is much narrower than the first neural arch. Johnson and Patterson (1996: pp 278) reported that the accessory neural arch of the osmerid Spirinchus is reduced to a “minute nubbin”. In gonostomatids the ANA is trapezoid and smaller than the first neural arch which is rather rectangular and angled posteriorly (Harold 2002). Also, in aulopiforms the shape of the ANA differs from that of the first neural arch as it is narrower.
The development of the accessory neural arch in the herein examined species Clupea harengus, Coregonus maraena, Osmerus eperlanus, and Thymallus thymallus matches previous reports for ANA formation in Pellona harroweri and Alosa sapidissima (de Pinna and Grande 2003). In these species, the ANA forms as paired cartilages anterior to the neural arch of vertebra 1 and later ossifies. Both, cartilage formation and ossification, are delayed in relation to the anterior neural arches. Only in Esox we observed a more or less simultaneous formation of the ANA and anterior neural arches. De Pinna and Grande (2003) reported that the delay is less pronounced in Esox and Coregonus. We on the other hand found the timing of formation to be very different between these two genera. Coburn and Chai (2003) reported the presence of rudiments of an ANA during a brief period in ontogeny in the gonorynchiform Chanos chanos. In the gonorynchiform Kneria stappersii we found a similar structure like the ANA in few larval specimens. However, this seems to be the result of a malformation during which the first vertebral centrum fuses to the occiput in some specimen and the ‘true’ first neural arch develops dorsally to the occipital condyle.
Evolution and homology
In previous studies, it was assumed that the accessory neural arch is the same structure in the examined species, although no clear homology assumptions were given (Fink and Fink 1981; Rosen 1985; Patterson and Johnson 1995). Johnson and Patterson (1996: pp 278) were “convinced that ANA was primitively present in that group [Euteleostei] and has been repeatedly lost”. The similar composition, development, and position of the accessory neural arch, fulfil all criteria formulated by Remane (1952) to determine homologous structures. Analyzing the presence and absence of the accessory neural arch based on the phylogeny in Betancur et al. (2017), resulted in different outcomes depending on the presence of the ANA or a homologous structure in Otophysi. Even considering the claustrum in Otophysi is homologous to the ANA, only one tested model (model 3), which of the herein described models is the least preferable, provided a reasonable estimation in which the accessory neural arch is homologous within the Clupeocephala (Fig. 4c). Model 1, which is the most preferable and that suggests the evolution of the ANA in a common ancestor to all actinopterygians, can be neglected as no extant non-teleost actinopterygian taxon has an ANA (Grande and Bemis 1998; Britz and Johnson 2010; Grande 2010) and fossil data give no indication of an ANA in stem actinopterygians (Bemis and Forey 2001). Model 2 provides concluding estimations only for some nodes while others are ambiguous between absence and presence of the ANA. However, it needs to be assumed that under this model the ANA is not homologous for a larger set of taxa. Accepting the absence of the accessory neural arch or a homologous structure in Otophysi, the ancestral character state estimation showed multiple independent origins of the ANA within non-acanthomorph teleosts rather than a single evolutionary event and the continuous presence in teleosts independent of the applied model (Fig. 1). Because of the dubiousness of homology between the ANA and the claustrum in Otophysi as well as the dependence of a possible continuous presence of the ANA in Clupeocephala on a specific and not preferable model, we proceed on the assumption that the accessory neural arch is not generally homologous in Teleostei.
Other phylogenomic studies resulted in slightly different phylogenetic relationships for non-acanthomorph teleosts (e.g., Hughes et al. 2018; Straube et al. 2018) than retrieved in Betancur et al. (2017). Mainly the position of the Galaxiiformes varies and they were positioned as sister taxon to the Stomiatii (Hughes et al. 2018) or as sister taxon to the Neoteleostei (Straube et al. 2018). Although we did not calculate ancestral character state estimations with these phylogenies, no different result due to the altered position of galaxiiforms is expected. Hence, several convergent evolutions of the ANA need to be assumed for all these phylogenies. However, such a scenario seems hard to comprehend because of the very similar morphology of the ANA as well as the matching development in these distantly related species. Especially, as no clear functional effect can be attributed to the ANA and an evolutionary advantage is questionable.
