Abstract
We examined the cranial morphology and cranial kinesis of the common toads Bufo bufo and B. spinosus with micro-computed tomography and geometric morphometrics and compared the results with published data for related species in a phylogenetic context. The species significantly diverge in skull shape. The skull of B. spinosus is shorter and higher, with a ventral arm of the squamosal bone and the jaw articulation point positioned perpendicular to the braincase, in comparison with a more lateral position in B. bufo. In either species, females have a shorter snout and a higher and wider skull at the jaw articulation point that is positioned more posteriorly, in comparison with conspecific males. High variation in the amount of bone ossification was recorded in both species, ranging from scarcely ossified and loosely connected bones to highly ossified and firmly connected bones. We also found that skull shape and inferred kinetic properties of the skull are highly variable across the Bufonini tribe. However, sample sizes are mostly small and intraspecific variation is high, which might compromise the analyses. Overall, the results suggest that developmental plasticity produces high variation in ossification and cranial kinesis, affecting individuals’ feeding performances. At the population level, this variation supports an efficient exploitation of the habitat and may promote morphological adaptation in a changing environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vertebrate skull is an anatomically and developmentally complex skeletal structure with multiple functions. The skull form (size and shape) may be constrained by development and by descent and by a variety of mechanical demands imposed by its functional role in perception, food gathering and vocalisation as well as locomotion and defence (Hanken & Hall, 1993). For several groups it has been shown that skull shape reflects selection pressures from functional requirements, such as food intake, in mammals (Galatius et al., 2020; Van Cakenberghe et al., 2002; Wroe & Milne, 2007), birds (Felice et al., 2019), snakes (Andjelković et al., 2016; Klaczko et al., 2016) and frogs (Paluh et al., 2020). Another property of the vertebrae skull that can be directly related with feeding is cranial kinesis. Cranial kinesis denotes the mobility between cranial elements, not including the articulation of the lower jaw (e.g. Kardong, 1977; Herrel et al., 2007). As here applied to anuran amphibians it refers to just the mobility of the upper jaw and the palate relative to the braincase (Iordansky, 1989).
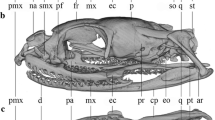
The description of the anuran skull morphology is necessarily general, due to the remarkable diversity and variation in the development of skull skeletal elements (Hanken & Hall, 1984; Herrel et al., 2019; Smirnov, 1990, 1994; Trueb, 1973, 1985; Trueb & Alberch, 1985; Weisbecker & Mitgutsch, 2010). Anurans have a broad, dorsoventrally flattened and fenestrated skull, with a posterior position of the jaw articulation joints. The bones of all three skull complements (neurocranium, dermatocranium and viscerocranium) are largely reduced. The dermal complement consists of the dorsal roofing bones (paired nasals and frontoparietals) and the ventral palatal bones (paired vomers, palates and pterygoids and medial parasphenoid). The neurocranium consist of the sphenethmoid and paired prootics and exoccipitals (Fig. 1). Laterally the ventral arm of squamosal bone reaches the quadrate and its upper arm (the otic ramus) reaches the otic region of the braincase. The quadrate (an element of the viscerocranium) articulates to the lower jaw and together with the pterygoid and squamosal it forms the suspensorium, i.e. the structure that connects the jaw to the braincase. Additional functional support is provided by the palatine that stabilises and connects the upper jaw to the braincase (Roček, 2003; Trueb, 1973; Trueb et al., 1993). The existence of distinct developmental stages (aquatic larvae and metamorphosed juveniles and adults) requires distinct functional adaptations. During metamorphosis an extensive tissue remodelling takes place, including the de novo formation of skull bones, and the full (adult) complement of cranial bones does not form until after metamorphosis is complete (Hanken & Gross, 2005; Kerney et al., 2012). However, the skull morphologies of adults may be affected by diet specialisation of larvae (Herrel et al., 2019, and references therein). The anuran skulls may further diversify in shape and ossification level during juvenile and adult growth (Ponssa & Vera Candioti, 2012). Moreover, the level of ossification of the skull varies from cartilaginous to largely ossified, adding to the disparity in anuran cranial morphology (Paluh et al., 2020).
The skull of Bufo bufo in dorsal, ventral and lateral view. Cranial bones are labelled for the neurocranium (in green), the dermatocranium (in grey) and the suspensorium (in yellow). Note the full set of 12 bilaterally symmetric landmarks that was used for analyses of divergences between B. bufo and B. spinosus species and sexes. A reduced set of six bilaterally symmetric landmarks (marked by solid dots) was used for the phylogeny-based analyses of 50 Bufonini species
In terms of cranial kinesis, the anuran skull can be classified as rhynchokinetic and/or pleurokinetic. Rhynchokinesis describes the mobility of the snout, particularly on account of the mobility of the premaxilla. In anurans this mobility allows the closing and opening of the nostrils (Iordansky, 1989). Pleurokinesis describes the mobility of the suspensorium and characterise all lissamphibians (De Villiers, 1938; Iordansky, 2000; Wake & Hanken, 1982). This mobility involves small-scale movements of the upper jaw and palate relative to the braincase and allows for greater flexibility. Conversely, ossification tightens the inter-bone connections and reduces cranial kinesis (Iordansky, 2000; Wake & Hanken, 1982). The skull shape across the family Bufonidae is largely conserved, notwithstanding some major evolutionary changes including ear loss and the reduction of the columella bone (Womack et al., 2018a, b). The genus Bufo is characterised by a well-ossified skull, which may in some species co-ossify with the superficial skin (Duellman & Trueb, 1994; Paluh et al., 2020). Cranial kinesis across the family is less well studied, but the reduction of pleurokinesis due an increase in ossification during postmetamorphic growth has been described only for Rhinella marina (also known as Bufo marinus).
