Abstract
Many pelagic shark species change body and fin shape isometrically or by positive allometry during ontogeny. But some large apex predators such as the white shark Carcharodon carcharias or the tiger shark Galeocerdo cuvier show distinct negative allometry, especially in traits related to feeding (head) or propulsion (caudal fin). In particular, changes in propulsion are attributed to a shift in swimming mode. The more heterocercal caudal fin of younger individuals with its large caudal fin span seemingly aids in hunting small, agile prey. In contrast, the less heterocercal caudal fin with a larger fin area in larger individuals aids a long-distance slow swimming mode. We were interested if negative allometric effects can be observed in a planktivorous shark, the basking shark Cetorhinus maximus, a large species adapted to long-distance slow swimming. To address this question, we compared three size classes, specifically < 260 cm (juveniles), 299–490 cm (subadults), and from adults > 541 cm total length. Comparing literature data, we found negative allometric growth of the head and of the caudal fin, but a more rapid decrease of relative caudal fin size than of relative head length. Hereby, we provide the first evidence for early negative allometric growth of the caudal fin in a large pelagic filter-feeding shark. Our study further demonstrates that ecomorphological approaches may add valuable insight into the life history of animals that are challenging to study in their natural habitat, including large roving sharks such as the basking shark.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent investigations have shown size-related spatial and trophic guild differences within and between shark species (Irschick and Hammerschlag 2015; Fu et al. 2016; Irschick et al. 2017). These studies revealed that small-bodied sharks seemingly undergo isometric morphological changes during ontogeny, i.e., they maintain a similar body shape throughout their life as opposed to many large shark species. In contrast, large apex predators such as the great white shark Carcharodon carcharias (Linnaeus 1758) or the tiger shark Galeocerdo cuvier (Péron and Lesueur 1822) undergo a distinct change in body proportions during ontogeny (allometric changes sensu Gould 1966). Most obvious is a negative allometry in the dimensions of the caudal fin. The distinctly larger dorsal lobe in juveniles is an indicator of ontogenetic change in the kinematics of swimming during ontogeny (Lingham-Soliar 2005; Irschick and Hammerschlag 2015; Fu et al. 2016).
Such allometric changes during ontogeny are closely linked to ecological demands (Gisbert 1999; Gratwicke et al. 2006; Lingham-Soliar 2005). Especially the anterior (feeding, respiration) and posterior (locomotion) parts of the body of many fish species show distinct ontogenetic allometry (Gisbert 1999; Irschick and Hammerschlag 2015). Contrary to isometric growth, these changes drive the shape of morphological structures related to, e.g., feeding and locomotion and thus ensuring the survival of early ontogenetic stages occupying a distinct ecological niche (Reiss and Bonnan 2010; Richardson et al. 2011; Higham et al. 2018). More specifically, the differences in the shape of the caudal fins in the tiger and in the white shark are believed to relate to a shift in swimming mode in search for prey seemingly require a change in locomotor ability (Irschick and Hammerschlag 2015). Indeed, younger individuals hunt small, agile prey (e.g., fishes). Older and larger individuals show a slow swimming mode in search for predominantly large prey (e.g., marine mammals) (Lingham-Soliar 2005).
Reaching a total length of more than 10 m, the basking shark, Cetorhinus maximus (Gunnerus 1765) is the second largest extant fish species (Kunzlik 1988), exceeded only by the whale shark Rhincodon typus Smith 1828 (> 16 m) (Pauly 2002). Both species are filter feeders and ovoviviparous (Kunzlik 1988; Compagno 2002; Sims 2008).
Only few datasets of body proportions are available for adult basking sharks, but even fewer (only three) for juvenile specimens (Izawa and Shibata 1993; Lipej and Mavrič 2015). In this study, we present information of body measurements of a fourth, very young specimen of C. maximus, a juvenile female of 207 cm total length (TL) deposited at the Naturhistorisches Museum Wien (Vienna, Austria). The age of basking sharks of 250 cm TL is estimated to be about 6 months (Izawa and Shibata 1993; Lipej et al. 2000). Since juvenile basking sharks measure already 160–180 cm (and possibly even more) at birth (Kunzlik 1988; Compagno 2002; Sims 2008), we assume that the Vienna specimen was only a few months old when captured. It constitutes the smallest basking shark known to date. Using morphological data from three size classes (juveniles, subadults, adults) extracted from the extant literature, we examined if negative allometric changes as described for large predatory sharks adapted to a long-distance slow swimming mode also occur in the large filter feeding basking shark which exhibits a similar swimming mode (Lingham-Soliar 2005; Sims 1999, 2008; Irschick and Hammerschlag 2015). Such observed allometric change can be used to infer unobserved (putative) life history changes, such as function and behavior (LaBarbera 1989; Carrier 1996; Lingham-Soliar 2005; Gratwicke et al. 2006; Carlisle et al. 2015). Thereby, inferring life history from ecomorphological approaches may add valuable insight into the life history of animals that are challenging to study in their natural habitat, including large roving sharks such as the basking shark.