Homology itself as well as homology theories are still under lively discussion (e.g., Assis 2015; Hejnol and Lowe 2015; Richter 2017; Vogt 2017; DiFrisco 2021; DiFrisco and Jaeger 2021). Following a classical approach that structures are homologous if the ancestor of recent taxa exhibiting this structure already possessed it too (Mayr 1982; Roth 1984; Wagner 1989; Brigandt 2003), the ANA cannot be considered homologous between all taxa exhibiting it (Fig. 2). However, different concepts discussed in the context of homologies and convergences may provide an explanation for the oddly distribution of the accessory neural arch within non-acanthomorph teleosts. Such a concept is “Atavism, or the re-expression of ancestral morphologies” (Stiassny 1992: pp 260). Like homology theories, the principles of atavism were discussed for decades (e.g., Raikow et al. 1979; Hall 1984, 2012; Stiassny 1992; Meyer 1999; Witten and Hall 2015). Atavisms can occur in different forms, such as individual or induced anomalies, or permanent characters manifested in the phenotype of a taxon, which Stiassny (1992) termed taxic atavism. Different examples for taxic atavisms were subsequentially found in a variety of taxa (e.g., Gatesy et al. 2003; Huysseune et al. 2009; Grünbaum and Cloutier 2010; Zander 2010). Hall (1984) hypothesized that there is a genetic basis of atavistic characters because they can be selected for. Somewhat following this hypothesis of genetical based atavisms, Meyer (1999) proposed an evolutionary retention of genetic potentiality, which could lead to convergent morphologies due to re-awakening of retained genetical networks or pathways. Hall (2003) later distinguished between atavisms, which only occur infrequently or sporadically on an individual basis, and parallelism, which describes the appearance of homoplastic characters in more closely related taxa that, in contrast to convergencies, are using a similar (homologous) developmental pathway. The concept of parallelism, therefore, combines Meyers (1999) hypothesis of retained genetical pathways and Stiassnys (1992) taxic atavism. Hall (2003) referred to Gould (2002), who stated that parallelism is a “gray zone between homology and convergence” (Gould 2002: pp 1088), because parallel characters are not fully independent in their evolution (DiFrisco 2021).
The accessory neural arch may be an example of a parallel character. Its gross morphology is very similar in the taxa that exhibit this character and at least the phenotypic development is the same in the examined species. However, its discontinuous presence suggests multiple independent evolutions. Therefore, the accessory neural arch fits the concept of parallelism. Going forward, the underlying developmental pathways of ANA formation need to be studied and compared to confirm this hypothesis.
Availability of data and material
Supplement 1: List of all examined specimen with indication if an ANA is present or absent. Supplement 2: Data matrix used for the ancestral character state estimation with data sources.
Code availability
Not applicable.
References
Assis LCS (2015) Homology assessment in parsimony and model-based analyses: two sides of the same coin. Cladistics 31:315–320. https://doi.org/10.1111/cla.12085
Assis LC, Rieppel O (2011) Are monophyly and synapomorphy the same or different? Revisiting the role of morphology in phylogenetics. Cladistics 27:94–102
Assis LCS, Santos LM (2014) Phylogenetics is not phylogenomics. Cladistics 30:8–9. https://doi.org/10.1111/cla.12028
Baldwin CC, Johnson GD (1996) Interrelationships of aulopiformes. In: Stiassny MLJ, Parenti LR, Johnson DG (eds) Interrelationships of fishes. Academic Press Inc, New York, pp 355–404
Bemis W, Forey P (2001) Occipital structure and the posterior limit of the skull in actinopterygians. In: Ahlberg PE (ed) Major events in early vertebrate evolution. Taylor and Francis, London and New York, pp 350–369
Betancur-R R, Broughton RE, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton Ii JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Ortí G (2013) The tree of life and a new classification of bony fishes. PLoS Curr. https://doi.org/10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Orti G (2017) Phylogenetic classification of bony fishes. BMC Evol Biol 17:162. https://doi.org/10.1186/s12862-017-0958-3