Here, we explore the skull morphology and between-bone connections of two toad species, the common toad, Bufo bufo (Linnaeus, 1758) and the spined toad, B. spinosus Daudin, 1803. Genus Bufo is positioned within the family Bufonidae (true toads), subfamily Bufoninae and within the Bufonini tribe. The Bufonini tribe holds four highly supported branches including the subtribe Bufonina, which comprise genus Bufo (Dubois, Ohler, & Pyron, 2021).
Bufo spinosus is present in North Africa, the Iberian Peninsula and the southwest of France, whereas B. bufo has a large range extending from France deep into Scandinavia, Russia and Turkey. These species are morphologically and ecologically similar, yet genetically well differentiated, with independent evolutionary histories since the Late Miocene (Recuero et al., 2012; Arntzen et al., 2013a, b). The external morphology of these toads is variable which hampers species identification (Cvetković et al., 2009; Gingras et al., 2013; Lüscher et al., 2001), although they can be told apart by the degree of posterior divergence of the parotoid glands and by the size and shape of the metatarsal tubercle, at least in some regions (Arntzen et al., 2013b, 2018, 2020). Recent studies described some divergences in skull shape between the sexes in B. bufo (Üzüm et al., 2021) and between B. bufo and B. spinosus (Sanna, 2019). There is a large variation in size, especially in B. bufo (Cvetković et al., 2009) with larger body sizes in the south than in the north and with females being larger than males. Despite this marked variation in size, similar diets were recorded, not only for sexes and populations (Crnobrnja-Isailović et al., 2012), but also between B. bufo and B. spinosus (Vallvé & Sánchez-Iglesias, 2018), indicating that they are opportunistic feeders.
To explore differences that might exist between B. bufo and B. spinosus, we gathered and analysed data with micro‐CT scanning and geometric morphometrics. Micro‐CT scanning allows the detailed reconstruction of between-bone interconnections that form the morphological basis to the kinetic properties of the skull (Natchev et al., 2016; Waltenberger et al., 2021). We here provide an in-depth analysis of interspecific differences and sexual dimorphism of B. bufo and B. spinosus in skull size and shape, ossification level and cranial kinesis. To estimate the amount and directions of evolutionary changes in skull between B. bufo and B. spinosus relative to other toad species, we integrate our results on common toads with those for other species in the Bufonini tribe using published three-dimensional models (Womack et al., 2018a, b) under reference to species phylogenetic relationships (Jetz & Pyron, 2018).
Materials and methods
Three-dimensional models of toad skulls were obtained for 27 adult B. bufo individuals (13 females and 14 males) and 18 B. spinosus (nine females and nine males), preserved in ethanol at the Naturalis Biodiversity Center, Leiden, The Netherlands (Supplementary Table S1). Specimens were scanned with the Carl Zeiss Xradia Versa 520 (Carl Zeiss X-Ray Microscopy, Inc., Pleasanton, CA, USA), with 80-kV source voltage and 86–88-mA intensity. The surface 3D model reconstructions of skulls were made with Avizo 9.5 software (FEI, Thermo Fisher Scientific), with some missing data in the case of incomplete skulls (see Supplementary Table S1). To explore the divergence in skull morphology of common toads relative to other toad species, we included 87 skull models of 48 species from Bufonini tribe available from https://doi.org/10.5061/dryad.6tn2n (Womack et al., 2018a, b). In this sample nine species were represented by a single individual and 39 species were represented by two individuals, all with sexes unknown.
Skull size and shape analyses were based on three-dimensional landmark configurations obtained directly from individual surface models using IDAV Landmark version 3.0 (http://graphics.idav.ucdavis.edu/research/EvoMorph). To represent anatomical positions that cover the skull entirely, we applied 12 bilaterally symmetric landmarks amounting to 24 landmarks in total. For comparisons with other toad species, we used a reduced set of six bilaterally symmetrical landmarks, so 12 landmarks in total (Fig. 1). Brief anatomical descriptions of the landmarks are provided in Supplementary Table S2. Skull size was expressed as centroid size (CS) that was calculated as the square root of the sum of squared distances from the centroid. A general Procrustes analysis was performed and the symmetric component was calculated as the averages of the original and mirrored landmark configurations in order to remove the redundancy amongst the bilaterally homologous landmarks (Dryden & Mardia, 1998; Klingenberg et al., 2002; Rohlf & Slice, 1990).
