Abstract
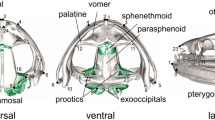
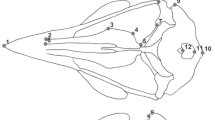
We examined morphological differences in cranium size and shape between closely related snake species, Natrix natrix and Natrix tessellata (Natricinae, Colubroidea), as well as variation within species. These two snake species have similar ecology and habitat preferences but differ in feeding strategies. Our hypothesis was that divergence in size and shape of cranial elements between species depends on their functional role and anatomical relationships. To analyse complex, kinetic crania, we applied computed microtomography and 3D geometric morphometrics. We analysed size and shape of six cranial elements separately. We selected two “non-trophic” structures (akinetic braincase and mobile nasals) and four movable “trophic” skeletal elements (maxillae, quadrates, pterygoids and compound bones) which are involved in prey capture and swallowing. Our results showed that N. natrix and N. tessellata significantly differ in size and shape of all analysed cranial elements. In both species, cranium is significantly larger in females than in males. To account for possible differences in shape due to differences in size, we estimated allometric and non-allometric component of shape variation. For all elements, except nasals, allometry accounted for a significant proportion of the variance in shape. The analysis of non-allometric component of shape variation revealed significant dimorphism in shape of the braincase and maxilla between N. tessellata females and males, and marginally significant sexual dimorphism in shape of maxilla in N. natrix. These results indicated that sexual dimorphism in skull shape is species specific and not entirely caused by selection for larger size in females.




Similar content being viewed by others
References
Adams DC, Rohlf FJ (2000) Ecological character displacement in Plethodon: biomechanical differences found from a geometric morphometric study. Proc Natl Acad Sci 97:4106–4111. doi:10.1073/pnas.97.8.4106
Arnold SJ (1993) Foraging theory and prey size-predator size relations in snakes. In: Seigel RA, Collins JT (eds) Snakes: ecology and behavior. McGraw-Hill, New York, pp 87–115
Beebe T, Griffiths RA (2000) Amphibians and reptiles: a natural history of the British herpetofauna. Harper Collins, London
Bellairs ADA (1949) The anterior brain-case and interorbital septum of Sauropsida, with a consideration of the origin of snakes. J Linn Soc Lond Zool 41:482–512. doi:10.1111/j.1096-3642.1940.tb02418.x
Boltt R, Ewer R (1964) The functional anatomy of the head of the puff adder, Bitis arietans (Merr.). J Morphol 114:83–105. doi:10.1002/jmor.1051140105
Borczyk B (2015) Allometry of head size and shape dimorphism in the grass snake (Natrix natrix L.). Turk J Zool 39:340–343. doi:10.3906/zoo-1402-9
Camilleri C, Shine R (1990) Sexual dimorphism and dietary divergence: differences in trophic morphology between male and female snakes. Copeia 1990:649–658. doi:10.2307/1446430
Cox RM, Skelly SL, John-Alder HB (2003) A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 57:1653–1669. doi:10.1111/j.0014-3820.2003.tb00371.x
Cundall D (1983) Activity of head muscles during feeding by snakes: a comparative study. Am Zool 23:383–396. doi:10.1093/icb/23.2.383
Cundall D (2000) Drinking in snakes: kinematic cycling and water transport. J Exp Biol 203:2171–2185
Cundall D, Gans C (1979) Feeding in water snakes: an electromyographic study. J Exp Zool 209:189–207. doi:10.1002/jez.1402090202
Cundall D, Greene HW (2000) Feeding in snakes. In: Schwenk K (ed) Feeding: form, function, and evolution in Tetrapod vertebrates. Academic Press, San Diego, pp 293–333
Darwin C (1971) The descent of man and selection in relation to sex. Murray, London
Dryden IL, Mardia KV (1998) Statistical shape analysis. Wiley, New York
Dwyer CM, Kaiser H (1997) Relationship between skull form and prey selection in the thamnophiine snake genera Nerodia and Regina. J Herpetol 31:463–475. doi:10.2307/1565597
Fitch HS (1981) Sexual size differences in reptiles. Misc Publ Mus Nat Hist Univ Kans 70:1–72
Forsman A, Lindell L (1993) The advantage of a big head: swallowing performance in adders, Vipera berus. Funct Ecol 7:183–189. doi:10.2307/2389885