Materials and methods
This study is mainly based on a review of the literature on juvenile, subadult and adult Cetorhinus maximus. Overall, we reviewed 52 publications for morphological information on body shape of basking sharks. Of these, only 15 publications provided data other than total length or estimations of the size from sightings, which were not included in our analysis. Finally, we retrieved data from following 11 studies: Bigelow and Schroeder (1948), Mathews and Parker (1950), Springer and Gilbert (1976), Casadevall and Escriche (1987), Tomaś and Gomez (1989), Izawa and Shibata (1993), Soldo et al. (1999), Lipej et al. (2000), Capapé et al. (2003), Ali et al. (2012) and Lipej and Mavrič (2015). Additionally, the data of a very small and young specimen stored at the Naturhistorisches Museum Wien (NMW) (register number NMW 94393) are presented. The numbers of individuals (n) assessed, and the numerical distribution of data points over measured distances and size classes are presented in Table 1.
Including our measurements from the Vienna specimen, we were able to compile a data set of overall 29 specimens which we grouped into three distinct size classes (reflecting ontogeny): size class one (hereafter termed “juveniles”), ranging from 207 to 260 cm TL (mean 233 ± 28.0 cm SD; n = 4); size class two (“subadults”), ranging from 299 to 490 cm TL (380 ± 66.7 cm; n = 8); and size class three (“adults”), ranging from 541 to 871 cm TL (782 ± 89.3 cm; n = 16) (Table 2; Tables S1–S3). In the following, we use the terms “juveniles”, “subadults” and “adults”. Information on sex and ontogenetic allometry were available for most of the 28 specimens (Tables S1–S3). Absolute (cm) and relative measurements (% TL) of all specimens are presented in Table 2.
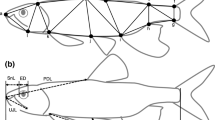
The juvenile female basking shark (pelvic fins without claspers) deposited at the Natural History Museum Vienna was collected in the Mediterranean Sea. The exact collection station is unknown. The specimen arrived at the museum between 1880 and 1900. It was preserved in 75% ethanol and stored in a cylindrical storage glass container. Due to its length, the specimen was bent two times, one time at the snout and the second time immediately anterior to the pelvic fins to fit the container (Fig. 1a, b). It was not possible to unfurl it without potentially inflicting damage. Therefore, all measurements along the body axis exceeding these bending marks constitute composite measurements between given points. Furthermore, the skin showed signs of shrinkage due to preservation. Nevertheless, because of the extremely rare documentation of young basking sharks of this size, we included the measurements into the dataset.
Cetorhinus maximus (NMW 94393), juvenile female, 207 cm TL. Due to its length, the specimen was bent two times, one time at the snout and the second time immediately anterior to the pelvic fins to fit in a cylindrical storage glass container. a Specimen in dorso-lateral view. b Head in lateral view (snout upturned due to storage). c Caudal fin
General notes to morphometrics
We are aware that our dataset of basking sharks, nearly completely retrieved from the literature, is a composition of measurements done by several authors. In the present paper, however, we focus on long distances like head and trunk size along with length of the caudal fin. Therefore, because of the sheer size of the specimens investigated, we assume that observer bias will be minimal, and will have no major effects on our results (all measurements provided in the supplementary material as Tables S1–S3).
Measurements and their nomenclature follow the second volume of the revised and updated version of the original FAO Catalogue of sharks of the world, “Sharks of the world, volume 2” (Compagno 2002) (Fig. 2; Table 1). Since these standards were not available to publications prior to 1984, some measurements are missing in these studies or other landmarks were used. Nevertheless, it was possible to retrieve a series of measurements for comparison from the available data. The most significant source we used is Matthews and Parker (1950), who provided measurements of five adult males and five adult females (Table S1). Due to this significant contribution, we have added our measurements accordingly. For instance, Matthews and Parker (1950) did not state the preorbital length from the tip of the snout to the anterior margin of the orbit, but rather measured to the center of the eye. Since the eye of adult basking sharks is very small (0.8% of the TL), we included these data into our preorbital dataset. Other distances like the TL or the length of the first dorsal fin were not explicitly stated in Matthews and Parker (1950). We, therefore, reconstructed the TL by combining the two distances “Centre of caudal emargination to tip of dorsal fluke” to “Tip of snout to caudal emargination”. The length of the first dorsal fin base we retrieved by subtracting the distance “tip of snout to anterior end of base” from “tip of snout to posterior end of base”.
Morphometric characteristics of Cetorhinus maximus used in this study [following Compagno (2002)]. a Body measures. b Head measures. c Caudal fin measures
One measurement which is commonly absent in the morphometric characterization of basking sharks is the head width, which is, however, an important morphometric character. In basking sharks head, width measurements are not straightforward due to the sheer size of their gill slits. As the gill membranes will collapse and extend laterally once the shark is outside the water, head width will ultimately be affected. This is probably the reason why head width is generally a feature missing in basking shark datasets. As the gill membranes were deformed due to storage in the Vienna specimen, we excluded this measurement from our data.
