Abstract
Introduction
The treatment approach for recently diagnosed advanced non-small cell lung cancer (NSCLC) with EGFR mutations primarily relies on confirming the tissue diagnosis as non-squamous NSCLC. This routine clinical practice of tissue diagnosis imposes several barriers and delays in turnaround time (TAT) for biomarker testing, significantly delaying the time to treatment. The objective of this study is to investigate the ‘plasma first’ approach for detection of EGFR mutation in advanced stage treatment naïve NSCLC patients.
Methods
We prospectively collected blood samples of treatment naïve patients with clinical and radiological suspicion of advanced stage NSCLC prior to obtaining tissue biopsy. Plasma cfDNA was tested for EGFR mutation using two different methods. We compared the sensitivity and TAT of liquid biopsy with tissue biopsy.
Results
In total, we analyzed plasma cell-free DNA (cfDNA) of 236 patients suspected of having advanced NSCLC for EGFR mutations. We observed a notably shorter turnaround time (TAT) of 3 days, which was significantly quicker compared to the 12-day TAT for tissue biopsy (p < 0.05). The ddPCR method had a sensitivity of 82.8%, which was higher than 66.34% sensitivity of ARMS-PCR. The current study also highlights that there is no significant difference in the clinical outcome of the patients whether treated based on liquid biopsy only or tissue biopsy (median progression-free survival of 11.56 vs. 11.9 months; p = 0.94).
Conclusions
Utilizing a ‘plasma first’ strategy, given its shorter turnaround time, strong positive concordance and comparable outcomes to tissue biopsy, emerges as a highly specific and reliable method for detecting EGFR mutations in advanced-stage NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the primary cause of cancer deaths globally, often diagnosed at an advanced inoperable stage (Hung et al. 2019). Recent treatment advancements have shifted from the traditional chemotherapeutic approach to personalized targeted approaches (Chan and Hughes 2015; Jones and Baldwin 2018) based on identifying specific driver mutations like epidermal growth factor receptor (EGFR) and fusions in anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) (Maemondo et al. 2010; Chuang and Neal 2015). The field of precision oncology revolves around the comprehensive molecular characterization of the most common adenocarcinoma subtype of non-small cell lung cancer (NSCLC). EGFR and its downstream signalling pathways have been the most extensively studied key player in the tumor development of NSCLC (Chan and Hughes 2015). EGFR mutations are more prevalent in Asia than other geographical regions and have been reported in up to 49.1% of Asian NSCLC patients with advanced stage (Benbrahim et al. 2018; Melosky et al. 2022). Studies have reported the occurrence of EGFR mutations in the Indian population ranging from 23 to 44% (Sahoo et al. 2011; Chougule et al. 2013; Singh et al. 2020). Tumor acquisition is vital and testing time for drivers is the current standard for the selection of treatment in EGFR-mutation positive advanced stage NSCLC (Lindeman et al. 2018; Panchard et al. 2018; Singh et al. 2022). However, difficulty in acquiring tumor tissue, delay in diagnosis, inadequate tumor tissue available for molecular testing and rapid deterioration of patient’s general condition hinders timely molecular testing and early initiation of therapy (Pisapia et al. 2019).
Liquid biopsy provides a minimally invasive alternative for genotyping, overcoming limitations of conventional biopsies (Diaz and Bardelli 2014). Detecting targetable mutations from circulating tumor DNA (ctDNA), a component of circulating cell-free DNA (cfDNA) has opened up new possibilities in therapeutic decision making, offering the choice between the ‘tissue first’ versus ‘plasma first’ approach (Rolfo et al. 2021). In advanced NSCLC, ctDNA detection has been limited to patients who have either progressed on EGFR TKIs or have inadequate tumor tissue for molecular analysis (Canale et al. 2019). Liquid biopsy holds great potential for rapid diagnosis, prognosis and predicting treatment response (Kawahara et al. 2015). EGFR mutations are usually detected from tumor DNA in the form of formalin-fixed paraffin-embedded (FFPE) diagnostic blocks or ctDNA from plasma. The standard clinically applicable method of EGFR detection in FFPE is polymerase chain reaction (real-time PCR-based), while various methods have been developed for liquid biopsy samples. Next-generation sequencing based (NGS) approaches have significantly outperformed other methods with greater sensitivity but requires sophisticated computational methods and bioinformatic expertise (Lee et al. 2020). Real-time PCR-based and droplet digital PCR (ddPCR) based methods are the two most feasible clinically applicable methods that can be applied as a quick screening test for liquid biopsy samples for tumor genotyping. This strategy is more pragmatic in ethnic populations like Asians where the frequency of EGFR mutation is high. Hence, the aim of the present study is to evaluate the ‘plasma first’ approach using liquid biopsy in advanced stage treatment naïve NSCLC patients for early detection of EGFR mutation and compare it with ‘tissue first’ approach.