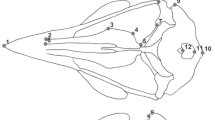
A factorial analysis of variance (ANOVA) was used to test the contribution of independent factors (species and sex) and their interaction (species × sex) to differences in skull size, along with Tukey’s HSD post hoc test. A principal component (PC) analysis was performed to explore the differences in skull shape between species and sexes. Because of relatively small sample sizes, the PC scores of nine principal components which describe approximately 85% of total shape variation were used as dependent variables in a multivariate factorial analysis of variance (MANCOVA) instead of the original variables. The MANCOVA was performed with species and sex as independent grouping variables and with skull size as covariate. Statistical tests were done using R version 4.0.2 (R Core Team, 2021). Principal component analyses and skull shape change visualisation were done using the geomorph package version 4.0 (Adams et al., 2021). We examined five between bones connections (Fig. 2) that together are taken as a measure of cranial kinesis. We scored character states as either unconnected (value 0) or connected (value 1) and summed these values to obtain an individual’s cranial kinesis score (CKi, range 0–5). The Fisher’s exact test for count data was performed to test for statistical significance in character state frequency distribution using R version 4.0.2 (R Core Team, 2021).
Variation in the degree of ossification of the parasphenoid and prootic in common toads. The arrows indicate the adjacent cranial bones for which character states (‘connected’ or ‘unconnected’) were scored as follows: prootic to exoccipital (P-E), prootic to squamosal (P-Sq), sphenethmoid to parasphenoid (Sp-Ps), sphenethmoid to palatine (Sp-Pa) and exoccipital to parasphenoid (E-Ps)
To explore the amount of ossification and cranial kinesis in a phylogenetic context, we scored the previously described between-bone connections (Fig. 2) on the models from Womack et al. (2018b) dataset. The between-bone connections were scored binary as described above, with the value 0.5 assigned when both character states were recorded within a species. The sum of scores represents a measure of ossification and cranial kinesis at the species level (CKs). The phylogenetic relationships of altogether 50 representatives of the tribe Bufonini (Dubois et al., 2021) with morphological data available were represented by a tree that was constructed by pruning the amphibian Tree of Life (Jetz & Pyron, 2018). For this we used the extraction tool in https://vertlife.org/phylosubsets/. Five-thousand trees from the pseudo-posterior distribution of trees were downloaded and a maximum clade credibility consensus tree was constructed with TreeAnnotator version 1.10.1 (distributed as part of the BEAST software package; Suchard et al., 2018) and used at all downstream analyses. The species average of symmetric component of shape variation (data for one species missing) and CKs were mapped onto the phylogenetic tree. The strength of phylogenetic signal for skull shape was calculated using Blomberg’s K statistics (Blomberg et al., 2003) adapted for highly multivariate data (Adams, 2014). The strength of phylogenetic signal for CKs was tested using the phytools package version 1.0–3 (Revell, 2012). For major clades, including the Atelopodina with nine species, Phryniscities with 20 species, Bufonities with 12 species, and Stephopaedities with six species, the amount of between-species variation or morphological disparity (MD) in skull shape and CKs was calculated and tested by pairwise permutation tests. These analyses were done in R using the geomorph version 4.0 (Adams et al., 2021) and RRPP packages version 1.3.0 (Collyer & Adams, 2021) for skull shape and with the bootstrap version of Levene’s test in the lawstat package version 3.4 for CKs (Gastwirth et al., 2020).
Results
Skull size and shape
A factorial ANOVA showed that the species and sexes significantly differed in skull size with a significant species × sex interaction (Fspecies = 49.37, P < 0.0001; Fsex = 56.38, P < 0.0001 and Fspecies × sex = 17.34, P = 0.0002). The sexual dimorphism in skull size was confirmed by post hoc tests, with females possessing larger skulls than males and with a CS ratio of 1.17 in B. bufo (P = 0.0134) and 1.47 in B. spinosus (P < 0.0001). Bufo spinosus females were larger than B. bufo females with a CS ratio = 1.41 (P < 0.0001). No differences were found between males of both species (CS ratio = 1.13, P = 0.1316).
The MANCOVA test showed a significant impact of size, species, and sex on skull shape variation. A non-significant species × sex interaction and significant CS × sex and CS × species interactions were recorded, indicating that species and sexes diverge in the pattern of size-related shape changes (Table 1).
The position of individuals along the first and second PC axes indicated a difference in skull shape amongst species and sexes. Species were best separated along the first axis (that described 26.26% of the total variation) and sexes were best separated along the second axis (20.10%) (Fig. 3). This figure also illustrates the mean skull shape for species and sexes, highlighting that the skull of B. spinosus is higher, with a ventral arm of squamosal bone and the jaw articulation point perpendicular to the braincase compared to more lateral position in B. bufo. The dorsal arm of the squamosal bone (otic ramus) is positioned laterally, more distant from the mid-dorsal line in B. spinosus than in B. bufo. Females of both species have a shorter snout and slightly wider and higher skulls at the jaw articulation point that has a more posterior position compared to males (Fig. 3).