Although adult basking sharks can commonly be observed in boreal to warm temperate marine waters, observations of juveniles < 300 cm in situ are rare (Kunzlik 1988; Compagno 2002). Further, only few datasets of body proportions are available for adults, but even fewer (only three) for juvenile specimens ranging from 217 to 260 cm in total length (Izawa and Shibata 1993; Lipej et al. 2000; Lipej and Mavrič 2015). Similarly, for adult basking sharks, detailed measurements were rarely reported. This is mostly due to the fact that decomposition is commonly too far progressed in the majority of specimens washed ashore dead (e.g., Hernández et al. 2010; Fahmi and White 2015) or incidentally captured in gill nets (e.g., Soldo et al. 1999; Hernández et al. 2010; Ali et al. 2012), not permitting detailed morphological assessments. In some instances, too little time is allocated to the investigation of captured specimens, as fishermen need to process the flesh. Therefore, often only the long large morphological distances, such as total length (TL), precaudal length, or head length were taken in these cases (Soldo et al. 1999; Capapé et al. 2003; Kabaskal 2013). As a result, only a limited dataset of body proportions for adult (Mathews and Parker 1950; Ali et al. 2012), subadult (Bigelow and Schroeder 1948; Springer and Gilbert 1976; Capapé et al. 2003), and juvenile (Izawa and Shibata 1993; Lipej and Mavrič 2015) basking sharks was available for the present investigation.
Out of 100 observations of basking sharks in the Adriatic Sea, only 6% were on specimens smaller than 300 cm TL (Lipej and Mavrič 2015). Indeed, morphological data on very young basking sharks are extremely rare. To date, morphological data of only three specimens of a size < 260 cm are known (Izawa and Shibata 1993; Lipej et al. 2000; Lipej and Mavrič 2015), including the Vienna specimen presented in the current study.
Ontogenetic allometry is the relationship between size and shape across different age stages. Generally, two types (directions) of allometry are discerned: (1) positive allometry—the relative size increases; (2) negative allometry—the relative size decreases. Contrary to allometry, in isometry the relative size is maintained (Gould 1966).
Statistical analysis
Prior to all analyses, all traits were standardized to relative values by dividing each trait by the total length of the respective specimen (% of TL). Due to the issues outlined above and the resulting scarcity of measurements, the sample sizes for some trait/size class combinations is very small, ruling out the use of most commonly used statistical analyses. Nevertheless, to statistically corroborate our conclusions, we performed multiple t tests with Bootstrapping (1000) for all traits with at least three measurements within a size class. We are aware of the problems associated with small sample sizes, such as poor statistical power. However, although under discussion, some researchers support the use of t tests at very low sample sizes (e.g., de Winter 2013).
To reveal possible allometric changes of morphological traits and their directions (i.e., positive or negative allometry) we conducted linear regression analyses for all traits. As the regression integrates over all samples within a trait, the slope of the regression line and the coefficient of determination (R2) constitute a valuable addition to the t tests and may provide a more reliable indication of positive or negative allometry in cases where sample sizes were low within one or more size classes. We are aware that low sample sizes may cause problems for linear regression analysis, as the slope may be affected by single values or outliers within size classes. Furthermore, ontogenetic changes may be non-linear. However, we are confident that, even though the results for some individual traits may not be very informative, meaningful and robust conclusion may be drawn from the synopsis of all traits and analyses. For instance, when all traits of a body region (e.g., the caudal fin) exhibit the same patterns (e.g., a negative slope), we can be confident that an ontogenetic allometric trend is indeed biological, not artificial (e.g., negative allometric growth of the caudal fin).
To visualize whether the age groups can be separated based on the body measurements we have conducted a principal component analysis (PCA) using the prcomp function implemented in R (version 3.5.1). We used standardized values (i.e., divided by the total length) and replaced missing values with the mean for each trait.
We want to encourage all readers to cautiously interpret the here presented statistical results and suggest that the provided p-, R2-, or mean values should best be considered as indicators of certain ontogenetic trends that should be assessed in combination. Nevertheless, we think that the rarity of morphometric data available for this species and available data justifies our approach.
Statistical analyses were conducted using SPSS Statistics 23 (IBM Corporation, Armonk, NY). Regression analyses were conducted using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA).
Results
Size and body proportions
Head, abdomen, dorsal fins and caudal fin exhibited relative proportional changes with increasing TL in 26 measured traits (Table 2). Specifically, we observed negative allometry in all head traits, all but one body trait, as well as all measurements on the upper lobe of the caudal fin and positive allometry for all traits on the lower lobe of the caudal find and the dorsal fin (Fig. 3). In most traits, the mean follows the line of the linear regression well, however, in some cases deviations reveal a non-linear course of allometry. Hereby, rapid changes from juveniles to subadults, but only minor changes from subadults to adults, or vice versa, indicate an accelerated or slowed down development in the respective trait.