Subjects and methods
Sample collection and processing
This prospective study was approved by institute ethics committee (IECPG-740/23-12-2021) and all patients given written informed consent for blood collection. All patients enrolled (n = 285) in the study were presented to lung cancer clinic of the institute with clinical and radiological suspicion of lung cancer. 10mL of peripheral blood sample was collected aseptically in K2-EDTA vial prior to obtaining tissue biopsy.
Peripheral blood drawn was gently mixed by inverting the vial several times immediately and processed for plasma separation within 1 h of collection. To obtain plasma, the collected whole blood sample was centrifuged in an optimized two-step centrifugation process and stored at -80 °C until processed further for cfDNA extraction. 4mL of stored plasma was processed to isolate cfDNA using the Maxwell® RSC ccfDNA Plasma kit (Promega, USA) as per slight modifications in the manufacturer’s instructions. DNA isolated was quantified using Nanophotometer (Implen N60, US) and Quantus Fluorometer (Promega, USA) using QuantiFluor® dsDNA kit.
Detection of EGFR mutations using ARMS-PCR
Real-time polymerase chain reaction (ARMS-PCR) was performed to detect clinically relevant EGFR hotspot mutations using EGFR RGQ PCR IVD kit (Qiagen, Manchester, UK). This is a ready to use kit which can qualitatively detect 29 clinically relevant hotspot mutations in the exon 18, 19, 20 and 21 of the EGFR gene. The qPCR assay was performed as per manufacturer’s instructions and the final interpretation of data was done according to recommended kit guidelines.
Detection of EGFR hotspot mutations using droplet digital PCR (ddPCR)
All droplet digital PCR (ddPCR) consumables including droplet PCR supermix, droplet generation oil for probes, droplet generator cartridges and gaskets, droplet reader oil and ddPCR 96-well plates were procured from Bio-Rad Laboratories Inc. (Hercules, CA, USA). ddPCR was performed with three commercially available PrimePCR™ ddPCR™ Mutation Detection Assay Kit for E746_A750del, L858R and T790M (Bio-Rad; Hercules, CA). The PCR reaction was performed according to the manufacturers’ instructions and the droplets were read by Bio-Rad QX200 ddPCR droplet reader system and finally analysed using Quantasoft version 1.7.
Statistical analysis
Data analysis was performed using Stata statistics version 14.2 (StataCorp LLC, USA). The Chi-Square test/Fisher Exact test was used to analyse the baseline categorical variables. Percentage of concordance between both techniques was determined from matched EGFR positive patients only either by tissue or liquid biopsy. We estimated turnaround time (TAT) as the time defined between the registration of the samples (tissue or liquid biopsy) in the pathology or molecular biology laboratory and the EGFR molecular test performed. Progression free survival (PFS) was studied using Kaplan-Meier curves, defined as the period of time from the start of TKI therapy until disease progression or death of the patient from any reason.
Results
Patient selection
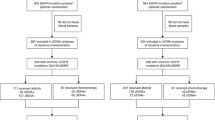
This was a prospective study with 285 newly diagnosed/suspected treatment naïve patients. Patients’ peripheral blood was collected and plasma cfDNA isolated was quantified and checked for quality by using both spectrophotometer and fluorometer. All patients tested by liquid biopsy were diagnosed at an advanced metastatic stage of the disease with involvement of bone and brain (58.6%) as the most common metastatic sites followed by concurrent effusions and other sites such as liver, adrenal and pancreas.