Bivariate plot on skull shape variables in Bufo bufo (blue symbols) and B. spinosus (red symbols) over the first (PC1) and second axes (PC2) of a principal component analysis, with females shown by solid symbols and males by open symbols. The amount of variation explained over the axes is shown within parentheses. The shape changes along PC axes are summarised by wireframe graphs depicting the dorsal (top row), posterior (middle row) and lateral (bottom row) skull shape. The images of 3D models showing mean skull shape were presented
Bone connections and ossification of skull elements
The bone connection characters showed a marked variation (Fig. 4; Table 2), with no significant differences between species and sexes (P > 0.05 in all comparisons). The cranial kinesis (CKi) was also highly variable (Table 3) with significant differences for species and sexes (Fisher’s exact test, P = 0.035). This test also indicated a significant difference between B. spinosus females and males (P = 0.032) on account of the absence of the between-bone connections in two females (CKi = 0; Table 3).
The degree of bone connectedness in Bufo bufo skulls illustrated in ventral view. The individuals with different cranial kinesis scores (CKi) are shown as follows: left CKi = 1, middle CKi = 2 and right CKi = 5. Arrows point to connected bones. The abbreviations are the same as in Fig. 2. Note that ossification increases with increasing CKi values. The three individuals shown are males from England, Leicestershire, Gaddesby
Morphological differentiation of common toads relative to other species
In the wider set of taxa the four Bufo species (apart from B. bufo and B. spinosus these are B. japonicus and B. bankorensis) had a broad skull and a wide snout (see shapes described by PC1 in Fig. 5) and represented a small amount of the variation documented for the subclade Bufonities or the entire Bufonini tribe (Fig. 5). The lowest level of disparity in skull shape was found in the Atelopodina (MD = 0.004) and the highest level was found in the Bufonities (MD = 0.010), whereas the Stephopaedities and the Phryniscities presented intermediate values (MD = 0.009 and MD = 0.007, respectively). A marginally insignificant difference in disparity was found for the Bufonities versus Atelopodina (P = 0.0501).
The first and second principal components of average skull shape of 49 toad species. The shape changes are summarised by wireframe graphs along the axes. Recognised clades are the Atelopodina in brown, Phryniscities in orange, Bufonities in green and Stephopaedities in purple. Four species that are outside these clades are shown by white symbols. For clades and species names see Fig. 6
The ossification scores across the Bufonini tribe ranged from CKs = 1.5 (a kinetic skull) to CKs = 5 (a rigid skull). The lowest scores were recorded for Wolterstorffina parvipalmata in the Bufonities and Amietophrynus mauritanicus in the Stephopaedities whereas high scores were found in many species, especially amongst the Atelopodina and the Phryniscities (Fig. 6). A high level of variation in the data was suggested by the observation that amongst 39 species with a sample size of two, ten are polymorphic. The disparity in CKs varied from MD = 1.21 in the Bufonities to MD = 0.48 in the Atelopodina with no significant difference between groups (P > 0.05 in all comparisons). Blomberg’s K for the phylogenetic signal in skull shape shows a slight deviation from a Brownian motion model (K = 0.9, P < 0.001) and a larger deviation for ossification scores (K = 0.5, P < 0.01), indicating a tendency for less signal than expected under a Brownian motion model.
Phylogenetic tree of 50 toad species in the tribe Bufonini with cranial kinesis character states (CKs) plotted over the tree (for details see text). The values of CKs range from 1.5 (in deep red) indicating a flexible skull to 5 indicating a rigid skull (in deep blue), with intermediate values shown by intermediate colours (see colour bar). The ancestral states over the tree were interpolated by a Brownian model of evolution under the likelihood criterion. Tree depth is proportional to time. The two species that we studied are indicated by an arrow
Discussion
The interspecific divergences and sexual dimorphism in skull shape and cranial kinesis in common toads
We observed significant differences in skull size and shape between species and sexes as well as a high level of variation in ossification and cranial kinesis of common toads (B. bufo and B. spinosus). In some individuals the suspensorium and braincase were poorly ossified and loosely connected, whereas in others, they were largely ossified and firmly connected forming rigid skulls (Fig. 4). Studied populations showed the entire range of cranial kinesis (Fig. 4 and Table 3) with some species- and sex-specific differences. For example, a movable connection between the exoccipital to parasphenoid was only found in B. spinosus females. The intraspecific variation might not have been uncovered if sample sizes had been small, as is not uncommon in present-day micro‐CT scanning studies (Natchev et al., 2016), especially when the number of species is large (e.g. Bardua et al., 2021; Paluh et al., 2020). In spite of this variation, skull shapes of species (B. bufo and B. spinosus) and males and females within species were significantly different. Species and sexes differ most in the relative position of the jaw articulation point and the depth of the skull (Fig. 3).