Bird NC, Hernandez LP (2007) Morphological variation in the Weberian apparatus of Cypriniformes. J Morphol 268:739–757. https://doi.org/10.1002/jmor.10550
Bird NC, Mabee PM (2003) Developmental morphology of the axial skeleton of the Zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn 228:337–357. https://doi.org/10.1002/dvdy.10387
Brigandt I (2003) Homology in comparative, molecular, and evolutionary developmental biology: the radiation of a concept. J Exp Biol Part B Mol Dev Evolut 299:9–17. https://doi.org/10.1002/jez.b.36
Britz R, Johnson GD (2010) Occipito-vertebral fusion in actinopterygians: conjecture, myth and reality. Part 1: non-teleosts. In: Nelson JS, Schultze H-P, Wilson MVH (eds) Origin and phylogenetic interrelationships of teleosts. Verlag Dr. Friedrich Pfeil, München, pp 77–94
Britz R, Hoffmann M (2006) Ontogeny and homology of the claustra in otophysan Ostariophysi (Teleostei). J Morphol 267:909–923. https://doi.org/10.1002/jmor.10447
Brower AV, Schawaroch V (1996) Three steps of homology assessment. Cladistics 12:265–272
Brühl CB (1956) Ueber ein bisher unbekanntes, accessorisches, Bogenelement der Occipitalgegend einiger Knochenfische. In: Brühl CB (ed) Osteologisches aus dem Pariser Pflanzengarten. Selbstverlag des Verfassers, Wien, pp 1–7
Coburn MM, Chai P (2003) Development of the anterior vertebrae of Chanos chanos (Ostariophysi: Gonorynchiformes). Copeia 2003:175–180
Coburn MM, Futey LM (1996) The ontogeny of supraneurals and neural arches in the cypriniform Weberian apparatus (Teleostei: Ostariophysi). Zool J Linn Soc 116:333–346
Davis MP, Sparks JS, Smith WL (2016) Repeated and widespread evolution of bioluminescence in marine fishes. PLoS ONE 11:e0155154. https://doi.org/10.1371/journal.pone.0155154
de Pinna MC (1991) Concepts and tests of homology in the cladistic paradigm. Cladistics 7:367–394
de Pinna MC, Grande T (2003) Ontogeny of the accessory neural arch in pristigasteroid clupeomorphs and its bearing on the homology of the otophysan claustrum (Teleostei). Copeia 2003:838–845
DiFrisco J, Jaeger J (2021) Homology of process: developmental dynamics in comparative biology. Interface Focus 11:20210007. https://doi.org/10.1098/rsfs.2021.0007
DiFrisco J (2021) Developmental homology. Evolutionary developmental biology: a reference guide, vol 11, pp 85–97. https://doi.org/10.1098/rsfs.2021.0007
Dingerkus G, Uhler LD (1977) Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol 52:229–232. https://doi.org/10.3109/10520297709116780
Fink SV, Fink WL (1981) Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc 72:297–353
Fink SV, Fink WL (1996) Interrelationships of Ostariophysan. In: Stiassny MLJ, Parenti LR, Johnson DG (eds) Interrelationships of fishes. Academic Press Inc, New York, pp 209–249
Fink WL, Weitzman SH (1982) Relationships of the stomiiform fishes (Teleostei), with a description of Diplophos. Bull Mus Comp Zool 150:31–93
Forey PL (1973) A revision of the Elopiformes fishes, fossil and recent. Bulletin of the British Museum (Natural History). Suppl Geol 10:1–222
Forey PL, Littlewood D, Ritchie P, Meyer A (1996) Interrelationships of elopomorph fishes. In: Stiassny MLJ, Parenti LR, Johnson DG (eds) Interrelationships of fishes. Academic Press Inc, New York, pp 175–191
Gatesy J, Amato G, Norell M, DeSalle R, Hayashi C (2003) Combined support for wholesale taxic atavism in gavialine crocodylians. Syst Biol 52:403–422. https://doi.org/10.1080/10635150390197037
Gould SJ (2002) The structure of evolutionary theory. Harvard University Press, Cambridge
Grande L, Bemis WE (1998) A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. J Vertebr Paleontol 18:1–696
Grande T, de Pinna M (2004) The evolution of the Weberian apparatus: a phylogenetic perspective. In: Arratia G, Tintori A (eds) Mesozoic fishes. Verlag Dr. Friedrich Pfeil, Munich, pp 429–448
Grande T, Young B (2004) The ontogeny and homology of the Weberian apparatus in the zebrafish Danio rerio (Ostariophysi: Cypriniformes). Zool J Linn Soc 140:241–254
