Abstract
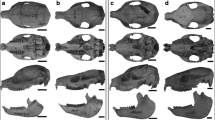
The study of cranial ontogeny is important for understanding the relationship between form and function in developmental, ecological, and evolutionary contexts. The transition from lactation to the diet of adult carnivores must be accompanied by pronounced modifications in skull morphology and feeding behavior. Our goal was to study relative growth and development in the skull ontogeny of the canid Lycalopex culpaeus, and interpret our findings in a functional context, thereby exploring the relationship between changes in shape and size with dietary habits and age stages. We performed quantitative analyses, including multivariate allometry and geometric morphometrics. Our results indicate that shape changes are related to functional improvements of the jaw mechanics related for food catching/processing. Estimates of full muscle size, mechanical advantage, and adult cranial shape are reached after sexual maturity, while adult mandible and skull size are reached after weaning, which is related to diet change (incorporation of meat and other food items). The ontogenetic pattern observed in L. culpaeus is similar to those observed in Canis familiaris and C. latrans. However, the magnitude of change seen in L. culpaeus is smaller than those seen in the felid Puma concolor and considerably smaller than those seen in the bone cracker hyaenid Crocuta crocuta. These patterns are associated with dietary habits and specializations in skull anatomy, as L. culpaeus, domestic dog and coyote are generalist species compared with hypercarnivores such as C. crocuta and P. concolor.



Similar content being viewed by others
References
Abdala F, Flores DA, Giannini NP (2001) Postweaning ontogeny in the skull in Didelphis albiventris. J Mamm 82:190–200
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Bekoff M (1974a) Social play in coyotes, wolves, and dogs. Bioscience 24:225–230
Bekoff M (1974b) Social play and play-soliciting by infant canids. Am Zool 14:323–340
Bekoff M, Jamieson R (1975) Physical development in coyotes (Canis latrans), with a comparison to other canids. J Mamm 56:685–692
Berta A (1987) Origin, diversification, and zoogeography of the south American Canidae. Fieldiana Zool 39:455–471
Biben M (1982) Object play and social treatment of prey in bush dogs and crab-eating foxes. Behaviour 79:201–211
Biben M (1983) Comparative ontogeny of social behaviour in three South American canids, the maned wolf, crab-eating fox and bush dog: implications for sociality. Ani Behav 31:814–826
Biknevicius AR, Leigh SR (1997) Patterns of growth of the mandibular corpus in spotted hyenas (Crocuta crocuta) and cougars (Puma concolor). Zool J Linn Soc 120:139–161
Binder WJ, Van Valkenburgh B (2000) Development of bite strength and feeding behavior in juvenile spotted hyenas (Crocuta crocuta). J Zool 252:273–283
Bookstein FL (1991) Morphometric tools for landmark data. Geometry and biology. Cambridge University Press, USA
Bookstein FL (1997) Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal 1:225–243
Crespo JA, De Carlo JM (1963) Estudio ecológico de una población de zorros colorados Dusicyon culpaeus. Rev Mus Argent Cienc Nat 1:1–55
Drake AG (2011) Dispelling dog dogma: an investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evol Dev 13:204–213
Emerson SB, Bramble DM (1993) Scaling, allometry and skull design. In: Hanken J, Hall BK (eds) The skull. The University of Chicago Press, Chicago, pp 384–416
Evans HE (1993) Miller’s anatomy of the dog, 3rd edn. W.B. Saunders Company, Philadelphia
Ewer R (1973) The carnivores. Cornell University Press, Ithaca
Finarelli JA, Goswami A (2009) The evolution of orbit orientation and encephalization in the carnivora (Mammalia). J Anat 214:671–678
Flores DA, Giannini NP, Abdala F (2003) Cranial ontogeny on Lutreolina crassicaudata (Didelphidae): a comparison with Didelphis albiventris. Acta Theriol 48:1–9
