Abstract
Objective
This study aims to investigate the correlation between serum testosterone levels after one month of treatment and prognosis in patients with high-volume disease metastatic prostate cancer (mPCa) who are undergoing combined androgen blockade therapy (CAB).
Methods
The clinical data of 199 patients with high-volume disease mPCa, diagnosed through biopsy pathology and imaging, were retrospectively analyzed from January 2010 to October 2022 in the Department of Urology at the First Affiliated Hospital of Xinjiang Medical University. Among these patients, 111 cases had a deep reduction in serum testosterone (< 0.7 nmol/l) after one month of treatment, while 88 cases did not achieve a deep reduction (≥ 0.7 nmol/l). The study utilized the Kaplan–Meier method to plot survival curves and employed the multifactor COX regression model to analyze independent risk factors. The risk factors with a significance level of P < 0.05 in the multivariate analysis were included in the nomogram prediction model. The accuracy of the model was assessed using the ROC curve and the calibration curve, while the net benefit for patients was evaluated through the decision curve analysis (DCA).
Results
The group that achieved deep testosterone reduction(DTR) had a higher proportion of PSA < 0.2 ng/ml and a greater PSA decline rate after six months of treatment (P < 0.05). The group that achieved DTR and the group that did not achieve DTR had a progression to castration resistant prostate cancer(CRPC) time of 17.93 ± 6.68 months and 13.43 ± 6.12 months, respectively (P < 0.001). The median progression-free survival time for the 2 groups were 18 months and 12 months, respectively (P < 0.001). The median overall survival times were 57 months and 32 months, respectively (P < 0.001). The median progression-free survival times were 18, 15, and 10 months for the group that achieved DTR within 1 month, the group that achieved DTR beyond 1 month but within 1 year, and the group that did not achieve DTR within 1 year, respectively (P < 0.001), and the median survival times were 57, 45, and 26 months, respectively (P < 0.001). COX multivariate analysis revealed that a testosterone level of ≥ 0.7 nmol/l at 1 month of treatment is an independent risk factor for the progression to CRPC and prognosis in patients with high-volume disease mPCa (P < 0.05). The risk of death in patients with a testosterone level of ≥ 0.7 nmol/l at 1 month of treatment was 2.087 times higher than that of patients with a level of < 0.7 nmol/l (P < 0.05). A nomogram prediction model was developed using independent risk factors, with the area under the ROC curve (AUC) for progression-free survival (PFS) at 12, 15, 18, and 21 months being 0.788, 0.772, 0.760, and 0.739, respectively. For 3 and 5 years, the AUCs for overall survival (OS) were 0.691 and 0.624. The calibration curve demonstrated good consistency between the model’s predicted values and the actual outcomes.
Conclusion
Patients with high-volume disease mPCa who receive CAB treatment may experience extended progression-free survival and overall survival if their serum testosterone levels are below 0.7 nmol/l after one month of treatment. The longer it takes to achieve DTR, the worse the patient’s prognosis may be. The nomogram prediction model developed in this study demonstrates good predictive ability in assessing the progression and prognosis of high-volume disease mPCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common cancer in men worldwide, with the highest incidence rate in the Americas, Europe, Australia, and the Caribbean (Bergengren et al. 2023). In the United States, PCa is the leading cancer that poses a threat to men's health (Siegel et al. 2023). While the incidence of PCa in Asia is lower than in Europe and the United States, it has been increasing in recent years (Culp et al. 2020). Age, family history, and genetic susceptibility are among the risk factors for PCa. Metastatic prostate cancer (mPCa) is a critical stage that significantly impacts patient survival, and those with a high-volume disease tend to have a worse prognosis (Clarke et al. 2019).
Since the discovery of the role of androgen deprivation therapy (ADT) in the treatment of PCa by Huggins and Hodges, ADT has remained a crucial aspect of PCa treatment, whether through surgical castration in the past or current mainstream medical castration. Currently, hormone blockade therapy, specifically combined androgen blockade (CAB), is considered the first-line treatment option for mPCa (Sathianathen et al. 2020). Testosterone and prostate specific antigen (PSA) are both important indicators used to monitor the prognosis of PCa patients. However, in the past, more emphasis was placed on monitoring PSA levels, while the role of testosterone in assessing patient recurrence and prognosis was overlooked. The clinical treatment goal after receiving ADT is to reduce testosterone levels to castration levels (< 50 ng/dl). It is important to note that this standard was defined based on testosterone testing standards from the last century. The cutoff point of < 50 ng/dl for serum testosterone level after treatment and its relationship to the prognosis of PCa patients is still a topic of controversy. Bertaglia et al. (Bertaglia et al. 2013) found that the cutoff value of 50 ng/dl had no prognostic significance for the entire patient cohort. However, the cutoff value of 20 ng/dl was significantly associated with survival rate. The determination of the most clinically significant castration level during ADT treatment is still uncertain. Nevertheless, both the European Association of Urology and the Canadian Consensus recommend that lowering testosterone below 20 ng/dl should be considered as the new castration level, also known as deep testosterone reduction (DTR) (Cornford et al. 2021; Klotz et al. 2018). mPCa patients with high-volume disease often progress to castration-resistant prostate cancer (CRPC) more quickly and have a poorer prognosis. This study aims to identify early monitoring indicators that can guide personalized medication for these patients. Specifically, the study aims to investigate the relationship between serum testosterone levels after one month of treatment and prognosis in mPCa patients with high-volume disease who received CAB treatment. Additionally, the role of serum testosterone as a prognostic marker after one month of CAB treatment will be evaluated. The findings of this study can provide valuable insights into the management and treatment of mPCa patients with high-volume disease.