Scatterplot of all traits with linear regression (solid line) and changes in mean values (grey diamonds and dashed line) for each size class. The star indicates the specimen stored at the NMW, which also represents the smallest known measured individual. All traits are relative lengths calculated against the total length (TL). See Table 1 for abbreviations. Note that y-axes are differently scaled
Body measures
All but one body trait exhibited negative allometry (Fig. 3). R2 values were variable, ranging from 0.06 (preventral fin length) to over 0.50 (precaudal and predorsal fin 1 length). Significant differences between size groups were found for precaudal, prepelvic, and predorsal fin 1 length, with juveniles having longer relative trait lengths than subadults and adults. Significant differences were also found between subadults and adults in predorsal fin 1 length (Tables 2, 3, S1).
Head measures
All linear regressions of the head traits had negative slopes, indicating negative allometry (Fig. 3). Generally, R2 values were high, especially for prebranchial, prespiracular, preorbital, and preoral length, for which the relative trait length explained more than 70% of the variation. In contrast, R2 values were low for all ventrally measured head traits (internarial distance, prenarial length, and mouth width). Statistically significant differences between size groups were exclusively found between juveniles and adults in the traits head, prebranchial, and preorbital length (Tables 2, 3, S2). Low sample sizes in the subadult size group impeded the calculation of p values, however, in some traits (esp. head, prebranchial, and eye length) the means suggests an accelerated course of allometry, with rapid changes from juveniles to subadults and only small changes afterwards (Fig. 3).
Caudal fin measures
The linear regressions showed negative allometry at all traits measured on the upper lobe of the caudal fin, but positive allometry at all traits on the lower lobe (Fig. 3). R2 values were generally high for upper lobe traits, ranging from 0.33 to 0.57, and low for lower lobe traits (< 0.20). Significant differences were only found for dorsal caudal fin margin, with juveniles having higher relative lengths than subadults and adults, which did not differ significantly (Tables 2, 3, S3). As a consequence, the caudal fin of juvenile basking sharks is less symmetric than in subadult and adult specimens (Figs. 1c, 4).
Dorsal fin measures
Based on the linear regression, we found positive allometry in the height and length of both dorsal fins. However, length of dorsal fin base showed low R2 values and no significant differences between size classes (Tables 2, 3, S3). In contrast, R2 values were high for dorsal fin height, but were probably affected by the low sample size of adult individuals (n = 1). No significant differences of dorsal fin height were found between size classes (Table 3).
Age groups show a clear clustering in the PCA (Fig. 5). Age groups primarily differ along PC1 while PC2 reflects within-group variation. Younger fish were characterized by higher values of most traits, such as head length or eye size; larger fish had a greater precaudal length and lower postcaudal margin (for a biplot with all factors see Fig. S1). Within-group differences concerned measurements such as internarial space or mouth width. Variation in relative body measurements decreases with age.
Principal component analysis of relative body measurements of basking shark specimens. Ellipses are 90% probability ellipses. Numbers (individual code) correspond to Table 2 and, in detail, to Tables S1–S3
Discussion
In the present study, we provide the most extensive compilation of morphometric measurements and features of three size classes of the basking shark, C. maximus, representing three ontogenetic stages (juveniles, subadults and adults) to date. Further, we provide the first morphological evidence for allometric change in some body regions of C. maximus during ontogeny. Contrary to isometry, allometric change influences the relative shape of morphological structures this way ensuring that a fish is able to cope with the relevant environmental constraints during growth. Therefore, allometric growth not only closely matches specific ecological requirements but also allows shift in resource use, e.g., in feeding (e.g., Gisbert 1999; Karachle and Stergiou 2011; and Richardson et al. 2011) and in locomotion (e.g., Morrow 1950; Irschick and Hammerschlag 2015; and Higham et al. 2018).
We observed negative allometry with increasing body size for structures important for locomotion (caudal fin) and for feeding (head). The observed allometric change can be used to infer unobserved changes in life history, and may, therefore, indirectly provide critical insight into strategies of how species adjust to, e.g., different habitats or to different behavior as they grow during ontogeny (LaBarbera 1989; Carrier 1996; Gratwicke et al. 2006; Carlisle et al. 2015).
We found strong negative allometry in the dorsal lobe of the caudal fin. The results from dorsal caudal fin margin, for which all size groups have high sample sizes, indicate pronounced changes early in ontogeny, i.e., from juveniles to subadults. This indicates a rapid decrease of relative caudal fin length with increasing body length, coinciding with rapid development to a more symmetric caudal fin, contrary to head length, which exhibited a continuous change with increasing TL.
Any change in the dimensions of the propulsion system (tail and caudal fin) will ultimately affect speed, maneuverability, and acceleration (Webb 1984; Blake 2004). In two large predatory sharks, the tiger shark G. cuvier and the white shark C. carcharias, it is widely assumed that the more heterocercal shape of the caudal fin in juveniles conveys the ability for greater relative swimming speed compared to adults (Lingham-Soliar 2005; Irschick and Hammerschlag 2015; Fu et al. 2016). Two potential benefits may, therefore, arise in juveniles. First, escape behavior: negative allometric growth may be an indication of a higher predation pressure on juveniles than on subadults and adults due to differences in body size in large predatory sharks (Carrier 1996; Irschick and Hammerschlag 2015). In this context, the large caudal fin likely enables juvenile basking sharks to escape from large predators such as the white shark, or orca. The large (assumed) size of the newborns (150–180 cm) (Kunzlik 1988; Compagno 2002; Sims 2008) makes them already less vulnerable to smaller predators. Since commercial fishing vessels have only documented non-pregnant females, it is widely assumed that females give birth in deep waters of remote areas (Fowler 2009; Campana et al. 2008), which would explain the rare sighting of juveniles (Kunzlik 1988; Sims et al. 1997; Compagno 2002). It is assumed that basking sharks segregate by sex or maturity (Campana et al. 2008). The latter is indicated by a later appearance of young individuals (< 300 cm TL) than larger individuals (> 400 cm TL) during zooplankton blooms in coastal areas (Sims et al. 1997).