Flores DA, Giannini NP, Abdala F (2006) Comparative postnatal ontogeny of the skull in an Australidelphian Metatherian, Dasyurus albopunctatus (Marsupialia: Dasyuromorpha: Dasyuridae). J Morphol 267:426–440
Flores DA, Giannini NP, Abdala F (2010) Cranial ontogeny of Caluromys philander (Didelphidae: Caluromyinae): a qualitative and quantitative approach. J Mamm 91:539–550
Fox MW (1964) The ontogeny of behaviour and neurologic responses in the dog. Ani Behav 12:301–310
Fox MW (1969) Ontogeny of prey-killing behavior in Canidae. Behaviour 35:259–272
Gay SW, Best TL (1996) Age-related variation in skulls of the puma (Puma concolor). J Mamm 77:191–198
Giannini NP, Abdala F, Flores DA (2004) Comparative postnatal ontogeny of the skull in Dromiciops gliroides (Marsupialia: Microbiotheriidae). Am Mus Novit 3460:1–17
Giannini NP, Segura V, Giannini MI, Flores D (2010) A quantitative approach to the cranial ontogeny of the puma. Mamm Biol 75:547–554
Goodall C (1991) Procrustes methods in the statistical analysis of shape. J R Stat Soc 53:285–339
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4:1–9. http://palaeo-electronica.org/2001_1/past/past.pdf. Accessed 28 February 2011
Holekamp KE, Kolowski JM (2009) Family Hyaenidae (Hyenas). In: Wilson DE, Mittermeier RA (eds) Handbook of the mammals of the world 1 carnivores. Lynx Editions, Barcelona, pp 234–260
Jiménez JE, Novaro AJ (2004) Pseudalopex culpaeus (Molina, 1782). In: Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) Canids: foxes, wolves, jackals and dogs. Status Survey and Conservation Action Plan, IUCN/SSC Canid Specialist Group, Gland, pp 44–49
Johnson WE, Franklin WL (1994) Role of body size in the diets of sympatric gray and culpeo foxes. J Mamm 75:163–174
Jolicoeur P (1963a) The multivariate generalization of the allometry equation. Biometrics 19:497–499
Jolicoeur P (1963b) The degree of generality of robustness in Martes americana. Growth 27:1–27
Kraglievich L (1930) Craneometría y clasificación de los cánidos sudamericanos, especialmente los argentinos actuales y fósiles. Physis 10:35–73
Kremenak CR (1969) Dental eruption chronology in dogs: deciduous tooth gingival emergence. J Dent Res 48:1177–1184
Kremenak CR, Russell LS, Christensen RD (1969) Tooth-eruption ages in suckling dogs as affected by local heating. J Dent Res 48:427–430
La Croix S, Holekamp KE, Shivik JA, Lundrigan BL, Zelditch ML (2011) Ontogenetic relationships between cranium and mandible in coyotes and hyenas. J Morphol 272:662–674
Manly BFJ (1997) Randomization, bootstrap, and Monte Carlo methods in biology. Chapman & Hall, New York
Moore WJ (1981) The mammalian skull. Cambridge University Press, UK
Noble VE, Kowalski EM, Ravosa MJ (2000) Orbit orientation and the function of the mammalian postorbital bar. J Zool 250:405–418
Novaro AJ (1997) Pseudalopex culpaeus. Mamm Species 558:1–8
Prevosti FJ, Lamas L (2006) Variation of cranial and dental measurements and dental correlations in the pampean fox Dusicyon gymnocercus. J Zool 270:636–649
Radinsky LB (1981) Evolution of skull shape in carnivores. I. Representative modern carnivores. Biol J Linn Soc 15:369–388
Ravosa MJ, Noble V, Hylander W, Johnson K, Kowalski E (2000) Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. J Hum Evol 38:667–693
Rohlf FJ (1999) Shape statistics: procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Rohlf FJ (2003a) TpsRegr version 1.28. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook. http://life.bio.sunysb.edu/morph/. Accessed 2 March 2011
Rohlf FJ (2003b) TpsRelw version 1.35. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook. http://life.bio.sunysb.edu/morph/. Accessed 2 March 2011
Rohlf FJ (2008a) TpsUtil version 1.40. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook. http://life.bio.sunysb.edu/morph/. Accessed 2 March 2011
Rohlf FJ (2008b) TpsDig version 2.12. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook. http://life.bio.sunysb.edu/morph/. Accessed 2 March 2011
Scott JP (1967) The evolution of social behavior in dogs and wolves. Am Zool 7:373–381