Liquid biopsy for EGFR molecular testing using ARMS-PCR
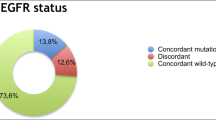
All patients enrolled in the study were tested for EGFR mutation on liquid biopsy using ARMS-PCR prior to obtaining tissue biopsy (Fig. 1). Sixty-nine (24.2%) of total 285 treatment naïve patients with suspected NSCLC were found to be positive for EGFR mutation by liquid biopsy only using ARMS-PCR. In these 69 cases, 48 (69.5%) showed exon 19 deletions followed by 17 (24.6%) cases of exon 21 L858R mutation. Among the ten plasma only positive patients with exon 19 deletions, four samples had insufficient tissue for molecular analysis, and the tissue EGFR status was unknown for the remaining six cases. Similarly, for the cases where L858R mutation was detected in plasma, the tissue status was not known for two cases. There were a few uncommon mutations such as exon 20 insertions (2), exon 21 L861Q (1) and 1 of compound mutation (exon 21 L858R and exon 20 T790M) (Fig. 2; Table 1). The median turnaround time (TAT) for detection of EGFR mutation using liquid biopsy was found to be 3 days (range 1 to 12 days).
Summary of samples collected for final analysis (*excluding cases in which tissue biopsy was scant for molecular testing; Δ patients tested negative on liquid biopsy by ARMS-PCR but tested positive on tissue biopsy; #excluded a case of small cell lung cancer tested positive for EGFR mutation by liquid biopsy)
Tissue sampling and EGFR molecular testing by ARMS-PCR
All 285 patients enrolled in the study were followed in the clinic for availability of matched tissue biopsy and confirmed EGFR mutation status. Twenty-eight (9.8%) cases were excluded because of non-availability of matched tissue biopsy, inconclusive biopsy, cancer of primary origin other than lung or patient lost to follow up after diagnosis without taking treatment. One case was additionally excluded diagnosed as small cell lung cancer on tissue biopsy which however detected to have EGFR mutation (exon 19 deletion) on liquid biopsy testing and then confirmed on tissue biopsy. Out of the remaining 256 patients, 187 (73%) tested negative for EGFR mutation using liquid biopsy. Among these, 132 (70.5%) cases were found to be EGFR wild type when tested using both tissue and liquid biopsy (Fig. 1). Twenty (10.6%) of 187 cases tested negative for EGFR mutation using liquid biopsy but their matched tissue biopsy EGFR status was scant for molecular testing. However, 35 (18.7%) of 187 were found to be positive for EGFR mutation on tissue biopsy using ARMS-PCR and plasma cfDNA of these cases was further tested for EGFR mutation using droplet digital PCR (ddPCR).
Overall, 236 treatment naïve patients tested for EGFR mutation and subdivided into two groups, 104 (44.06%) patients with EGFR mutations and 132 (55.94%) patients with wild-type EGFR (Fig. 1). Patients tested for EGFR mutation using tissue biopsy by ARMS-PCR showed exon 19 deletions in 59/104 (56.7%), exon 21 L858R in 25/104 (24%) and uncommon mutations in 5 (3 of exon 20 insertions, 1 of exon 18 G719x and 1 of exon 21 L861Q). Additionally, 3 cases showed compound mutation of which 2 had exon 21 L858R and exon 20 T790M while 1 had exon 21 L858R and exon 20 S768I. Of these 104 cases, tissue biopsy in 12 (11.5%) was either scant or not known for EGFR molecular testing but their matched liquid biopsy tested positive either using ARMS-PCR or ddPCR (Table 1). The median turnaround time (TAT) for detection of EGFR mutation using tissue biopsy was found to be 12 days (range 7 to 57 days).