Forsman A, Shine R (1997) Rejection of non-adaptive hypotheses for intraspecific variation in trophic morphology in gape-limited predators. Biol J Linn Soc 62:209–223. doi:10.1111/j.1095-8312.1997.tb01623.x
Gans C (1961) The feeding mechanism of snakes and its possible evolution. Am Zool 1:217–227. doi:10.1093/icb/1.2.217
Gentilli A, Cardini A, Fontaneto D, Zuffi MAL (2009) The phylogenetic signal in cranial morphology of Vipera aspis: a contribution from geometric morphometrics. Herpetol J 19:69–77
Gloyd HK, Conant R (1990) Snakes of the Agkistrodon complex: a monographic review. Society for the Study of Amphibians and Reptiles (SSAR), Oxford
Good P (1994) Permutation tests: a practical guide to resampling methods for testing hypotheses. Springer, New York
Gregory PT, Isaac LA (2004) Food habits of the grass snake in southeastern England: is Natrix natrix a generalist predator? J Herpetol 38:88–95. doi:10.1670/87-03A
Gruschwitz M, Lenz S, Mebert K, Lanka V (1999) Natrix tessellata (Laurenti, 1768)–Würfelnatter. In: Bohme W (ed) Handbuch der reptilien und amphibien Europas. AULA-Verlag, Wiesbaden, pp 581–644
Guicking D, Lawson R, Joger U, Wink M (2006) Evolution and phylogeny of the genus Natrix (Serpentes: Colubridae). Biol J Linn Soc 87:127–143. doi:10.1111/j.1095-8312.2006.00561.x
Hampton PM (2011) Comparison of cranial form and function in association with diet in natricine snakes. J Morphol 272:1435–1443. doi:10.1002/jmor.10995
Hanken J, Hall BK (1993) Mechanisms of skull diversity and evolution. In: Hanken J, Hall BK (eds) The skull. Functional and evolutionary mechanisms, vol 3. The University of Chicago Press, Chicago, pp 1–36
Herrel A, Schaerlaeken V, Meyers JJ, Metzger KA, Ross CF (2007) The evolution of cranial design and performance in squamates: consequences of skull-bone reduction on feeding behavior. Integr Comp Biol 47:107–117. doi:10.1093/icb/icm014
Herrel A, Vincent S, Alfaro M, Van Wassenbergh S, Vanhooydonck B, Irschick DJ (2008) Morphological convergence as a consequence of extreme functional demands: examples from the feeding system of natricine snakes. J Evolut Biol 21:1438–1448. doi:10.1111/j.1420-9101.2008.01552.x
Hibbitts TJ, Fitzgerald LA (2005) Morphological and ecological convergence in two natricine snakes. Biol J Linn Soc 85:363–371. doi:10.1111/j.1095-8312.2005.00493.x
Kabisch K (1999) Natrix natrix (Linnaeus, 1758)–Ringelnatter. In: Bohme W (ed) Handbuch der reptilien und amphibien Europas. AULA-Verlag, Wiesbaden, pp 513–580
Kardong KV (1977) Kinesis of the jaw apparatus during swallowing in the cottonmouth snake, Agkistrodon piscivorus. Copeia 1977:338–348. doi:10.2307/1443913
Kardong KV (1979) “Protovipers” and the evolution of snake fangs. Evolution 33:433–443. doi:10.2307/2407632
Kardong KV (1980) Evolutionary patterns in advanced snakes. Am Zool 20:269–282. doi:10.1093/icb/20.1.269
King RB (1989) Body size variation among island and mainland snake populations. Herpetologica 45:84–88
King RB (2002) Predicted and observed maximum prey size–snake size allometry. Funct Ecol 16:766–772. doi:10.1046/j.1365-2435.2002.00678.x
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357. doi:10.1111/j.1755-0998.2010.02924.x
Klingenberg CP, Barluenga M, Meyer A (2002) Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56:1909–1920. doi:10.1111/j.0014-3820.2002.tb00117.x
Kramer E (1980) Zum Skelett der Aspisviper, Vipera aspis (Linnaeus, 1758). Rev Suisse Zool 87:3–16
Krause MA, Burghardt GM, Gillingham JC (2003) Body size plasticity and local variation of relative head and body size sexual dimorphism in garter snakes (Thamnophis sirtalis). J Zool 261:399–407. doi:10.1017/S0952836903004321
Luiselli L, Rugiero L (1991) Food niche partitioning by water snakes (genus Natrix) at a freshwater environment in central Italy. J Freshw Ecol 6:439–444. doi:10.1080/02705060.1991.9665323
Madsen T (1983) Growth rates, maturation and sexual size dimorphism in a population of grass snakes, Natrix natrix, in southern Sweden. Oikos 40:277–282. doi:10.2307/3544592
Mebert K (2011) The dice snake, Natrix tessellata: biology, distribution and conservation of a palaearctic species. Mertensiella. Deutsche Gesellschaft fur Herpetologie und Terrarienkunde (DGHT), Rheinbach