Data and methods
Subjects and methods of study
This retrospective analysis examined the clinical data of 199 patients with high-volume disease mPCa. The patients were admitted to the Department of Urology, the First Affiliated Hospital of Xinjiang Medical University, between January 2010 and October 2022. The patients were confirmed to have mPCa with high-volume disease through biopsy pathology and imaging. Based on their total serum testosterone level in the first month after treatment, the patients were divided into 2 groups: a group that achieved DTR (< 0.7 nmol/l, 111 cases) and a group that did not achieve DTR (≥ 0.7 nmol/l, 88 cases). The study analyzed the differences in clinical and prognostic data between these two groups. Survival curves were generated using the Kaplan–Meier method, and multivariate COX regression analysis was performed to identify independent risk factors influencing the prognosis of patients with high-volume disease mPCa. Risk factors with a significance level of P < 0.05 in the multivariate analysis were included in the nomogram prediction model. The accuracy of the model was assessed using the receiver operating characteristic curve (ROC) and calibration curve. Additionally, the decision curve analysis (DCA) was employed to evaluate the accuracy of the nomogram prediction model in predicting clinical benefit.
Follow-up and observation indicators
Clinical data includes various factors such as age, ethnicity, smoking history, drinking history, history of hypertension, history of diabetes, T stage, nerve invasion and visceral metastasis, Gleason score (the highest score in the biopsy pathology report), initial PSA, initial testosterone, and serum testosterone levels after 1 month of treatment. Additionally, PSA levels and PSA decline rate after 6 months of treatment, patient progression time to CRPC, and overall survival (OS) are also considered. Venous blood samples were collected to measure testosterone levels and PSA. Testosterone levels were measured using chemiluminescence immunoassay, while PSA levels were measured using the enzyme luminescence method. OS was the primary outcome, with time to CRPC as the secondary outcome. The time to progress to CRPC is defined as the duration from the start of ADT to the diagnosis of CRPC. OS is measured from the diagnosis of mPCa and the initiation of regular endocrine therapy until death from any cause or the length of the last follow-up.
Related definitions
-
1.
The article refers to total serum PSA and total serum testosterone when mentioning PSA and testosterone, unless stated otherwise.
-
2.
A high-volume disease is defined as having four or more bone metastases, with at least one metastasis located outside the pelvis or spine, and/or visceral metastasis (Shiota et al. 2021).
-
3.
Testosterone castration level and deep castration level are defined as follows: Castration level is when the serum testosterone is less than 50 ng/dl (1.7 nmol/l), and deep castration is when the serum testosterone is less than 20 ng/dl (0.7 nmol/l).
-
4.
CRPC definition: castration conditions, i.e. serum testosterone T < 50 ng/dl or < 1.735 nmol/l; also accompanied by one of the following conditions: ①Biochemical progression: three consecutive elevations of PSA at 1-week intervals, two of which are > 50% higher than the lowest value and the absolute value of PSA elevation is > 2 ng/ml; ②Imaging progression: two or more new bone metastases or one or more soft tissue lesions detected on bone scan (Cai et al. 2023).
-
5.
Combined Androgen Blockade Therapy (CAB): In this study, CAB therapy is redefined as ADT combined with either traditional or new endocrine therapy drugs. But not all patients in the study received the same CAB drugs, with the most common combination being goserelin or leuprolide with abiraterone. Additionally, the ADT drugs administered were predominantly in long-acting dosage forms.
Inclusion and exclusion criteria
Inclusion criteria: ①Patients diagnosed with mPCa with a high-volume disease based on biopsy pathology and imaging during the initial diagnosis, patients with pathologic type of prostatic acinar adenocarcinoma; ②patients who have not received any ADT and endocrine therapy before or at the beginning of the study, as these treatments may affect the observed indicators; ③patients who receive regular combined androgen blockade treatment after diagnosis; ④patients with complete clinical data.