Segura V, Flores D (2009) Aproximación cualitativa y aspectos funcionales en la ontogenia craneana de Puma concolor (felidae). Mastozool Neotrop 16:169–182
Sheets HD (2002) IMP-integrated morphometrics package. Department of Physics, Casius College, Buffalo
Slater GJ, Dumont E, Van Valkenburgh B (2009) Implications of predatory specialization for cranial form and function in canids. J Zool 278:181–188
Tanner JB, Zelditch ML, Lundrigan BL, Holekamp KE (2010) Ontogenetic change in skull morphology and mechanical advantage in the spotted hyena (Crocuta crocuta). J Morphol 271:353–365
R Development Core Team (2004) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.rproject.org. Accessed 26 Feb 2011
Travaini A, Juste J, Novaro A, Capurro A (2000) Sexual dimorphism and sex identification in the South American culpeo fox, Pseudalopex culpaeus (Carnivora: Canidae). Wildlife Res 27:669–674
Tseng ZJ (2009) Cranial function in a late Miocene Dinocrocuta gigantea (Mammalia: Carnivora) revealed by comparative finite element analysis. Biol J Linn Soc 96:51–67
Tseng ZJ, Binder WJ (2010) Mandibular biomechanics of Crocuta crocuta, Canis lupus, and the late Miocene Dinocrocuta gigantea (Carnivora, Mammalia). Zool J Linn Soc 158:683–696
Tseng ZJ, Wang X (2010) Cranial functional morphology of fossil dogs and adaptation for durophagy in Borophagus and Epicyon (Carnivora, Mammalia). J Morphol 271:1386–1398
Tukey JW (1956) Bias and confidence in not quite large samples. Ann Math Stat 23:614
Van Valkenburgh B (1988) Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14:155–173
Van Valkenburgh B (1989) Carnivore dental adaptations and diet: a study of trophic diversity within guilds. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Cornell University Press, Ithaca, pp 410–436
Van Valkenburgh B (2007) Déjà vu: the evolution of feeding morphologies in the carnivora. Integr Comp Biol 47:147–163
Van Valkenburgh B, Koepfli KP (1993) Cranial and dental adaptations to predation in canids. Symp Zool Soc Lond 65:15–37
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40:243–261
Zapata SC, Funes M, Novaro A (1997) Estimación de la edad en el zorro colorado patagónico (Pseudalopex culpaeus). Mastozool Neotrop 4:145–150
Zar JH (1984) Biostatistical analysis. Prentice-Hall Inc, Englewood Cliff
Zelditch ML, Swiderski D, Sheets H, Fink W (2004) Geometric morphometrics for biologists: a primer. Elsevier Academic Press, London
Acknowledgments
We thank David Flores for the permission to study the material under his care; to Pablo Teta for his drawings of L. culpaeus skulls; to Erika Hingst-Zaher, Amelia Chemisquy, and David Flores for their critical revision of the preliminary version of this manuscript and to Cecilia Morgan for her revision of English grammar. We also thank to three anonymous reviewers who provided many helpful suggestions to this study. This research was partially supported by CONICET (PIP 01054) and ANPCyT (PICT 2008-1798).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Bartolomaeus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
435_2012_145_MOESM2_ESM.tif
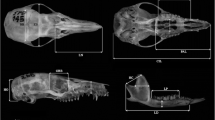
Fig. S1. Boxplots of skull centroid size vs. age classes of Lycalopex culpaeus, for dorsal (A), lateral (B), ventral (C), and mandible (D) view. The boxplots include median, upper, and lower quartiles (75 and 25%, respectively), minimum and maximum. Supplementary material 2 (TIFF 382 kb)
435_2012_145_MOESM3_ESM.tif
Fig. S2. Boxplots of skull procrustes distance of each specimen of all age classes Lycalopex culpaeus to the mean of J1 class, for dorsal (A), lateral (B), ventral (C), and mandible (D) view. The boxplots include median, upper, and lower quartiles (75 and 25%, respectively), minimum and maximum. Supplementary material 3 (TIFF 358 kb)
435_2012_145_MOESM4_ESM.tif