Second, energy expenditure: juveniles and adults share the same food source, and have similar limitations when ram feeding (drag). Smaller specimens will have to maintain a higher rate of tail beats, which might result in higher energetic expenditure (Bainbridge 1958; Webb 1984; Blake 2004). Possibly the more heterocercal fin mitigates some of the expenditure. After all, juveniles need their energy to grow. An energy-saving swimming mode may, therefore, be of advantage. Nevertheless, this remains speculative and needs to be tested.
The juveniles of predatory sharks prey on agile small animals (e.g., fish) (Irschick and Hammerschlag 2015; Fu et al. 2016). This was supported by stomach content analysis on juvenile white sharks, which prey on nearshore pelagic and benthic fishes (Weng et al. 2007). In contrast, a more symmetric caudal fin enables adults to cruise steadily for long distances in search for large prey (e.g., marine mammals) (Lingham-Soliar 2005; Maia et al. 2012). Thereby, negative allometric growth may not only be an indication of differences in trophic niches, but possibly also of a higher predation pressure on juveniles than on adults (Carrier 1996; Irschick and Hammerschlag 2015).
As body size increases in pelagic sharks, tail beat frequency decreases, resulting in reduced swimming speed and hydrodynamic lift which is compensated for by a change in caudal fin morphology. This phenomenon has been observed in a variety of phylogenetic distant aquatic vertebrates such as sharks, billfishes or cetaceans but also in fossil groups such as placoderms and ichthyosaurs (summarized in Ferrón et al. 2017). Additionally, in large fast swimming lamniform sharks, the compensation of lower buoyancy by increase in body size can also be reached by an increase in size of the lipid-rich liver in combination with reduction in tissue densities (Gleiss et al. 2017). [As the head of lamniforms (and other fast swimming pelagic vertebrates, e.g., scombrids or dolphins) is conical, it is negligible as a lift generating structure (Thomson and Simanek 1977)]. In many shark species, the increase of liver volume is subject to positive allometry, and thus increasingly contributing to buoyancy with increasing size (Iosilevskii and Papastamatiou 2016; Gleiss et al. 2017). Positive allometry of liver size was postulated for the basking shark (Gleiss et al. 2017) with adults having livers making up 15–30% of total body volume (Lingham-Soliar 2005; Sims 2008). Nevertheless, no information on liver volume is available for juvenile specimens, and only one measure for subadult basking sharks [11.9% in a 375 cm specimen (Kruska 2004)]. From this available literature, it is obvious that the huge liver volume of basking sharks (Gleiss et al. 2017) can be highly variable (Lingham-Soliar 2005; Sims 2008) and seemingly fluctuating, with specimens caught in areas of low plankton density having a reduced liver volume (Fairfax 1998). This high variability of liver volume to total body mass reported for basking sharks supports the view that the liver holds nutritional reserve, and hence, its volume may potentially strongly fluctuate in adult basking sharks depending on availability of their patchily distributed food source. The relative caudal fin length decreases rapidly, already reaching the adult shape with high aspect ratio in subadult basking sharks. Therefore, the development to a more symmetric caudal fin is possibly not as strongly correlated to the relative increase of liver mass than in other lamniform apex predators. But considering the lack of data especially of non-adult specimens, the potential contribution of liver mass to hydrodynamic lift in basking sharks must remain speculative at this point.
The shape of the caudal fin of basking sharks is unique among lamniforms and can be considered “transitional” between the plesiomorphic heterocercal type (e.g., sand tiger shark or megamouth shark) and a nearly homocercal type (e.g., short fin mako or lemon shark) (Kim et al. 2013). Considering (1) the rapid ontogenetic change of the caudal fin to a fin type suited for sustained swimming, (2) a caudal fin type unique among lamniform sharks, (3) a very elongated body cavity which contains the huge liver (Compagno 2002), (4) an elongate, “cigar shaped” body (Kunzlik 1988) likely not altered in its hydrodynamic efficiency by the huge liver as it is reported for large, fast swimming lamniform sharks (Gleiss et al. 2017), and (5) an obligate ram feeding mode (in contrast, the whale shark and megamouth shark are gulp or suction feeders), the basking shark seemingly occupies a rather unique position within the large pelagic marine vertebrates from an ecomorphological point of view. Taken together, these morphological traits have potentially highly interesting ramifications for basking shark ecophysiology.