Exclusion criteria: ①Patients previously diagnosed with PCa and received treatment; ②patients who received other treatments before progressing to CRPC, such as palliative surgery, radiotherapy, molecular targeted therapy, or docetaxel chemotherapy; ③patients with other malignant tumors at the time of diagnosis; ④patients with other serious diseases; ⑤patients who were lost to follow-up or had missing data during the follow-up process; ⑥patients with a history of prostate surgery or urinary tract infection/prostatitis within the past month; ⑦patients who have used 5α reductase inhibitors, testosterone replacement drugs, and other external factors that may affect testosterone; ⑧individuals with a history of ejaculation within 24 h; ⑨and those who have undergone surgical castration.
Based on strict inclusion and exclusion criteria, 199 patients with high-volume disease mPCa were eventually included in this study. The flow chart of the study selection process is shown in Fig. 1.
Ethical review
This study is retrospective, and the subjects studied were informed or supplemented with the purpose of the study, the content of the study, and the potential patient benefits and risks of the study, and the patients themselves or their families were informed by telephone or signed the informed consent in writing, and the study complied with the principles of the Declaration of Helsinki and the principles of the Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University(No.20220308–166).
Statistical methods
Data processing and analysis were conducted using SPSS 25.0 and R 4.2.2 software. Measurement data were described using \(\overline{x }\pm s\), and a t-test was performed for comparison between two groups. Count data were described using ratio, and a χ2 test was performed for comparison between groups. Survival curves were drawn using the Kaplan–Meier method. Multi-factor analysis was conducted using the COX proportional hazards regression model. Risk factors with a significance level of P < 0.05 in the multivariate analysis were included in the nomogram prediction model. The accuracy of the model was assessed using the ROC curve and calibration curve. Additionally, the DCA was employed to evaluate the accuracy of the nomogram prediction model in predicting clinical benefit. P < 0.05 indicated statistical significance.
Results
Patients who achieve DTR within one month have greater PSA decline
There were no statistically significant differences between the two groups in terms of age, ethnicity, smoking, drinking, hypertension, diabetes, Gleason score, T stage, visceral metastasis, nerve invasion, initial PSA, and initial testosterone (P > 0.05). However, there were statistical differences in PSA levels after six months of treatment, PSA decline rates, and the time it took for the disease to progress to CRPC. The group with DTR had a higher proportion of patients with PSA levels below 0.2 ng/ml after six months of treatment, a greater PSA decline rate, and a longer time to progress to CRPC (P < 0.05). Please refer to Table 1 for more details.
Longer progression-free survival in patients who achieve DTR within one month
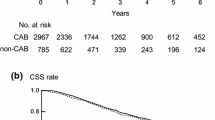
The time to progression to CRPC was 17.93 ± 6.68 months in the group that achieved DTR and 13.43 ± 6.12 months in the group that did not achieve DTR. These results were statistically significant (P < 0.001), as shown in Table 1. Additionally, the median progression-free survival time was 18 months in the group that achieved DTR and 12 months in the group that did not achieve DTR. These differences were also statistically significant (P < 0.001), as shown in Fig. 2.
Patients who achieve DTR in one month have longer OS
The median survival time for the group that achieved DTR was 57 months, while it was 32 months for the group that did not achieve DTR. The survival rate was significantly higher in the group that achieved DTR compared to the group that did not (P < 0.001), as shown in Fig. 3.
Patients with shorter time to achieve DTR have longer progression-free survival time
The median progression-free survival times were 18 months for the group that achieved DTR within 1 month, 15 months for the group that achieved DTR beyond 1 month but within 1 year, and 10 months for the group that did not achieve DTR within 1 year. These differences were found to be statistically significant (P < 0.001). Please refer to Fig. 4 for more details.
Patients with shorter time to reach DTR have longer OS
The median survival times for the group that achieved DTR within 1 month, the group that achieved DTR beyond 1 month but within 1 year, and the group that did not achieve DTR within 1 year were 57, 45, and 26 months, respectively. These differences were found to be statistically significant (P < 0.001). Please refer to Fig. 5 for visualization.
Testosterone ≥ 0.7 nmol/L at 1 month of treatment is an independent risk factor for progression to CRPC in patients with high-volume disease mPCa.
The univariate COX regression analysis revealed that T-stage, Gleason score, initial PSA, PSA at 6 months of treatment, and testosterone after 1 month of treatment were associated with progression to CRPC in patients with high-volume disease mPCa (P < 0.05). Subsequently, the significant variables from the univariate analysis were included in the multifactorial COX regression analysis using the forward stepwise method. The analysis showed that T-stage > 3, Gleason score ≥ 9, initial PSA > 200 ng/ml, and testosterone ≥ 0.7 nmol/l after 1 month of treatment were independent risk factors for progression to CRPC in patients with high-volume disease mPCa (P < 0.05). Please refer to Table 2 for more details.