Mertens R (1947) Studien zur eidonomie und taxonomie der ringelnatter (Natrix natrix). Abh Senck- Enbergischen Naturforschenden Ges 476:1–38
Mori A, Vincent SE (2008) An integrative approach to specialization: relationships among feeding morphology, mechanics, behaviour, performance and diet in two syntopic snakes. J Zool 275:47–56. doi:10.1111/j.1469-7998.2007.00410.x
Olsson M, Shine R, Wapstra E, Ujvari B, Madsen T (2002) Sexual dimorphism in lizard body shape: the roles of sexual selection and fecundity selection. Evolution 56:1538–1542. doi:10.1111/j.0014-3820.2002.tb01464.x
Polachowski KM, Werneburg I (2013) Late embryos and bony skull development in Bothropoides jararaca (Serpentes, Viperidae). Zoology 116:36–63. doi:10.1016/j.zool.2012.07.003
Pough FH, Groves JD (1983) Specializations of the body form and food habits of snakes. Am Zool 23:443–454. doi:10.1093/icb/23.2.443
Rieppel O (1980) The evolution of the ophidian feeding system. Zool Jahrbucher Abt Anat Ontog Tiere 103:551–564
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rieppel O (2007) The naso-frontal joint in snakes as revealed by high-resolution X-ray computed tomography of intact and complete skulls. Zool Anz J Comp Zool 246:177–191. doi:10.1016/j.jcz.2007.04.001
Schoener TW (1977) Competition and the niche. In: Gans C, Tinkle DW (eds) Biology of the Reptilia. Academic Press, London, pp 35–136
Shine R (1986) Sexual differences in morphology and niche utilization in an aquatic snake, Acrochordus arafurae. Oecologia 69:260–267. doi:10.1007/BF00377632
Shine R (1989) Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol 64:419–461. doi:10.1086/416458
Shine R (1991) Why do larger snakes eat larger prey items? Funct Ecol 5:493–502. doi:10.2307/2389631
Shine R (1993) Sexual dimorphism in snakes. In: Seigel RA, Collins JT (eds) Snakes: ecology and behavior. McGraw-Hill, New York, pp 49–86
Shine R (1994) Sexual size dimorphism in snakes revisited. Copeia 1994:326–346. doi:10.2307/1446982
Shine R, Olsson MM, Lemaster MP, Moore IT, Mason RT (2000) Effects of sex, body size, temperature, and location on the antipredator tactics of free-ranging gartersnakes (Thamnophis sirtalis, Colubridae). Behav Ecol 11:239–245. doi:10.1093/beheco/11.3.239
Slatkin M (1984) Ecological causes of sexual dimorphism. Evolution 38:622–630. doi:10.2307/2408711
Smith KK (1993) The form of the feeding apparatus in terrestrial vertebrates: studies of adaptation and constraint. In: Hanken J, Hall BK (eds) The skull. Functional and evolutionary mechanisms, vol 3. The University of Chicago Press, Chicago, pp 150–196
Šukalo G, Đorđević S, Gvozdenović S, Simović A, Anđelković M, Blagojević V, Tomović L (2014) Intra-and inter-population variability of food preferences of two Natrix species on the Balkan peninsula. Herpetol Conserv Biol 9:123–136
Thorpe R (1979) Multivariate analysis of the population systematics of the ringed snake, Natrix natrix (L.). Proc R Soc Edinb Sect B Biol Sci 78:1–62. doi:10.1017/S026972700001294X
Vincent SE, Herrel A (2007) Functional and ecological correlates of ecologically-based dimorphisms in squamate reptiles. Integr Comp Biol 47:172–188. doi:10.1093/icb/icm019
Vincent S, Brandley M, Herrel A, Alfaro M (2009) Convergence in trophic morphology and feeding performance among piscivorous natricine snakes. J Evol Biol 22:1203–1211. doi:10.1111/j.1420-9101.2009.01739.x
Acknowledgments
We thank anonymous reviewer for helpful comments on earlier version of the manuscript. This research was financed by the Ministry of Education, Science and Technological Development of Republic of Serbia (Grant No. 173007 and 173043). AI acknowledges grants from SyntheSys (NL-TAF 3082 and 3926) and a Naturalis Biodiversity Center “Temminck fellowship”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 3931 kb)
Supplementary material 2 (AVI 3987 kb)
Rights and permissions
About this article
Cite this article
Andjelković, M., Tomović, L. & Ivanović, A. Variation in skull size and shape of two snake species (Natrix natrix and Natrix tessellata). Zoomorphology 135, 243–253 (2016). https://doi.org/10.1007/s00435-016-0301-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0301-3