Liquid biopsy for EGFR molecular testing using ddPCR
All 104 patients tested positive for EGFR mutations either using liquid or tissue biopsy had their plasma cfDNA tested for EGFR common mutations only using ddPCR except five cases with uncommon EGFR mutations. Using ddPCR, 55/99 (55.5%) cases showed exon 19 deletions followed by 24/99 (24.2%) of exon 21 L858R and 3 cases of compound mutation. Among the plasma negative cases, ddPCR identified 17 out of 33 false negative cases that were undetected by the less sensitive ARMS-PCR technique. However, two false negative cases, one with exon 20 insertions and one with exon 18 G719x could not be technically assessed using ddPCR. Detailed representation of matched tissue-plasma samples detected using ddPCR has been depicted in Fig. 2 and Supplementary Fig. 1.
Concordance and turnaround time (TAT) of tissue vs. plasma cfDNA EGFR mutation status
Matched plasma cfDNA from 104 EGFR mutant group when primarily tested using ARMS PCR, 69/104 (66.34%) cases positively correlated with tissue EGFR status. All 69 patients’ plasma samples that tested positive for EGFR mutations with ARMS-PCR also showed positive results with ddPCR, except for mutations that were technically challenging to detect. We performed ddPCR further for common EGFR mutations and the assay sensitivity increased to 82.8% (82 out of 99 cases) when compared with ARMS-PCR (Fig. 2).
All patients tested for EGFR mutation using liquid biopsy had shorter TAT (the time when the patient is presented to the clinic till the revelation of EGFR mutation status to the treating clinician) with a median TAT of 3 days (range 1–12 days) in comparison to the tissue EGFR testing with a median TAT of 12 days (range 7–57 days) with a significant p-value of < 0.05 (Fig. 3). There was no discordance in the target hotspot between matched plasma and tissue. The study showed that cfDNA testing for EGFR mutation detection using ddPCR had 82.8% sensitivity, 100% specificity, 100% positive predictive value (PPV) and 88.5% negative predictive value (NPV).
Clinical features of EGFR mutant and EGFR wild type patients
Demographic details of EGFR mutated and EGFR wild type patients (Table 2) showed overall male predominance (male to female ratio of 1.22:1) of treatment naïve patients tested for EGFR mutations. However, it was found that females with EGFR mutation were more prevalent than males (p = 0.01). The median age of overall cohort was 55 years (range 28–84 years). Smoking history was available for 220/236 (93.2%) patients of which frequency of non-smoker was similar in both EGFR mutant and EGFR wild type group. However, the proportion of non-smokers in the EGFR mutant group was significantly higher than in the EGFR wild type group (p = 0.001).
Treatment outcomes and survival of EGFR mutated patients
Out of 104 EGFR mutant patients, treatment details of 96 (92.3%) patients were available (Supplementary Table 1). Among these, 85/96 (88.5%) patients received EGFR TKIs of which 66 (77.6%), 10 (11.8%), 3 (3.5%) and 6 (7.1%) were treated with gefitinib, erlotinib, afatinib and osimertinib, respectively. In the remaining 96 cases, eight (8.34%) patients received combination of EGFR TKI (gefitinib) and chemotherapy while three (3.1%) received chemotherapy only. Kaplan-Meier survival analysis was performed based on EGFR status detected by liquid biopsy only and by tissue biopsy with or without liquid biopsy. After the median follow-up of 12.6 months, progression-free survival (PFS) of all patients undergoing EGFR TKI therapy was found to be 11.67 months (95% CI 9.34–16.24; Fig. 4A). Those patients treated only on the basis of liquid biopsy EGFR status had similar PFS (11.56 months 95% CI 5.26-NR) when compared with those where EGFR status was detected by tissue biopsy with or without liquid biopsy (median PFS of 11.9 months 95% CI 9.34–16.23) (log rank p = 0.94) (Fig. 4B).