Testosterone ≥ 0.7 nmol/L at 1 month of treatment is an independent risk factor for death in patients with high-volume disease mPCa
Univariate COX regression analysis revealed that smoking, PSA at 6 months of treatment, and testosterone at 1 month of treatment were significantly associated with the prognosis of mPCa patients with high-volume disease (P < 0.05). Variables that demonstrated statistical significance in the univariate analysis were included in the multivariate COX regression analysis using the forward stepwise method. The results indicated that smoking and testosterone levels ≥ 0.7 nmol/l at 1 month of treatment were independent risk factors affecting the prognosis of patients with high-volume disease mPCa (P < 0.05). The mortality risk for smoking patients was 1.707 times higher than that of non-smoking patients, while the risk of death for patients with testosterone levels ≥ 0.7 nmol/l at 1 month of treatment was 2.087 times higher than that of patients with testosterone levels < 0.7 nmol/l. Please refer to Table 3 for further details.
Nomogram prediction model has good predictive ability for progression-free survival time in patients with high-volume disease mPCa
The independent risk factors identified through COX multi-factor analysis were visually analyzed to construct a nomogram prediction model using R4.2.2 software. This model is based on T stage, Gleason score, initial PSA, and testosterone levels after 1 month of treatment. The progression of high-volume disease mPCa patients to CRPC at 12, 15, 18, and 21 months was evaluated using this model, as depicted in Fig. 6. The areas under the ROC curve for the progression-free survival time prediction models at 12, 15, 18, and 21 months were 0.788, 0.772, 0.760, and 0.739 respectively. These results indicate that the model exhibits good predictive capabilities, as illustrated in Fig. 7. The results of the calibration curve indicate a good fit between the actual curve and the ideal curve. There is a good level of consistency observed between the predicted 12, 15, 18, and 21 month progression-free survival times from the model and the corresponding true values, as shown in Fig. 8. Following the evaluation of the model, DCA was employed to assess the net benefit for the patient. The findings indicated that the prediction model utilizing the nomogram held particular significance in enhancing clinical benefits for the patient, as illustrated in Fig. 9.
Nomogram prediction model has good predictive ability for OS in patients with high-volume disease mPCa
Visual analysis was conducted on the independent risk factors identified through the COX multifactor analysis mentioned above. The R4.2.2 software was utilized to develop a nomogram prediction model incorporating smoking history and testosterone levels after 1 month of treatment. This model was employed to assess the 3 year and 5 year survival rates of high-volume disease mPCa patients, as illustrated in Fig. 10. The AUCs of the 3 year and 5 year overall survival prediction models are 0.691 and 0.624 respectively, providing valuable reference points (see Fig. 11). The calibration curve analysis demonstrates a good fit between the actual and ideal curves, indicating good consistency between the predicted 3 year and 5 year overall survival times and the true values (see Fig. 12). Additionally, the Decision Curve Analysis (DCA) results highlight the clinical benefit of the prediction model based on the nomogram for patients (see Fig. 13).
Discussion
Metastatic burden plays a crucial role in determining the prognosis of patients with mPCa. Patients with a high-volume disease tend to have a shorter time to progression to CRPC and overall survival compared to those with a low-volume disease. A multicenter study revealed that the median progression-free survival time for patients with low-volume disease was 44.5 months, while for those with high-volume disease, it was 16.1 months. Similarly, the median survival time for patients with low-volume disease was 103.2 months, whereas for those with high-volume disease, it was 62.7 months. Various factors such as PSA levels, Gleason score, T stage, N stage, and treatment methods are closely associated with the progression-free survival time and overall survival of patients with high-volume disease mPCa (Shiota et al. 2021). Although CAB treatment can enhance progression-free survival time and overall survival in mPCa patients with high-volume disease, most patients will progress to the CRPC stage within 16–24 months of treatment (Nagumo et al. 2022; Achard et al. 2022). Some studies have found that serum testosterone levels during ADT are associated with the overall survival of mPCa patients. However, there is insufficient clinical evidence to support the relationship between the traditional castration cutoff point and the prognosis of PCa patients. Additionally, the clinically significant castration level has not yet been determined (Perachino et al. 2010). This study aimed to assess the correlation between serum testosterone levels below 0.7 nmol/l after one month of treatment and the prognosis in patients with high-volume disease mPCa treated with CAB. The findings of this study may provide valuable insights for adjusting therapeutic strategies in patients with high-volume disease mPCa.