Even small differences in skull shape and cranial kinesis can have direct functional consequences, in particular on feeding performance (Hanken & Hall, 1983). An increase in cranial kinesis allows the processing of larger pray, whereas firmly connected, robust constructions provide better muscle support (Herrel et al., 2007). Feeding involves coordinated movements of the cranium, the lower jaw, hyoid and tongue. Toads do not chew their prey and have limited tongue protraction which is directly associated with the lower jaw movements (Deban et al., 2001, Nishikawa & Guns, 1992, 1996). Eye retraction is an important accessory mechanism that assists the primary tongue-based swallowing (Levine et al., 2004). Common toads feed mostly on small- to medium-sized prey including beetles, ants, leaches and worms (Cornish et al., 1995; Crnobrnja-Isailović et al., 2012), but intake may vary depending on pray availability. Occasionally larger and hard prey is devoured, such as molluscs, lizards and mice (Sinsch et al., 2009; Vallvé & Sánchez-Iglesias, 2018). In B. bufo a dietary niche partitioning in pray size was found, with females consuming medium-sized pray in larger quantities compered to predominantly smaller sized pray in males (Crnobrnja-Isailović et al., 2012). For food generalists such as common toads, the observed high variation in cranial morphology may help to efficiently exploit their habitat. A wide head and a loose construction of the skull would increase their capability to process large prey, but might also affect feeding performance negatively, in particular bite force, due to a reduced muscle support (Herrel et al., 2007). The increase in cranial kinesis probably does not affect the eye-retraction swallowing mechanism, because the m. retractor bulbi that moves the eyeballs into the buccal cavity is attached to the generally well-ossified, posterior part of parasphenoideum (Witzmann & Werneburg, 2017).
Phylogenetic aspects of the toad skull morphology
The divergence in skull shape of Bufo bufo and B. spinosus is small in comparison with the shape changes inferred for the Bufonini tribe and the subclade Bufonities (including the genus Bufo) in particular (Figs. 5 and 6). The species also have high intraspecific variation in cranial kinesis. This similarity may be due to the species close phylogenetic relatedness as well as to their limited ecological differentiation (Arntzen et al., 2013a, b, 2020). To this it may be noted that in ten species of the subclade Bufonities, when just two individuals were studied, variation in cranial kinesis was nevertheless observed.
In amphibians, developmental trajectories may vary within species (Roček, 2003) and developmental plasticity plays an important role in the evolution of the cranial skeleton (Trueb & Alberch, 1985; Duellman & Trueb, 1994; Smirnov, 1997; Weisbecke & Mitgutsch, 2010; Bardua et al., 2021). For anurans it has been suggested that diversity in ossification sequences and loss of ossification are unrelated to changes in skull shape (Trueb, 1993). Conversely, recent studies found that ossification sequences (Bardua et al., 2021) and change in the ossification level (Paluh et al., 2020) may both affect evolutionary changes and diversity of the anuran skull. Either way, given the observed intraspecific variation, the role of developmental plasticity has to be taken into account which requires large sample sizes.
In line with other authors (Levis & Pfennig, 2019; West-Eberhard, 2003, 2005), we suggest that the high developmental plasticity which produces a wide range of cranial morphologies may be adaptive and that it would increase evolvability. Testing these hypotheses would involve a biomechanical study of individuals and species with different levels of ossification and connectedness of the skull bones. The pronounced divergences in shape and the high intraspecific and interspecific variation in the kinetic properties of the skull make toads a promising model for the study of functional morphology.
Data availability
The skull models available at https://datadryad.org/stash/share/a7wJ4WjrBjSwGqvFKr7pRF8hHF0gLYXh4Tck7he2wM0.
References
Adams, D. C. (2014). A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology, 63(5), 685–697. https://doi.org/10.1093/sysbio/syu030
Adams, D., Collyer, M., Kaliontzopoulou, A., & Baken, E. (2021). “Geomorph”: Software for geometric morphometric analyses. R package version 4.0. https://cran.r-project.org/package=geomorph
Andjelković, M., Tomović, Lj., & Ivanović, A. (2016). Variation in skull size and shape of two snake species (Natrix natrix and Natrix tessellata). Zoomorphology, 135, 243–253. https://doi.org/10.1007/s00435-016-0301-3
Arntzen, J. W., Recuero, E., Canestrelli, D., & Martínez-Solano, I. (2013a). How complex is the Bufo bufo species group? Molecular Phylogenetics and Evolution, 69(3), 1203–1208. https://doi.org/10.1016/j.ympev.2013.07.012
Arntzen, J. W., McAtear, J., Recuero, E., Ziermann, J. M., Ohler, A., van Alphen, J., & Martinez-Solano, I. (2013b). Morphological and genetic differentiation of Bufo toads: Two cryptic species in Western Europe (Anura, Bufonidae). Contributions to Zoology, 82(4), 147–169. https://doi.org/10.1163/18759866-08204001
Arntzen, J. W., McAtear, J., Butôt, R., & Martínez-Solano, I. (2018). A common toad hybrid zone that runs from the Atlantic to the Mediterranean. Amphibia-Reptilia, 39(1), 41–50. https://doi.org/10.1163/15685381-00003145
Arntzen J. W., de Vries W., Canestrelli D., & Martínez-Solano I. (2020). Genetic and morphological differentiation of common toads in the Alps and the Apennines. In P. Pontarotti (Ed.), Evolutionary Biology - A Transdisciplinary Approach (pp. 1–13). Springer, Cham. https://doi.org/10.1007/978-3-030-57246-4_1
Bardua, C., Fabre, A. C., Clavel, J., Bon, M., Das, K., Stanley, E. L., Blackburn, D. C., & Goswami, A. (2021). Size, microhabitat, and loss of larval feeding drive cranial diversification in frogs. Nature Communications, 12(1), 1–13. https://doi.org/10.1038/s41467-021-22792-y
Blomberg, S. P., Garland, T., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution, 57(4), 717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Collyer, M. L., & Adams, D. C. (2021). Phylogenetically aligned component analysis. Methods in Ecology and Evolution, 12(2), 359–372. https://doi.org/10.1111/2041-210X.13515
Cornish, C. A., Oldham, R. S., Bullock, D. J., & Bullock, J. A. (1995). Comparison of the diet of adult toads (Bufo bufo L.) with pitfall trap catches. Herpetological Journal, 5, 236–238.