It was also assumed that ontogenetic shift in prey and habitat of several shark species may reduce competition of juveniles with adult conspecifics (Ebert 2002; Carlisle et al. 2015). This strategy might strongly apply to the basking shark, a species in which juveniles and adults share the same feeding mode (Kunzlik 1988; Compagno 2002; Sims 2008).
Adult basking sharks maintain average cruising speeds (with mouth closed) of about 1.1 m s−1 (Sims 2000, 2008). This cruising speed is on average about 26% faster compared to large predatory sharks such as the white shark and the mako shark Isurus oxyrinchus Rafinesque 1810, which on average maintain 0.8 m s−1 when cruising, or almost twice as fast as the blue shark Prionace glauca (Linnaeus 1758) and the tiger shark which both on average cruise at a speed of 0.6 m s−1 (Klimley et al. 2002; Bruce et al. 2006; Ryan et al. 2015). Additionally, the relative larger dorsal fins of adult specimens, especially the first dorsal fin, may aid against roll during sustained swimming over long distances as has been reported for many fish species (e.g., Harris 1936; Lauder and Drucker 2004; and Lingham-Soliar 2005) and cetaceans (Fish 2004).
The course of the mean in dorsal caudal fin margin suggests that in basking sharks, the caudal fin changes rapidly during early ontogeny (i.e., in the transition from juveniles to subadults), but only slowly later on in specimens of about 300 cm total length. Interestingly, this happens to be the size at which basking sharks are commonly observed. Indeed, observations of this size class were reported three times more often compared to sightings of juvenile specimens (Lipej and Mavrič 2015), which might be an indication of habitat shift occurring at this size. We speculate that newborn basking sharks, because they are vulnerable to predatory pressure by large predatory sharks or whales, remain in deep water or at least offshore for the first year(s) of their life. However, as no, or only few measurements from subadults were available for the other traits on the caudal fin, conclusions have to remain speculative.
This study provides first morphologic evidence that allometric change occurs in different body regions of the basking shark, C. maximus, during ontogeny. These body regions, head and caudal fin, are first and foremost related to feeding, and locomotion, respectively, and might, therefore, have critical implications for energetic expenditure in these large pelagic sharks. As basking sharks start filter feeding immediately after birth, changes in the shape of the caudal fin, however, are unlikely due to a shift in foraging behavior, as known for some large predatory shark species, but is possibly related to habitat shifts. To verify this assumption, however, further studies on basking shark birthing grounds, as well as the early life history, physiology, and behavior of juveniles will be required. Our study demonstrates that while ecomorphological tools may not replace comprehensive in situ studies on living animals, they can provide important insight into the life history of large roving animals that are challenging to study in their natural habitat, such as the basking shark, and may spark new venues for further research.
References
Ali M, Saad A, Reynaud C, Capapé C (2012) Occurrence of basking shark, Cetorhinus maximus (Elasmobranchii: Lamniformes: Cetorhinidae), off the Syrian coast (Eastern Mediterranean) with description of egg case. Acta Ichthyol Piscat 42:335–339. https://doi.org/10.3750/AIP2012.42.4.07
Bainbridge R (1958) The speed of swimming of fish as related to size and to the frequency and amplitude of the tail beat. J Exp Biol 35:109–133
Bigelow HB, Schroeder WC (1948) Sharks. In: Tee-Van J, Breder CM, Hildebrand F, Parr AE, Schroeder WC (eds) Fishes of the western North Atlantic. Mem Seares Found Mar Res 1. Yale University, New Haven, pp 59–546
Blake RW (2004) Fish functional design and swimming performance. J Fish Biol 65:1193–1222. https://doi.org/10.1111/j.1095-8649.2004.00568
Bruce BD, Stevens JD, Malcolm H (2006) Movements and swimming behaviour of white sharks (Carcharodon carcharias) in Australian waters. Mar Biol 150:161–172. https://doi.org/10.1007/s00227-006-0325-1
Campana SE, Gibson J, Brazner J, Marks L, Joyce W, Gosselin J-F et al (2008). Status of basking sharks in Atlantic Canada. Canadian Science Advisory Secretariat, Research Document 2008/004, vol 66
Capapé C, Hemida F, Bensaci J, Saïdi B, Bradaï MN (2003) Records of basking sharks, Cetorhinus maximus (Gunnerus, 1765) (Chondrichthyes: Cetorhinidae) off the Maghrebin shore (Southern Mediterranean): a survey. Ann Ser Hist Nat 13:13–17