As early as 10 years ago, experts emphasized the importance of monitoring serum testosterone levels to verify the response to ADT treatment. However, testosterone monitoring has not been given due attention for many years. Currently, it is believed that DTR may become a new standard (Schulman et al. 2010). Our study demonstrates a significant difference in PSA and PSA decline rate at 6 months of treatment between the group that achieved DTR in serum testosterone after one month of treatment and the group that did not achieve DTR. The group that achieved DTR had a higher proportion of PSA < 0.2 ng/ml and a greater PSA decline rate, indicating that patients with low testosterone after treatment show a better PSA response. Morote et al. (Morote et al. 2007) established a direct correlation between testosterone increase and the progression of PCa. They were the first to report that a serum testosterone level of less than 32 ng/dl after treatment can help prolong the progression-free survival of patients. Dason et al. (Dason et al. 2013) also discovered that patients with a testosterone level of less than 32 ng/dl at 9 months of treatment had a significantly longer time to progress to CRPC (P = 0.001). The median progression-free survival time was 33.1 months for those with testosterone levels below 32 ng/dl and 12.5 months for those with levels above 32 ng/dl. However, their study did not find that a testosterone level below 20 ng/dl predicts the time to develop CRPC. Nevertheless, several studies have shown that a testosterone level below 20 ng/dl after ADT treatment is important for improving patient prognosis. In a study conducted by Ding et al. (Ding* M et al. 2019), it was found that patients with testosterone levels below 20 ng/dl at 6 months of treatment experienced a significantly longer time before progressing to CRPC. The median CRPC-free survival times were 48 months for patients with testosterone levels below 20 ng/dl and 24 months for those with levels above 20 ng/dl. Kamada et al. (Kamada et al. 2015) investigated the relationship between testosterone and prognosis in PCa patients who received CAB treatment. They discovered that patients with testosterone levels below 20 ng/dl after 6 months of treatment and the lowest value of testosterone below 20 ng/dl after treatment had a significantly prolonged overall survival. Similarly, Yamamoto et al. (Yamamoto et al. 2017) conducted studies that demonstrated how a post-treatment testosterone nadir below 20 ng/dl or a testosterone reduction of at least 480 ng/dl can lead to a longer progression-free survival and OS in Japanese male patients with advanced PCa. In our study, we found that patients with serum testosterone < 0.7 nmol/l at 1 month of treatment had significantly prolonged time to progression to CRPC (17.93 ± 6.68 months vs. 13.43 ± 6.12 months) and OS compared to those with ≥ 0.7 nmol/l. The median progression-free survival time in the two groups was 18 and 12 months, respectively, and the median survival time was 57 and 32 months. The results of the our study were consistent with the previous studies, however, the results of the our study showed shorter progression-free survival time and OS than the previous studies, which may be attributed to the inclusion of a population with high-volume disease mPCa. The results of the our study further support the idea that a strict control of testosterone < 20 ng/dl will result in a longer progression-free survival and OS. Studies have shown that when testosterone levels are below 20 ng/dl after ADT treatment, there is a more effective killing effect on PCa cells. Additionally, under these conditions, residual tumor cells are more likely to proliferate into hormone-sensitive PCa cells, leading to a longer progression to CRPC. Moreover, a lower testosterone levels after treatment is associated with a reduced incidence of testosterone escape, which may contribute to a better prognosis. This finding supports the prediction that testosterone levels below 20 ng/dl after ADT treatment are indicative of a favorable outcome (Klotz and Toren 2012). Some studies have indicated that an increase in testosterone levels above the target threshold or testosterone escape after the initial month of ADT could result in poorer clinical outcomes. Tan et al. reached similar conclusions in their cohort study on the population with CRPC. The ‘bounce’ phenomenon in testosterone levels is defined as a 10% increase in testosterone levels after initiating new anti-androgen therapy compared to levels before starting the therapy. Tan et al.’s research has shown that this ‘bounce’ phenomenon is linked to poorer PSA response, shorter PSA progression-free survival, and shorter overall survival (Tan et al. 2021; Saad et al. 2020). Tan et al.’s analysis of the testosterone ‘bounce’ phenomenon underscores the importance of testosterone as a predictive factor for prostate cancer treatment response, and provides valuable insights for the clinical monitoring of testosterone levels. The underlying mechanism of the testosterone ‘bounce’ phenomenon is currently unclear. Lower testosterone levels post-treatment are associated with a decreased incidence of testosterone escape. Additionally, lower testosterone levels post-treatment may help mitigate this 'bounce' phenomenon of testosterone, which could have a direct impact on the treatment and prognosis of patients with prostate cancer. Our study aimed to identify early predictors, therefore we evaluated testosterone levels after 1 month of treatment instead of after half a year of treatment or the nadir value of testosterone. This study further analyzed the differences in progression time to CRPC and overall survival time among patients who entered DTR at different time periods. The study revealed that the median progression-free survival time for patients who achieved DTR within 1 month, reached DTR more than 1 month but within 1 year, and those who did not achieve DTR within 1 year were 18, 15, and 10 months, respectively. Similarly, the median survival time for these groups were 57, 45, and 26 months, respectively. There was a correlation between patients’ progression-free survival time and overall survival with the time taken to reach DTR. As the time to reach DTR increased, both progression-free survival time and overall survival decreased. The relationship between the time of testosterone decline and the prognosis of PCa patients has shown varying results in previous studies. Previous studies by Kamada et al. (Kamada et al. 2015) suggest that the key factor influencing the prognosis of PCa is not the rapid decline of testosterone, but rather whether the lowest testosterone level is below 20 ng/dl. However, some studies suggest that a rapid reduction in testosterone during endocrine therapy may have a positive impact on prognosis.