Grande L (2010) An empirical synthetic pattern study of gars (Lepisosteiformes) and closely related species, based mostly on skeletal anatomy. The resurrection of Holostei. American Society of Ichthyologists and Herpetologists, supplementary issue of Copeia 10(2A)
Grünbaum T, Cloutier R (2010) Ontogeny, variation, and homology in Salvelinus alpinus caudal skeleton (Teleostei: Salmonidae). J Morphol 271:12–24
Hall BK (1984) Developmental mechanisms underlying the formation of atavisms. Biol Rev 59:89–122
Hall BK (2003) Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biol Rev 78:409–433
Hall BK (2012) Evolutionary developmental biology (Evo-Devo): past, present, and future. Evolut Educ Outreach 5:184–193. https://doi.org/10.1007/s12052-012-0418-x
Harold A (2002) Order stomiiformes gonostomatidae. Living Mar Resour Western Central Atlantic 2:889–892
Hejnol A, Lowe CJ (2015) Embracing the comparative approach: how robust phylogenies and broader developmental sampling impacts the understanding of nervous system evolution. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2015.0045
Herrel A, Moureaux C, Laurin M, Daghfous G, Crandell K, Tolley K, Measey J, Vanhooydonck B, Boistel R (2016) Frog origins: inferences based on ancestral reconstructions of locomotor performance and anatomy. Fossil Imprint 72:108–116. https://doi.org/10.14446/fi.2016.108
Hilton EJ (2002) Osteology of the extant North American fishes of the genus Hiodon Lesueur, 1818 (Teleostei: Osteoglossomorpha: Hiodontiformes). Fieldiana (zoology) New Ser 100:1–142
Hilton EJ (2003) Comparative osteology and phylogenetic systematics of fossil and living bony-tongue fishes (Actinopterygii, Teleostei, Osteoglossomorpha). Zool J Linn Soc 137:1–100
Hoffmann M, Britz R (2006) Ontogeny and homology of the neural complex of otophysan Ostariophysi. Zool J Linn Soc 147:301–330
Hughes LC, Orti G, Huang Y, Sun Y, Baldwin CC, Thompson AW, Arcila D, Betancur RR, Li C, Becker L, Bellora N, Zhao X, Li X, Wang M, Fang C, Xie B, Zhou Z, Huang H, Chen S, Venkatesh B, Shi Q (2018) Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc Natl Acad Sci USA 115:6249–6254. https://doi.org/10.1073/pnas.1719358115
Huysseune A, Sire JY, Witten PE (2009) Evolutionary and developmental origins of the vertebrate dentition. J Anat 214:465–476. https://doi.org/10.1111/j.1469-7580.2009.01053.x
Johnson GD, Britz R (2010) Occipito-vertebral fusion in actinopterygians: conjecture, myth and reality. Part 2: teleosts. In: Nelson JS, Schultze H-P, Wilson MVH (eds) Origin and phylogenetic interrelationships of teleosts. Verlag Dr. Friedrich Pfeil, München, pp 95–110
Johnson GD, Patterson C (1996) Relationships of lower euteleostean fishes. In: Stiassny MLJ, Parenti LR, Johnson DG (eds) Interrelationships of fishes. Academic Press Inc, New York, pp 251–332
Johnson GD, Paxton JR, Sutton TT, Satoh TP, Sado T, Nishida M, Miya M (2009) Deep-sea mystery solved: astonishing larval transformations and extreme sexual dimorphism unite three fish families. Biol Let 5:235–239. https://doi.org/10.1098/rsbl.2008.0722
Kanehira N, Imamura H, Yabe M (2012) Phylogenetic relationships of the suborder Notacanthoidei (Teleostei: Albuliformes) reassessed from osteological characters, with a proposed new classification. Mem Fac Fish Sci Hokkaido Univ 54:1–31
McDowall RM, Burridge CP (2011) Osteology and relationships of the southern freshwater lower euteleostean fishes. Zoosyst Evolut 87:7–185. https://doi.org/10.1002/zoos.201000020
Meyer A (1999) Homology and homoplasy: the retention of genetic programmes. In: Novartis foundation symposium. Wiley Online Library, pp 141–152
Near TJ, Dornburg A, Eytan RI, Keck BP, Smith WL, Kuhn KL, Moore JA, Price SA, Burbrink FT, Friedman M (2013) Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc Natl Acad Sci USA 110:12738–12743. https://doi.org/10.1073/pnas.1304661110
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. https://doi.org/10.1093/bioinformatics/btg412
Patterson C (1982) Morphological characters and homology. In: Joysey KA, Friday AE (eds) Problems of phylogenetic reconstruction. Academic Press, London and New York, pp 21–74