Carlisle AB, Goldman KJ, Litvin SY, Madigan DJ, Bigman JS, Swithenbank AM, Kline TC Jr, Block BA (2015) Stable isotope analysis of vertebrae reveals ontogenetic changes in habitat in an endothermic pelagic shark. Proc R Soc B 282:20141446. https://doi.org/10.1098/rspb.2014.1446
Carrier D (1996) Ontogenetic limits on locomotor performance. Phys Zool 69:467–488
Casadevall M, Suñer Escriche D (1987) Some data on the capture of a specimen of Cetorhinus maximus (Gunner, 1756) (Pisces, Ceterohinidae) at L’Estartit (Torroella de Montgri, Mar Català). Sci Gerund 13:149–151 (In Catalan with English and Spanish abstracts)
Compagno LJV (2002) FAO Species Catalogue for Fisheries Purposes 1. Sharks of the World. An annotated and illustrated catalogue of sharks known to date. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes), vol 2. FAO Species Catalogue for Fisheries Purposes, Rome
de Winter JCF (2013) Using the Student’s t-test with extremely small sample sizes. Practical assessment, research and evaluation 18:1–12. Available online: http://pareonline.net/getvn.asp?v=18&n=10. Accessed 10 Oct 2018
Ebert DA (2002) Ontogenetic changes in the diet of the sevengill shark (Notorynchus cepedianus). Mar Freshw Res 53:517–523. https://doi.org/10.1071/MF01143
Fahmi White WT (2015) First record of the basking shark Cetorhinus maximus (Lamniformes: Cetorhinidae) in Indonesia. Mar Biodivers Rec 8:e18. https://doi.org/10.1017/S1755267214001365
Fairfax D (1998) The basking shark in Scotland. Natural History, Fishery and Conservation. Tuckwell Press
Férron HG, Martínez-Pérez C, Botella H (2017) Ecomorphological inferences in early vertebrates: reconstructing Dunkleosteus terrelli (Arthrodira, Placodermi) caudal fin from palaeoecological data. PeerJ 5:e4081. https://doi.org/10.7717/peerj.4081
Fish FE (2004) Structure and mechanics of nonpiscine control surfaces. IEEE J Ocean Eng 29:60–621. https://doi.org/10.1109/JOE.2004.8332193
Fowler SL (2009) Cetorhinus maximus. IUCN Red List of Threatened Species 2009, e.T4292A10763893. https://doi.org/10.2305/IUCN.UK.2005.RLTS.T4292A10763893.en
Fu AL, Hammerschlag N, Lauder GV, Wilga CD, Kuo CY, Irschick DJ (2016) Ontogeny of head and caudal fin shape of an apex marine predator: the tiger shark (Galeocerdo cuvier). J Morphol 277:556–564. https://doi.org/10.1002/jmor.20515
Gisbert E (1999) Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. J Fish Biol 54:652–852
Gleiss AC, Potvin J, Goldbogen JA (2017) Physical trade-offs shape the evolution of buoyancy control in sharks. Proc R Soc B 284:20171345. https://doi.org/10.1098/rspb.2017.1345
Gould SJ (1966) Allometry and size in ontogeny and physiology. Biol Rev 4:587–640
Gratwicke B, Petrocic C, Speight MR (2006) Fish distribution and ontogenetic habitat preferences in non-estuarine lagoons and adjacent reefs. Eviron Biol Fish 76:191–210. https://doi.org/10.1007/s10641-006-9021-8
Harris JE (1936) Roles of the fins in the equilibrium of the swimming fish. I. Wind-tunnel tests on a model of Mustelus canis (Mitchill). J Exp Biol 13:476–493
Hernández S, Vögler R, Bustamente C, Lamilla J (2010) Review of the occurrence and distribution of the basking shark (Cetorhinus maximus) in Chilean waters. Mar Biodivers Rec 3:ec7. https://doi.org/10.1017/S1755267210000540
Higham TE, Seamone SG, Arnold A, Toews D, Janmohamed Z, Smith SJ, Rogers SM (2018) The ontogenetic scaling of form and function in the spotted ratfish, Hydrolagus colliei (Chondrichthyes: Chimaeriformes): fins, muscles, and locomotion. J Morphol 279:1408–1418. https://doi.org/10.1002/jmor.20876
Iosilevskii G, Papastamatiou YP (2016) Relations between morphology, buoyancy and energetics of requiem sharks. Roy Soc Open Sci 3(10):160406
Irschick DJ, Hammerschlag N (2015) Morphological scaling of body form in four shark species differing in ecology and life history. Biol J Linn Soc (London) 114:126–135
Irschick DJ, Fu A, Lauder G, Wilga C, Kuno C-Y, Hammerschlag N (2017) A comparative morphological analysis of body and fin shape for eight shark species. Biol J Linn Soc (London) 122:589–604
Izawa K, Shibata T (1993) A young basking shark, Cetorhinus maximus, from Japan. Jpn J Ichthyol 40:23–245
Kabaskal H (2013) Rare but present: status of basking shark, Cetorhinus maximus (Gunnerus, 1765) in Eastern Mediterranean. Ann Ser Hist Nat 23:127–132
Karachle PK, Stergiou KI (2011) Mouth allometry and feeding habits of some Mediterranean fishes. Acta Ichthyol Piscat 41:265–275. https://doi.org/10.3750/AIP2011.41.4.02
Kim SH, Shimada K, Rigsby CK (2013) Anatomy and evolution of heterocercal tail in lamniform sharks. Anat Rec 296(3):433–442
Klimley A, Beavers S, Curtis T, Jorgensen S (2002) Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environ Biol Fish 63:117–135