Wang et al. (Wang et al. 2017) conducted a multi-factor analysis and discovered that testosterone levels below 50 ng/dl after the first month of CAB treatment are not indicative of the effective hormone treatment duration. However, they observed that testosterone levels equal to or less than 25 ng/dl after the first month of treatment are significantly linked to a reduced risk of progression to CRPC (HR = 1.46, 95%CI 1.08–1.96, P = 0.013). Our study identified several independent risk factors for progression to CRPC in patients with high-volume disease mPCa. These risk factors include T stage > 3, Gleason score ≥ 9, initial PSA > 200 ng/ml, and testosterone ≥ 0.7 nmol/l at 1 month of treatment. Additionally, smoking and testosterone ≥ 0.7 nmol/l at 1 month of treatment were found to be independent risk factors for the prognosis of mPCa patients with high-volume disease. Smoking patients had a 1.707 times higher risk of death compared to non-smoking patients, while patients with testosterone ≥ 0.7 nmol/l at 1 month of treatment had a 2.087 times higher risk of death compared to those with < 0.7 nmol/l. These findings align with previous research conducted by Wang et al. and are supported by other studies (Bertaglia et al. 2013; Perachino et al. 2010; Kamada et al. 2015; Yamamoto et al. 2017). It is evident that maintaining low testosterone levels after treatment can significantly improve patient prognosis. According to another study, it was found that testosterone levels below 20 ng/dl can be used to predict the recovery of testosterone to castration levels in PCa patients undergoing external radiotherapy treated with ADT (HR = 0.35, 95%CI 0.14–0.79, P = 0.0112) (Takei et al. 2018). Previous studies have indicated that a level of 25 ng/dl can be used to differentiate patients who will progress to CRPC from those who will not progress in the short term (AUC = 0.59, 95%CI 0.51–0.66), with a sensitivity of 56% and specificity of 59%. On the other hand, a level of 30 ng/dl demonstrated the highest sensitivity (80%) and specificity (55%) in predicting patient survival (AUC = 0.69, 95%CI 0.58–0.79) (Bertaglia et al. 2013; Wang et al. 2017). Based on the results of COX multifactor analysis, this study developed a nomogram prediction model to forecast the progression and prognosis of patients with high-volume disease mPCa. This model demonstrates good predictive ability in assessing the progression and prognosis of patients with high-volume disease mPCa. This study will serve as a valuable reference point for evaluating patient progress and prognosis. It will play a crucial role in guiding clinicians to tailor treatment plans to individual patients, ultimately leading to improved patient outcomes and enhanced quality of life.
As the understanding of PCa advances, clinical practice is increasingly focusing on comprehensive management of endocrine therapy for PCa, with testosterone monitoring being a crucial component. Despite this, clinical emphasis still largely remains on monitoring PSA, with insufficient attention given to testosterone monitoring. It is worth noting that PSA monitoring alone has its limitations, as a small number of patients may still experience clinical progression despite no increase in PSA levels. There is a growing body of evidence indicating that a lower testosterone level during treatment is associated with a better prognosis for patients. Therefore, it is crucial to focus on monitoring and managing testosterone levels. This study discovered that patients who achieved DTR within 1 month had a more favorable prognosis. If the results of the current study can be replicated and confirmed, clinicians may want to consider implementing routine testing of serum testosterone at 1 month of treatment. By focusing on this early monitoring indicator, clinicians can optimize the effectiveness of castration treatment, make more precise decisions regarding treatment plans, decrease the likelihood of treatment failure, extend the patient's survival time effectively, and ultimately maximize the benefits for the patient. When monitoring patients with prostate cancer undergoing treatment, it may be observed that serum testosterone and PSA levels are not always ‘synchronized’. Patients who have achieved DTR may exhibit higher PSA levels, while patients who have not reached DTR may show lower PSA levels. The exact relationship between testosterone and PSA remains unclear at present, but it can be explored from the following perspectives. First of all, endocrine therapy drugs have a direct impact on testosterone levels and demonstrate good specificity. PSA is linked to PCa cells, and its gene transcription is regulated by androgens. This can result in an 'out-sync' between testosterone levels and PSA levels after treatment. Secondly, various pathological types of prostate cancer, as well as differing Gleason scores and individual characteristics, can result in varying sensitivities to treatment. These heterogeneities in prostate cancer can also lead to ‘out-sync’ between testosterone and PSA levels. Finally, variations in baseline levels of serum testosterone and PSA, the selection of treatment medications, and the patient's overall health condition could contribute to the lack of ‘synchronization’ between testosterone and PSA levels. Additionally, differences in the clearance rates of serum testosterone and PSA within the body should be taken into account (Zhang 2004; Saxena et al. 2012). The author's explanations for the lack of 'synchronization' between serum testosterone and PSA levels post-treatment are speculative. There is currently a lack of research establishing a clear link between the two factors and the underlying reasons for their 'non-synchronization'. The 'unsynchronized' phenomenon of testosterone and PSA highlights the complex relationship between the two in PCa. To effectively evaluate treatment outcomes and adjust strategies promptly, synchronous management of PSA and testosterone is crucial. Both biomarkers are essential for monitoring PCa patients.