Patterson C (1988) Homology in classical and molecular biology. Mol Biol Evol 5:603–625. https://doi.org/10.1093/oxfordjournals.molbev.a040523
Patterson C, Johnson GD (1995) The intermuscular bones and ligaments of Teleosteans Fishes. Smithson Contrib Zool 559:1–84
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raikow RJ, Borecky SR, Berman SL (1979) The evolutionary re-establishment of a lost ancestral muscle in the bowerbird assemblage. Condor 81:203–206
Remane A (1952) Die Grundlagen des natürlichen Systems, der vergleichenden Anatomie und der Phylogenetik. Akademische Verlagsgesellschaft Geest & Portig, Leipzig
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). (package version: 0.6-44). Methods Ecol Evol 3:217–233
Richter S (2017) Homology and synapomorphy-symplesiomorphy-neither synonymous nor equivalent but different perspectives on the same phenomenon. Cladistics 33:540–544. https://doi.org/10.1111/cla.12180
Rieppel O (1992) Homology and logical fallacy. J Evolut Biol 5:701–715. https://doi.org/10.1046/j.1420-9101.1992.5040701.x
Rosen DE (1985) An essay on euteleostean classification. Am Mus Novit 2827:1–57
Sanford CP (2000) Salmonoid fish osteology and phylogeny (Teleostei: Salmonoidei). ARG Gantner, Ruggell, Liechtenstein
Sato T, Nakabo T (2002) Paraulopidae and Paraulopus, a new family and genus of aulopiform fishes with revised relationships within the order. Ichthyol Res 49:25–46
Sauquet H, von Balthazar M, Magallon S, Doyle JA, Endress PK, Bailes EJ, Barroso de Morais E, Bull-Herenu K, Carrive L, Chartier M, Chomicki G, Coiro M, Cornette R, El Ottra JHL, Epicoco C, Foster CSP, Jabbour F, Haevermans A, Haevermans T, Hernandez R, Little SA, Lofstrand S, Luna JA, Massoni J, Nadot S, Pamperl S, Prieu C, Reyes E, Dos Santos P, Schoonderwoerd KM, Sontag S, Soulebeau A, Staedler Y, Tschan GF, Wing-Sze LA, Schonenberger J (2017) The ancestral flower of angiosperms and its early diversification. Nat Commun 8:16047. https://doi.org/10.1038/ncomms16047
Schnell NK, Britz R, Johnson GD (2010) New insights into the complex structure and ontogeny of the occipito-vertebral gap in barbeled dragonfishes (Stomiidae, Teleostei). J Morphol 271:1006–1022
Smith ND, Turner AH, Macleod N (2005) Morphology’s role in phylogeny reconstruction: perspectives from paleontology. Syst Biol 54:166–173. https://doi.org/10.1080/10635150590906000
Stiassny ML (1992) Atavisms, phylogenetic character reversals, and the origin of evolutionary novelties. Neth J Zool 42:260–276
Straube N, Li C, Mertzen M, Yuan H, Moritz T (2018) A phylogenomic approach to reconstruct interrelationships of main clupeocephalan lineages with a critical discussion of morphological apomorphies. BMC Evolut Biol 18:158. https://doi.org/10.1186/s12862-018-1267-1
Taylor WR, Van Dyke GC (1985) Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9:107–119
Vogt L (2017) Assessing similarity: on homology, characters and the need for a semantic approach to non-evolutionary comparative homology. Cladistics 33:513–539. https://doi.org/10.1111/cla.12179
Wagner GP (1989) The biological homology concept. Annu Rev Ecol Syst 20:51–69
Weitzman SH (1974) Osteology and evolutionary relationships of the Sternoptychidae, with a new classification of stomiatoid families. Bull Am Mus Nat Hist 153:327–478
Witten PE, Hall BK (2015) Teleost skeletal plasticity: modulation, adaptation, and remodelling. Copeia 103:727–739
Zander RH (2010) Taxon mapping exemplifies punctuated equilibrium and atavistic saltation. Plant Syst Evol 286:69–90
Zattara EE, Bely AE (2016) Phylogenetic distribution of regeneration and asexual reproduction in Annelida: regeneration is ancestral and fission evolves in regenerative clades. Invertebr Biol 135:400–414. https://doi.org/10.1111/ivb.12151
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
PT and TM composed the study, cleared and stained specimen, and wrote the manuscript. PT acquired data and images and analyzed the dataset.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thieme, P., Moritz, T. The accessory neural arch: development, morphology, and systematic distribution. Zoomorphology 141, 101–113 (2022). https://doi.org/10.1007/s00435-021-00548-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-021-00548-y