At disease progression till the last date of follow-up, repeat liquid biopsy with or without tissue biopsy could be performed in 17 of 96 cases (17.7%). (Supplementary Table 1). Repeat tissue biopsy could be performed in 8/96 (8.3%) patients with paired liquid biopsy in six patients. Overall, 19 patients were available with repeat liquid or tissue biopsy and tested for presence of T790M resistance mutation or histological transformation. Among these, 4 patients showed presence of EGFR T790M resistance mutation by both, ARMS-PCR and ddPCR. Additionally, 2 patients showed small cell transformation (SCT) and 13 showed either only the founder mutation or ctDNA cleared for founder mutation.
Kaplan-Meier survival analysis showing, A) Progression free survival (PFS) in all patients treated with EGFR TKI; B) Progression free survival (PFS) comparison of patients with EGFR status detected from liquid biopsy only and tissue biopsy with or without liquid biopsy (LB = liquid biopsy, TB = tissue biopsy)
Discussion
In recent years, treatment decisions of advanced stage unresectable NSCLC patients are mostly based on personalized medicine advancements. In advanced stage lung cancer, frontline liquid biopsy testing is recommended for EGFR mutation detection when tumor tissue is insufficient (Paweletz et al. 2016; Lindeman et al. 2018; Rolfo et al. 2021; Satapathy & Singh et al. 2021), while it was strongly recommended for detection in TKI resistance settings (Satapathy & Singh et al. 2021; Silveira et al. 2021; Filipits et al. 2023). The incidence of EGFR mutations in advanced stage NSCLC varies among different ethnicities, of which highest prevalence is observed among Asians (Benbrahim et al. 2018; Melosky et al. 2022; Hofman et al. 2023). Due to procedural and technical advantages of liquid biopsy, it has been widely accepted as an alternative to tumor tissue genotyping for detecting EGFR mutations in NSCLC (Paweletz et al. 2016; Leighl et al. 2019; Rolfo et al. 2021; Raez et al. 2023). Besides the limited availability of tissue biopsy for tumor genotyping, turnaround time is a critical factor for lung cancer patients with a heavy symptomatic disease burden. In such scenario, it is prudential to adopt testing strategies that help in quickly identifying patients eligible for targeted therapy by single-gene testing such as EGFR oncogenic driver mutations in comparison to the more comprehensive NGS based approaches. Furthermore, in regions of the world with high EGFR mutation rates, the initial molecular evaluation often involves limited PCR analysis for detecting EGFR mutations (Rolfo et al. 2021).
We performed single gene EGFR mutation testing by two methods, using real-time polymerase chain reaction based followed by ddPCR. Real-time PCR based methods have been widely used due to their cost-effectiveness and reliable results (Hofman et al. 2023). In our study, EGFR mutation detection using ARMS-PCR method had a lesser sensitivity of 66.34% (69/104) as shown by other trials in comparison to ddPCR (Li et al. 2019; Satapathy & Singh et al. 2021; Douillard et al. 2014; Hsiue et al. 2016; Suryavanshi et al. 2018; Satapathy & Singh et al. 2021). Real-time PCR based assays have been widely used for detecting EGFR mutations in tumors and has shown limited clinical sensitivity when it comes to detecting EGFR mutations in liquid biopsy samples. We observed false-negative plasma results using ARMS-PCR in 35 cases with EGFR mutations. This further highlights the challenge of detecting EGFR mutations in liquid biopsy samples due to the low quantity of cfDNA. Liquid biopsy has proven to be an invaluable tool in identifying EGFR mutations in NSCLC patients. Through the utilization of highly sensitive ddPCR and NGS techniques, previous studies have demonstrated the remarkable sensitivity and specificity of this approach (Pawaletz et al. 2016; Wei et al. 2019; Soria-Comes et al. 2020; Satapathy & Singh et al. 2021).