Conclusion
In patients with high-volume disease mPCa, the use of CAB and achieving a serum testosterone level of less than 0.7 nmol/l after one month of treatment has been associated with improved progression-free survival and overall survival. The longer it takes to achieve DTR, the worse the patient's prognosis may be. The nomogram prediction model developed in this study demonstrates good predictive ability in assessing the progression and prognosis of high-volume disease mPCa. These findings suggest that targeting the achievement of DTR should be considered in the treatment of high-volume disease mPCa patients, as it may have a positive impact on patient survival. Monitoring of testosterone in PCa is as important as PSA. This study has several limitations. Firstly, it is a retrospective study and the sample size included is limited. Secondly, it is important to note that testosterone levels are influenced by circadian rhythm, however, the time for collecting testosterone samples was not standardized in this study. Additionally, the study did not explore bioavailable testosterone. Furthermore, it should be acknowledged that not all patients in the study received the same drugs for CAB treatment. In future studies, we will increase the sample size, carefully control the collection of testosterone blood samples, focus on patients receiving ADT only, and investigate the differences in testosterone levels and prognosis between patients using only ADT and those using CAB. Additionally, we will examine the association between various testosterone types and PCa prognosis in individuals undergoing different CAB drug regimens. Furthermore, we will delve deeper into studying the impact of the timing of testosterone decline on PCa prognosis. Moving forward, it is crucial to conduct large-sample, multi-center, and prospective studies to further validate these findings.
Author contributors
Hengqing An and Ning Tao designed this study. Xin Huang, Zhuang Lei and Tao Zhuo collected and managed these data. Tao Zhuo, Xiangyue Yao and Yujie Wang completed the data analysis. Tao Zhuo, Hudie Yang and Xiangyue Yao drafted the manuscript. Hengqing An and Ning Tao checked and revised the manuscript. All authors contributed to the article and approved the submitted version.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
References
Achard V, Putora PM, Omlin A, Zilli T, Fischer S (2022) Metastatic prostate cancer: treatment options. Oncology 100(1):48–59
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L et al (2023) 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur Urol 84(2):191–206
Bertaglia V, Tucci M, Fiori C, Aroasio E, Poggio M, Buttigliero C, Grande S, Saini A, Porpiglia F, Berruti A (2013) Effects of serum testosterone levels after 6 months of androgen deprivation therapy on the outcome of patients with prostate cancer. Clin Genitourin Cancer 11(3):325-330.e321
Cai M, Song XL, Li XA, Chen M, Guo J, Yang DH, Chen Z, Zhao SC (2023) Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist Updat 68:100962
Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, Brawley CD, Calvert J, Chowdhury S, Cook A et al (2019) Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 30(12):1992–2003
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol 79(2):263–282
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A (2020) Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 77(1):38–52
Dason S, Allard CB, Tong J, Shayegan B (2013) Defining a new testosterone threshold for medical castration: results from a prospective cohort series. Can Urol Assoc J 7(5–6):E263-267
Ding M, Lee T, Di Lena R, Shayegan B (2019) MP22-04 INVESTIGATING THE IMPACT OF A LOWER TESTOSTERONE THRESHOLD ON CASTRATION-RESISTANT PROGRESSION IN PATIENTS ON CONTINUOUS ANDROGEN DE PRIVATION THERAPY. J Urol. https://doi.org/10.1097/01.JU.0000555588.43900.cf
Kamada S, Sakamoto S, Ando K, Muroi A, Fuse M, Kawamura K, Imamoto T, Suzuki H, Nagata M, Nihei N et al (2015) Nadir testosterone after long-term followup predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol 194(5):1264–1270
Klotz L, Toren P (2012) Androgen deprivation therapy in advanced prostate cancer: is intermittent therapy the new standard of care? Curr Oncol 19(Suppl 3):S13-21
Klotz L, Shayegan B, Guillemette C, Collins LL, Gotto G, Guérette D, Jammal MP, Pickles T, Richard PO, Saad F (2018) Testosterone suppression in the treatment of recurrent or metastatic prostate cancer - a canadian consensus statement. Can Urol Assoc J 12(2):30–37