Cvetković, D., Tomašević, N., Ficetola, G. F., Crnobrnja-Isailović, J., & Miaud, C. (2009). Bergmann’s rule in amphibians: Combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufo. Journal of Zoological Systematics and Evolutionary Research, 47(2), 171–180. https://doi.org/10.1111/j.1439-0469.2008.00504.x
Crnobrnja-Isailović, J., Ćurčić, S., Stojadinović, D., Tomašević-Kolarov, N., Aleksić, I., & Tomanović, Ž. (2012). Diet composition and food preferences in adult common toads (Bufo bufo) (Amphibia: Anura: Bufonidae). Journal of Herpetology, 46(4), 562–568. https://doi.org/10.1670/10-264
Deban, S., O’Reilly, J., & Nishikawa, K. (2001). The evolution of the motor control of feeding in amphibians. American Zoologist, 41(6), 1280–1298. https://doi.org/10.1093/icb/41.6.1280
De Villiers, C. G. S. (1938). A comparison of some cranial features of the East African gymnophiones Boulengerula boulengeri Tornier and Scolecomorphus uluguruensis Boulenger. Anatomischer Anzeiger, 86(1/4), 1–26.
Dryden, I. L., & Mardia, K. V. (1998). Statistical shape analysis. Wiley.
Dubois, A., Ohler, A., & Pyron, R. A. (2021). New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa, 5(1), 1–738. https://doi.org/10.11646/megataxa.5.1.1
Duellman, W. E., & Trueb, L. (1994). Biology of amphibians. Johns Hopkins University Press.
Felice, R. N., Tobias, J. A., Pigot, A. L., & Goswami, A. (2019). Dietary niche and the evolution of cranial morphology in birds. Proceedings of the Royal Society B, 286(1897), 20182677. https://doi.org/10.1098/rspb.2018.2677
Galatius, A., Racicot, R., McGowen, M., & Olsen, M. T. (2020). Evolution and diversification of delphinid skull shapes. Iscience, 23(10), 101543. https://doi.org/10.1016/j.isci.2020.101543
Gingras, B., Boeckle, M., Herbst, C. T., & Fitch, W. T. (2013). Call acoustics reflect size across four anuran clades. Journal of Zoology., 289(2), 143–150. https://doi.org/10.1111/j.1469-7998.2012.00973.x
Gastwirth, J. L., Gel., Y. R., Hui, W. L. W., Lyubchich, V., Miao, W., & Noguchi, K. (2020). lawstat: Tools for biostatistics, public policy, and law. R package version 3.4. https://CRAN.R-project.org/package=lawstat
Hanken, J., & Hall, B. (1984). Variation and timing of the cranial ossification sequence of the oriental fire-bellied toad, Bombina orientalis (Amphibia, Discoglossidae). Journal of Morphology, 182(3), 245–255. https://doi.org/10.1002/jmor.1051820302
Hanken, J., & Hall, B. (1993). The skull (Vol. 3). University of Chicago Press.
Hanken, J., & Gross, J. B. (2005). Evolution of cranial development and the role of neural crest: Insights from amphibians. Journal of Anatomy, 207(5), 437–446. https://doi.org/10.1111/j.1469-7580.2005.00481.x
Herrel, A., Schaerlaeken, V., Meyers, J. J., Metzger, K. A., & Ross, C. F. (2007). The evolution of cranial design and performance in squamates: Consequences of skull-bone reduction on feeding behavior. Integrative and Comparative Biology, 47(1), 107–117. https://doi.org/10.1093/icb/icm014
Herrel, A., O’Reilly, J. C., Fabre, A-C., Bardua, C., Lowie, A., Boistel, R., & Gorb, S. N. (2019). In V. Bels & Q. Whishaw (Eds.), Feeding in amphibians: Evolutionary transformations and phenotypic diversity as drivers of feeding system diversity (pp. 431–467). Springer Nature. https://doi.org/10.1007/978-3-030-13739-7_12
Iordansky, N. N. (1989). Evolution of cranial kinesis in lower tetrapods. Netherlands Journal of Zoology, 40(1–2), 32–54. https://doi.org/10.1163/156854289X00174
Iordansky, N. N. (2000). Cranial kinesis in the amphibia: A review. Zhurnal Obshcheĭ Biologii, 61(1), 102–118.