Kruska DCT (2004) The brain of the basking shark (Cetorhinus maximus). Brain Behav Evol 32(6):353–363
Kunzlik PA (1988) The basking shark. Scottish Fish Pam 14:1–21
LaBarbera M (1989) Analyzing body size as a factor in ecology and evolution. Ann Rev Ecol Syst 20:97–117
Lauder GV, Drucker EG (2004) Morphology and experimental hydrodynamics of fish fin control surfaces. IEEE J Ocean Eng 29:556–571. https://doi.org/10.1109/JOE.2004.833219
Lingham-Soliar T (2005) Caudal fin allometry in the white shark Carcharodon carcharias: implications for locomotory performance and ecology. Naturwissenschaften 92:231–236. https://doi.org/10.1007/s00114-005-0614-4
Lipej L, Mavrič B (2015) Juvenile basking shark Cetorhinus maximus caught in waters off Piran (northern Adriatic). New Med Biodiversity Rec. https://doi.org/10.12681/mms.1440
Lipej L, Makovec T, Orlando M (2000) Occurrence of the basking shark, Cetorhinus maximus (Gunnerus, 1765), in the waters off Piran (Gulf of Trieste, Northern Adriatic). Ann Ser Hist Nat 10:211–216
Maia AMR, Wilga CAD, Lauder GV (2012) Biomechanics of locomotion in sharks, rays and chimeras. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC, Boca Raton, pp 125–151. https://doi.org/10.1201/b11867-8
Mathews LH, Parker HW (1950) Notes on the anatomy and biology of the basking shark (Cetorhinus maximus (Gunner)). Proc Zool Soc (London) 120:535–576
Morrow JE (1950) Allometric growth in the striped marlin, Makaira mitsukurii, from New Zealand. Pac Sci 6:53–58
Pauly D (2002) Growth and mortality of the basking shark Cetorhinus maximus and their implications for management of whale sharks Rhincodon typus. In: Fowler SL, Reed TM, Dipper FA (eds) Elasmobranch biodiversity, conservation and management. Occ Paper IUCN spec sure Comm vol 25, pp 129–208
Reiss KL, Bonnan MF (2010) Ontogenetic scaling of caudal fin shape in Squalus acanthias (Chondrichthyes, Elasmobranchii): a geometric morphometric analysis with implications for caudal fin functional morphology. Anat Rec 293:1184–1191
Richardson TJ, Potts WM, Santos CV, Sauer WHH (2011) Ontogenetic shift and morphological correlates for Diplodus capensis (Teleostei: Sparidae) in southern Angola. Afr Zool 46:280–287
Ryan LA, Meeuwig JJ, Hemmi JA, Collin SP, Hart NS (2015) It is just size that matters: shark cruising speeds are species-specific. Mar Biol 162:1307–1318. https://doi.org/10.1007/s00227-015-2670-4
Sims DW (1999) Threshold foraging behavior of basking sharks on zooplankton: life on an energetic knife-edge? Proc R Soc Lond B 266:1437–1443. https://doi.org/10.1098/rspb.1999.0798
Sims DW (2000) Filter-feeding and cruising swimming speeds of basking sharks compared with optimal models: they filter-feed slower than predicted for their size. J Exp Mar Biol Ecol 249(1):65–76
Sims DW (2008) Sieving a living: a review of the biology, ecology and conservation status of the plankton-feeding basking shark Cetorhinus maximus. Adv Mar Biol 54:171–220. https://doi.org/10.1016/S0065-2881(08)00003-5
Sims DW, Fox AM, Merrett DA (1997) Basking shark occurrence off south-west England in relation to zooplankton abundance. J Fish Biol 51(2):436–440
Soldo M, Peharda M, Onofri V, Glavić N, Tutman P (1999) New record and some morphological data of basking shark, Cetorhinus maximus (Gunnerus, 1765), in the eastern Adriatic. Ann Ser Hist Nat 9:229–232
Springer S, Gilbert PW (1976) The basking shark, Cetorhinus maximus, from Florida and California, with comments on its biology and systematics. Copeia 1976:47–54
Thomson KS, Simanek DE (1977) Body form and locomotion in sharks. Am Zool 17(2):343–354
Tomaś ARG, Gomes UL (1989) On the presence of the basking shark, Cetorhinus maximus (Gunnerus, 1765) (Elasmobranchii, Cetorhinidae), in the southeastern and southern Brazil. Boletim do Instituto de Pesca 16:111–116 (In Portuguese with English abstract)
Webb PW (1984) Body form, locomotion and foraging in aquatic vertebrates. Am Zool 24(1):107–120
Weng KC, O’Sullivan JB, Lowe CG, Winkler CE, Dewar H, Block BA (2007) Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar Ecol Prog Ser 338:211–224. https://doi.org/10.3354/meps338211
Acknowledgements
Open access funding provided by University of Vienna.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
435_2019_464_MOESM1_ESM.jpg
Supplementary material 1 Principal component analysis biplot of relative body measurements of 28 basking shark specimens (JPEG 467 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahnelt, H., Sauberer, M., Ramler, D. et al. Negative allometric growth during ontogeny in the large pelagic filter-feeding basking shark. Zoomorphology 139, 71–83 (2020). https://doi.org/10.1007/s00435-019-00464-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-019-00464-2