However, various factors have been identified limiting the clinical sensitivity and false-negative results with plasma mutation analysis (Trigg et al. 2018; Markus et al. 2018; Aldae et al. 2020; Song et al. 2022). Plasma contains tumour-derived circulating tumor DNA (ctDNA), with the proportion of ctDNA in the bloodstream being influenced by the release from tumor cells undergoing apoptosis and necrosis. Aldae et al. have demonstrated a significantly lower shedding of ctDNA between NSCLC patients with central nervous system (CNS) metastases during disease progression and those without any CNS involvement. Various pre-analytical factors impact the quantity of cfDNA in the blood (Trigg et al. 2018; Markus et al. 2018). Additionally, patient related factors frequently contribute to the effectiveness of mutation detection, particularly in cases where there is a minimal presence of mutant DNA (Zhu et al. 2015).
The utilization of ddPCR assays to detect the low limit of detection enhances its suitability as a more sensitive approach for identifying mutations in liquid biopsy samples. ddPCR assays have demonstrated a sensitivity in detecting EGFR mutation as low as 0.04%, with the detection limit depending on the sample DNA input and the ratio of mutant copies to wild-type DNA template (Zhu et al. 2015). We experienced failure in detecting one-third (35 out of 104 cases) of total EGFR positive cases using ARMS-PCR. Of these 35 cases, 2 cases were positive for exon 20 insertions and exon 18 G719x mutation which were not technically feasible using ddPCR. In the remaining 33 cases, ddPCR successfully detected mutations in approximately 51.5% (17/33) of the cases. Out of these, 12 cases were positive for exon 21 L858R mutation, and ddPCR was able to detect 9 out of these 12 cases. However, ddPCR identified only 8 out of 22 false- negative cases with exon 19 deletions.
We have observed cases that were initially false-negative but later tested positive in plasma using ddPCR, with mutant DNA fractions ranges from 0.1 to 0.9%. Such cases with very low tumor fraction may be attributed to cfDNA contamination by non-tumor DNA reducing the fraction of tumor derived DNA and thereby, false negative plasma results with less sensitive ARMS-PCR method. However, a significant number of false negative plasma samples (14 out of 22 cases) with exon 19 deletions went undetected using ddPCR. This could also be attributed to the utilization of the E746_A750del mutation assay in ddPCR, which is the common subtype for del 19, (Rossi et al. 2019; Zhao et al. 2020). It remains unclear whether other uncommon subtypes of exon 19 deletion mutation differ from the common one in terms of tumor DNA shedding. In a recently published study, we observed a case of exon 19 deletion with uncommon EGFR subtype (Leu747-thr751delinsGln) with a mutant fraction as high as 87.5%. Surprisingly, this uncommon EGFR mutation subtype went undetected by the less sensitive PCR-based assay but was successfully identified through NGS (Thakur & Rathor et al. 2024). Similarly, another study identified unusual L858R mutation identified using NGS in liquid-based cytology indicating that NGS based methods are superior than PCR-based methods in detecting more mutation sites within a target region (Wu et al. 2020). Furthermore, within a group of patients who tested negative on liquid biopsy results, we identified 2.8% (3/104) of cases with exon 20 insertions, with only one of these cases showing a negative result on liquid biopsy. EGFR Exon 20 insertions are heterogenous short in-frame insertions which are the third most frequent EGFR mutations in NSCLC (Burnett et al. 2021). While traditionally these mutations are associated with a poorer prognosis compared to classical EGFR mutations (Chouaid et al. 2021), the recent approval of selective inhibitors has sparked renewed interest in studying and targeting these specific mutations (Passaro et al. 2022). Detection of EGFR exon 20 insertions has been earlier limited to the use of multiplex-based PCR kits, which have shown significant false-negative results when compared to NGS (Shen et al. 2022; Rolfo et al. 2023).