Jetz, W., & Pyron, R. A. (2018). The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nature Ecology and Evolution, 2(5), 850–858. https://doi.org/10.1038/s41559-018-0515-5
Kardong, K. V. (1977). Kinesis of the jaw apparatus during swallowing in the cottonmouth snake, Agkistrodon piscivorus. Copeia, 1977, 338–348. https://doi.org/10.2307/1443913
Kerney, R. R., Brittain, A. L., Hall, B. K., & Buchholz, D. R. (2012). Cartilage on the move: Cartilage lineage tracing during tadpole metamorphosis. Development, Growth & Differentiation, 54(8), 739–752. https://doi.org/10.1111/dgd.12002
Klaczko, J., Sherratt, E., & Setz, E. Z. (2016). Are diet preferences associated to skulls shape diversification in Xenodontine snakes? PLoS ONE, 11(2), e0148375. https://doi.org/10.1371/journal.pone.0148375
Klingenberg, C. P., Barluenga, M., & Meyer, A. (2002). Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution, 56(10), 1909–1920. https://doi.org/10.1111/j.0014-3820.2002.tb00117.x
Levine, R. P., Monroy, J. A., & Brainerd, E. L. (2004). Contribution of eye retraction to swallowing performance in the northern leopard frog, Rana pipiens. The Journal of Experimental Biology, 207(8), 1361–1368. https://doi.org/10.1242/jeb.00885
Levis, N. A., & Pfennig, D. W. (2019). Phenotypic plasticity, canalization, and the origins of novelty: Evidence and mechanisms from amphibians. Seminars in Cell & Developmental Biology, 88, 80–90. https://doi.org/10.1016/j.semcdb.2018.01.012
Lüscher, B., Grossenbacher, K., & Scholl, A. (2001). Genetic differentiation of the common toad (Bufo bufo) in the Swiss Alps. Amphibia-Reptilia, 22(2), 141–154. https://doi.org/10.1163/15685380152030373
Natchev, N., Handschuh, S., Lukanov, S., Tzankov, N., Naumov, B., & Werneburg, I. (2016). Contributions to the functional morphology of caudate skulls: Kinetic and akinetic forms. PeerJ, 4, e2392. https://doi.org/10.7717/peerj.2392
Nishikawa, K. C., & Gans, C. (1992). The role of hypoglossal sensory feedback during feeding in the marine toad, Bufo marinus. Journal of Experimental Biology, 264(3), 245–252. https://doi.org/10.1002/jez.1402640303
Nishikawa, K. C., & Gans, C. (1996). Mechanisms of tongue protraction and narial closure in the marine toad Bufo marinus. Journal of Experimental Biology, 199(11), 2511–2529. https://doi.org/10.1242/jeb.199.11.2511
Paluh, D. J., Stanley, E. L., & Blackburn, D. C. (2020). Evolution of hyperossification expands skull diversity in frogs. Proceedings of the National Academy of Sciences, 117(15), 8554–8562. https://doi.org/10.1073/pnas.2000872117
Ponssa, M. L., & Vera Candioti, M. F. (2012). Patterns of skull development in anurans: Size and shape relationship during postmetamorphic cranial ontogeny in five species of the Leptodactylus fuscus group (Anura: Leptodactylidae). Zoomorphology, 131(4), 349–362. https://doi.org/10.1007/s00435-012-0164-1
R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Recuero, E., Canestrelli, D., Voros, J., Szabo, K., Poyarkov, N. A., Arntzen, J. W., Crnobrnja-Isailović, J., Kidov, A. A., Cogalniceanu, D., Caputo, F. P., Nascetti, G., & Martinez-Solano, I. (2012). Multilocus species tree analyses resolve the radiation of the widespread Bufo bufo species group (Anura, Bufonidae). Molecular Phylogenetics and Evolution., 62(1), 71–86. https://doi.org/10.1016/j.ympev.2011.09.008
Revell, L. J. (2012). “phytools”: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3(2), 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Roček, Z. (2003). Larval development and evolutionary origin of the anuran skull. In H. Heatwole & M. Davies (Eds.), Amphibian Biology 5, Osteology (pp. 1878–1995). Surrey Beatty and Sons.
Rohlf, F. J., & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39(1), 40–59. https://doi.org/10.2307/2992207
Sanna, G. (2019). Ontogenetic and interspecific variation in skull morphology of two closely related species of toad, Bufo bufo and B. spinosus (Anura: Bufonidae). Acta Herpetologica, 14(2), 117–122. https://doi.org/10.13128/a_h-7749
Sinsch, U., Schneider, H., & Tarkhnishvili, D. (2009). Bufo bufo Superspezies - Erdkröten-Artenkreis - taxon bufo (Linnaeus, 1758) - Erdkröte - taxon gredosicola L. Müller und Hellmich 1935 - Gredoserdkröte - taxon spinosus Daudin, 1803 - Riesenerdkröte - taxon verrucosissumus (Pallas, 1811) - Kolchische Erdkröte. Pp. 191–335 in: Grossenbacher K., ed., Handbuch der Reptilien und Amphibien Europas, 5/II. Froschlurche (Anura) II (Hylidae, Bufonidae). Wiebelsheim: Aula-Verlag.
Smirnov, S. V. (1990). Evidence of neoteny: A paedomorphic morphology and retarded development in Bombina orientalis (Anura, Discoglossidae). Zoologischer Anzeiger, 225(5–6), 324–332.