Morote J, Orsola A, Planas J, Trilla E, Raventós CX, Cecchini L, Catalán R (2007) Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol 178(4 Pt 1):1290–1295
Nagumo Y, Onozawa M, Kojima T, Terada N, Shiota M, Mitsuzuka K, Yasumoto H, Matsumoto H, Enokida H, Sugiyama T et al (2022) Efficacy of combined androgen blockade therapy in patients with metastatic hormone-sensitive prostate cancer stratified by tumor burden. Int J Urol 29(5):398–405
Perachino M, Cavalli V, Bravi F (2010) Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int 105(5):648–651
Saad F, Fleshner N, Pickles T, Niazi T, Lukka H, Pouliot F, Martins I, Klotz L (2020) Testosterone breakthrough rates during androgen deprivation therapy for castration sensitive prostate cancer. J Urol 204(3):416–426
Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, Howard H, Kapoor J, Lawrentschuk N, Siva S et al (2020) Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol 77(3):365–372
Saxena P, Trerotola M, Wang T, Li J, Sayeed A, Vanoudenhove J, Adams DS, Fitzgerald TJ, Altieri DC, Languino LR (2012) PSA regulates androgen receptor expression in prostate cancer cells. Prostate 72(7):769–776
Schulman CC, Irani J, Morote J, Schalken JA, Montorsi F, Chlosta PL, Heidenreich A (2010) Testosterone measurement in patients with prostate cancer. Eur Urol 58(1):65–74
Shiota M, Terada N, Saito T, Yokomizo A, Kohei N, Goto T, Kawamura S, Hashimoto Y, Takahashi A, Kimura T et al (2021) Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer Sci 112(4):1524–1533
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48
Takei A, Sakamoto S, Wakai K, Tamura T, Imamura Y, Xu M, Maimaiti M, Kawamura K, Imamoto T, Komiya A et al (2018) Duration of androgen deprivation therapy and nadir of testosterone at 20 ng/dL predict testosterone recovery to supracastrate level in prostate cancer patients who received external beam radiotherapy. Int J Urol 25(4):352–358
Tan YG, Quek SZH, Huang HH, Ho HSS, Yuen JSP, Tay KJ, Tuan JKL, Chen K (2021) Serum testosterone levels and testosterone ‘bounce’ phenomenon predict response to novel anti-androgen therapies in castration-resistant prostate cancer. Urol Oncol 39(12):829.e829-829.e817
Wang Y, Dai B, Ye DW (2017) Serum testosterone level predicts the effective time of androgen deprivation therapy in metastatic prostate cancer patients. Asian J Androl 19(2):178–183
Yamamoto S, Sakamoto S, Minhui X, Tamura T, Otsuka K, Sato K, Maimaiti M, Kamada S, Takei A, Fuse M et al (2017) Testosterone reduction of ≥ 480 ng/dL predicts favorable prognosis of japanese men with advanced prostate cancer treated with androgen-deprivation therapy. Clin Genitourin Cancer 15(6):e1107–e1115
Zhang H: Ad5-(PSE-BC)-(GAL4-(VP16)(2))-(GAL4)(5)-sr39tk. In: Molecular Imaging and Contrast Agent Database (MICAD). edn. Bethesda (MD): National Center for Biotechnology Information (US); 2004.
Acknowledgements
We acknowledge the assistance of the data from the prostate cancer follow-up database of the Urology Center of the First Affiliated Hospital of Xinjiang Medical University. We also thank all the subject team members who participated in the study.
Funding
This study was supported by Key Program of Natural Science Foundation of Xinjiang Uygur Autonomous Region, China (2022D01D39), Outstanding Youth Program of Natural Science Foundation of Xinjiang Uygur Autonomous Region, China (2023D01E05), Regional Science Foundation of the National Natural Science Foundation of China (82360476), and Program of Tianshan Talents of Xinjiang Uygur Autonomous Region, China (2022TSYCCX0026).
Author information
Authors and Affiliations
Contributions
H.A. and N.T. designed this study. X.H., Z.L. and T. Z. collected and managed these data. T.Z., X.Y. and Y.W. completed the data analysis. T.Z., H.Y. and X.Y. drafted the manuscript. H.A. and N.T. checked and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
None declared.
Ethics approval
Ethical Approval was obtained from Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (No.20220308–166).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhuo, T., Yang, H., Yao, X. et al. Effect of deep testosterone reduction on the prognosis of metastatic prostate cancer with high-volume disease. J Cancer Res Clin Oncol 150, 444 (2024). https://doi.org/10.1007/s00432-024-05865-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05865-5