Fig. S3. Boxplots of mechanical advantage of masseter and temporal muscles (in-levers of masseteric and temporal muscles/out-lever at canine and carnassial) vs. age classes of Lycalopex culpaeus. Zygomatic breadth (A), mechanical advantage of masseter muscle measure at the canine (B), mechanical advantage of masseter muscle measure at the carnassial (C), mechanical advantage of temporal muscle measure at the canine (D), and mechanical advantage of temporal muscle measure at the carnassial (E). The boxplots include median, upper, and lower quartiles (75 and 25%, respectively), minimum and maximum. Supplementary material 4 (TIFF 429 kb)
Appendices
Appendix 1
Specimens of Lycalopex culpaeus of Museo Argentino de Ciencias Naturales Bernardino Rivadavia (MACN) used in this study
15022; 15024; 15025; 15028; 15033; 15037; 15040; 15044; 15045; 15049; 15050;15055; 15062; 15063; 15064; 15073; 15078; 15081; 15082; 15083; 15089; 15093; 15096; 15101; 15106; 15112; 15119; 15121; 15122; 15123; 15124; 15127; 15129; 15130; 15131; 15132; 15133; 15138; 15140; 15149; 15151; 15154; 15158; 15163; 15168; 15172; 15173; 15177; 15180; 15181; 15182; 15190; 15194; 15196; 15197; 15199; 15200; 15201; 15202; 15203; 15208; 15212; 15220; 15223; 15224; 15226; 15227; 15228; 15229; 15232; 15233; 15240; 15243; 15246; 15248; 15258; 15259; 15260; 15261; 15266; 15267; 15268; 23072; 23076; 23077; 23093; 23095; 23098; 23099; 23100; 23101; 23102; 23103; 23104; 23108; 23119; 23123; 23125; 23143; 23148; 23152.
Appendix 2
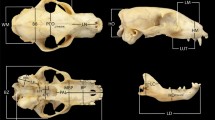
Definition of the landmarks and semi-landmarks used in the geometric morphometric analyses (see Fig. 1)
Dorsal landmarks: 1, tip of premaxilla in the sutura interincisiva; 2, anterior portion of the nasals in the sutura internasalis; 3, midline of sutura frontonasalis; 4, intersection between sutura coronalis, sutura sagittalis, and sutura interfrontalis; 5, tip of occipital plate; 6–16, semi-landmarks; 17, tip of the supraorbital process; 18–24, semi-landmarks; 25, lacrimal foramen; 26–31, semi-landmarks; 32, tip of the infraorbital process; 33–37, semi-landmarks; 38, apex of canine root; 39, nasal process; 40, anterior contact of sutura nasomaxillaris; 41, posterior contact of sutura nasomaxillaris; 42, apex of sutura frontomaxillaris.
Ventral landmarks: 1, anterior tip of premaxilla; 2, midline in Sutura incisivomaxillaris; 3, midline in Sutura palatomaxillaris; 4, posterior point of palatine torus; 5, anterior point of intercondyloid incisure; 6, internal apex of occipital condyle; 7, apex of jugular process. 8, tip of mastoid process; 9, internal apex of tympanic bulla; 10, anterior apex of tympanic bulla; 11–14, semi-landmarks; 15, tip of postglenoid process; 16, internal edge of masseteric fossa; 17, caudal apex of border of palatine; 18, external edge of masseteric fossa; 19, anterior edge of masseteric fossa; 20–30, semi-landmarks.
Lateral landmarks: 1, tip of premaxilla; 2–3, semi-landmarks; 4, apex of sutura frontomaxillaris; 5–8, semi-landmarks; 9, posterior point between sagittal and nuchal crests; 10, apex of occipital condyle; 11, tip of paracondylar process; 12, point between nuchal crest and mastoid process; 13, apex of tympanic bulla; 14–17, semi-landmarks; 18, tip of infraorbital process; 19–20, semi-landmarks; 21, lacrimal foramen; 22–23, semi-landmarks; 24, tip of the supraorbital process; 25, tip of Postglenoid process; 26, posterior point of pterygoid; 27–29, semi-landmarks; 30, posterior tip of dentary row; 31, notch of carnassial; and 32–33, semi-landmarks.
Mandibular landmarks: 1, anterior tip of body of mandible; 2–9, semi-landmarks; 10, posterior tip of coronoid process; 11, mandibular notch; 12, anterior point of masseteric fossa; 13, external point of condyloid process; 14, separation between condyloid and angular process; 15, tip of angular process; 16–22, semi-landmarks.
Rights and permissions
About this article
Cite this article
Segura, V., Prevosti, F. A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131, 79–92 (2012). https://doi.org/10.1007/s00435-012-0145-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-012-0145-4