Smirnov, S. V. (1994). Postmaturation skull development in Xenopus laevis (Anura; Pipidae): Late-appearing bones and their bearing on the pipid ancestral morphology. Russian Journal of Herpetology, 1(1), 21–29. https://doi.org/10.30906/1026-2296-1994-1-1-21-29
Smirnov, S. V. (1997). Additional dermal ossifications in the anuran skull: Morphological novelties or archaic elements? Russian Journal of Herpetology, 4(1), 17–27. https://doi.org/10.30906/1026-2296-1997-4-1-17-27
Suchard, M. A., Lemey, P., Baele, G., Ayres, D. L., Drummond, A. J., & Rambaut, A. (2018). Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution, 4(1), vey016. https://doi.org/10.1093/ve/vey016
Trueb, L. (1973). Bones, frogs, and evolution. In J. L. Vial (Ed.), Evolutionary biology of the Anurans: Contemporary research on major problems (pp. 65–132). University Missouri Press.
Trueb, L. (1985). A summary of osteocranial development in anurans with notes on the sequence of cranial ossification in Rhinophrynus dorsalis (Anura: Pípoidea: Rhinophrynidae). South African Journal of Science, 81(4), 181–185.
Trueb, L., & Alberch, P. (1985). Miniaturizatíon and the anuran skull: A case of heterochrony. In H. R. Dunker & G. Fleischer (Eds.), International symposium on vertebrate morphology, Fortschritte der Zoologie, 30, 113–121.
Trueb, L., Hanken, J., & Hall, B. K. (1993). Patterns of cranial diversity among the Lissamphibia. In J. Hanken & B. K. Hall (Eds.), The skull, Volume 2, Patterns of Structural and Systematic Diversity (pp. 255–343). University of Chicago Press.
Üzüm, N., Özdemir, N., Dursun, C., Kutrup, B., & Gül, S. (2021). Interspecific and intraspecific size and shape variation in skull of two closely related species Bufo bufo (Linnaeus, 1758) and Bufo verrucosissimus (Pallas, 1814) from Turkey. Turkish Journal of Zoology, 45, 91–101. https://doi.org/10.3906/zoo-2009-8
Vallvé, Á., & Sánchez-Iglesias, I. (2018). Composition of the diet of the Iberian common toad (Bufo spinosus, Daudin, 1803) in central Spain. Basic and Applied Herpetology, 32, 19–27. https://doi.org/10.11160/bah.95
Van Cakenberghe, V., Herrel, A., & Aguirre, L. F. (2002). Evolutionary relationships between cranial shape and diet in bats (Mammalia: Chiroptera). In P. Aerts, K. D’Août, A. Herrel, & R. Van Damme (Eds.), Topics in functional and ecological vertebrate morphology (pp. 205–236). Shaker Publishing.
Wake, M. H., & Hanken, J. (1982). The development of the skull of Dermophis mexicanus (Amphibia: Gymniophiona), with comments on skull kinesis and amphibian relationships. Journal of Morphology, 173(2), 203–223. https://doi.org/10.1002/jmor.1051730208
Waltenberger, L., Rebay-Salisbury, K., & Mitteroecker, P. (2021). Three-dimensional surface scanning methods in osteology: A topographical and geometric morphometric comparison. American Journal of Physical Anthropology, 174(4), 846–858. https://doi.org/10.1002/ajpa.24204
Weisbecker, V., & Mitgutsch, C. (2010). A large-scale survey of heterochrony in anuran cranial ossification patterns. Journal of Zoological Systematics and Evolutionary Research, 48(4), 332–347. https://doi.org/10.1111/j.1439-0469.2010.00570.x
West-Eberhard, M. J. (2003). Developmental plasticity and evolution. Oxford University Press. https://doi.org/10.1093/oso/9780195122343.001.0001
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences, 102(suppl 1), 6543–6549. https://doi.org/10.1073/pnas.0501844102
Witzmann, F., & Werneburg, I. (2017). The palatal interpterygoid vacuities of Temnospondyls and the implications for the associated eye and jaw musculature. The Anatomical Record, 300(7), 1240–1269. https://doi.org/10.1002/ar.23582
Womack, M. C., Fiero, T. S., & Hoke, K. L. (2018a). Trait independence primes convergent trait loss. Evolution, 72(3), 679–687. https://doi.org/10.1111/evo.13442
Womack, M. C., Fiero, T. S., & Hoke, K. L. (2018b). Data from: Trait independence primes convergent trait loss, Dryad, Dataset. Retrieved September 10, 2021, from https://doi.org/10.5061/dryad.6tn2n
Wroe, S., & Milne, N. (2007). Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution, 61(5), 1251–1260. https://doi.org/10.1111/j.1558-5646.2007.00101.x
Acknowledgements
We thank Antigoni Kaliontzopoulou for the help with the geomorph package, Dirk van der Marel for the advice on the CT scanning, Esther Dondorp for the collection management and an anonymous reviewer for the helpful comments.
Funding
This work was supported by a Temminck-Fellowship of Naturalis Biodiversity Center (A. I.) and the Serbian Ministry of Education, Science and Technological Development of the Republic of Serbia (A. I., M. C., T. V., grant numbers 451–03-9/2021–14/200007 and 451–03-9/2021–14/200178).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ivanović, A., Cvijanović, M., Vučić, T. et al. Differentiation of skull morphology and cranial kinesis in common toads. Org Divers Evol 23, 209–219 (2023). https://doi.org/10.1007/s13127-022-00585-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-022-00585-5