The present study suggests that the plasma first approach can overcome a major implementation barrier for personalized medicine i.e. long waiting time of invasive tissue biomarker results (Aggarwal et al. 2019; Hofman et al. 2023). The estimated TAT in the clinical guidelines for EGFR testing using tissue biopsy is 7–10 days (Hofman et al. 2023) however significantly shorter TAT can be achieved using the ‘plasma first’ approach in comparison to tissue biopsy (median TAT of 3 vs. 12 days, respectively; p=<<0.05). We have shown how implementing ‘plasma first’ approach is linked to a significant improvement in the TAT as short as 1 day to reveal EGFR status to the clinician. Similar studies were performed using NGS based testing that demonstrated dispensability of liquid biopsy in determining front-line therapy decision with shorter TAT and greater test success rate in comparison to tissue biopsy (Aggarwal et al. 2019; Cui et al. 2022; Raez et al. 2023; García-Pardo et al. 2023; Russo et al. 2024). The challenge lies in obtaining matched tissue biopsy samples for patients with poor clinical conditions or when biopsies are not feasible. Consequently, this has led to a biased increase of 44.06% in the EGFR mutations.
Additionaly, we evaluated progression-free survival (PFS) of patients who received EGFR TKI therapy. The PFS did not significantly differ between patients treated based on liquid biopsy alone versus those treated based on tissue biopsy with or without liquid biopsy (median PFS of 11.56 vs. 11.9 months, respectively; p = 0.94). The observed PFS with EGFR TKIs was similar as reported in various studies and treatment decision based on liquid biopsy do not affect clinical outcomes (Huang et al. 2021; Lu et al. 2023). The ‘plasma first’ approach allowed clinician to treat patients with positive cfDNA results for EGFR single oncogene test. Although tumor tissue is the ‘gold standard’ for tumor genotyping, it still remains undergenotyped in many patients (Smolle et al. 2021). In the present study, tissue EGFR status was either unknown or not sufficient for EGFR molecular testing in twelve cases however, in these patients liquid biopsy was the only tool for predicting EGFR status. The study showcased the PFS of patients who underwent treatment solely based on their EGFR status, utilizing a single-gene testing approach. We also observed that nearly one-third of the EGFR-positive patients who received EGFR TKI therapy experienced a PFS duration of less than 5 months. Recent studies have shed light on the correlation between co-mutations and unfavorable outcomes, as well as the underlying mechanism that promotes resistance in EGFR-mutant lung adenocarcinoma (Vokes et al. 2022; Liu et al. 2022).
In addition, the NSCLC subtype is dynamically evolving with current recommendations suggesting testing with a multigene NGS based approach (Mosele et al. 2020; Ettinger et al. 2022). The primary limitation of the current study lies in exclusive testing of the EGFR gene through liquid biopsy, instead of conducting comprehensive multi-gene testing that includes comutations. Studies using NGS on liquid biopsy in metastatic advanced stage have shown the potential of using liquid biopsy as a standard of care to complement tissue genotyping (Paweletz et al. 2016; Aggarwal et al. 2019; Leighl et al. 2019). Some recent NGS based studies evaluated the potential of using plasma NGS approach in subjects with suspected lung cancer prior to obtaining tissue biopsy (Cui et al. 2022; Raez et al. 2023; García-Pardo et al. 2023; Russo et al. 2024). García-Pardo et al. and Cui et al. demonstrated similar median turnaround time (TAT) of approximately one week for plasma-based NGS compared to tissue diagnosis, which had a median TAT of around three weeks in advanced nonsquamous NSCLC. We are conducting an investigative study utilizing NGS based approach to evaluate the potential of using liquid biopsy in the management of treating patients with concomitant mutations. The current project served to evaluate the feasibility of integrating liquid biopsy into standard patient care for the most prevalent predictive biomarker, EGFR.
Conclusions
Liquid biopsy is non-invasive, offers high specificity and an efficiently quick testing method compared to tissue biopsy sampling, which may not always be feasible or sufficient for molecular testing. It can serve as an alternative for biomarker evaluation during initial diagnosis to detect EGFR mutations in advanced NSCLC. The turnaround time (TAT) for EGFR molecular analysis using liquid biopsy is significantly faster than tissue biopsy, thereby resulting in reduced delays in treatment. The present study indicates that survival outcomes are similar between liquid biopsy and tissue biopsy, suggesting that liquid biopsy is a promising modality for early detection of EGFR mutations in advanced NSCLC, especially in parts of globe where EGFR mutation rate is high.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aggarwal C et al (2019) Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell Lung Cancer. JAMA Oncol 5(2):173–180. https://doi.org/10.1001/jamaoncol.2018.4305
Aldea M et al (2020) Circulating tumor DNA analysis for patients with Oncogene-Addicted NSCLC with isolated Central Nervous System Progression. J Thorac Oncology: Official Publication Int Association Study Lung Cancer vol 15(3):383–391. https://doi.org/10.1016/j.jtho.2019.11.024
Benbrahim Z, Antonia T, Mellas N (2018) EGFR mutation frequency in Middle East and African non-small cell lung cancer patients: a systematic review and meta-analysis. BMC Cancer 18(1):891. https://doi.org/10.1186/s12885-018-4774-y
Burnett H et al (2021) Epidemiological and clinical burden of EGFR exon 20 insertion in advanced non-small cell lung cancer: a systematic literature review. PloS One vol 16. 3 e02476208 Mar https://doi.org/10.1371/journal.pone.0247620
Canale M, Pasini L, Bronte G, Delmonte A, Cravero P, Crinò L, Ulivi P (2019) Role of liquid biopsy in oncogene-addicted non-small cell lung cancer. Translational lung cancer Res 8(Suppl 3):S265–S279. https://doi.org/10.21037/tlcr.2019.09.15
Cescon DW, Bratman SV, Chan SM, Siu LL (2020) Circulating tumor DNA and liquid biopsy in oncology. Nat cancer 1(3):276–290. https://doi.org/10.1038/s43018-020-0043-5
Chan BA, Hughes BG (2015) Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Translational lung cancer Res 4(1):36–54. https://doi.org/10.3978/j.issn.2218-6751.2014.05.01
Chouaid C et al (2021) A real-world study of patients with Advanced non-squamous non-small cell Lung Cancer with EGFR exon 20 insertion: clinical characteristics and outcomes. Target Oncol vol 16(6):801–811. https://doi.org/10.1007/s11523-021-00848-9
Chougule A, Prabhash K, Noronha V, Joshi A, Thavamani A, Chandrani P, Upadhyay P, Utture S, Desai S, Jambhekar N, Dutt A (2013) Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS ONE 8(10):e76164. https://doi.org/10.1371/journal.pone.0076164
Chuang JC, Neal JW (2015) Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Translational lung cancer Res 4(5):639–641. https://doi.org/10.3978/j.issn.2218-6751.2015.03.06
Cui W et al (2022) Up-front cell-free DNA next generation sequencing improves target identification in UK first line advanced non-small cell lung cancer (NSCLC) patients. Eur J cancer (Oxford England: 1990 171:44–54. https://doi.org/10.1016/j.ejca.2022.05.012
Detection and quantification of EGFR T790M mutation in liquid biopsies by droplet digital PCR. Translational lung cancer research, 10(3), 1200–1208. https://doi.org/10.21037/tlcr-20-1010
Diaz LA Jr, Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncology: Official J Am Soc Clin Oncol 32(6):579–586. https://doi.org/10.1200/JCO.2012.45.2011
Douillard J-Y et al Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 9,9 (2014): 1345–1353. https://doi.org/10.1097/JTO.0000000000000263
Ettinger DS et al (2022) Non-small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Cancer Network: JNCCN 20(5):497–530. https://doi.org/10.6004/jnccn.2022.0025
Filipits M et al (2023) Epidermal growth factor receptor T790M mutation testing in Non-small Cell Lung Cancer: An International Collaborative Study to Assess Molecular EGFR T790M testing in Liquid Biopsy. Cancers 15(13):3528. https://doi.org/10.3390/cancers15133528
García-Pardo M et al (2023) Association of circulating Tumor DNA Testing before tissue diagnosis with time to treatment among patients with suspected Advanced Lung Cancer: the ACCELERATE Nonrandomized Clinical Trial. JAMA Netw open 6(7):e2325332. https://doi.org/10.1001/jamanetworkopen.2023.25332
Hofman P et al (2023) Real-world EGFR testing practices for non-small-cell lung cancer by thoracic pathology laboratories across Europe. ESMO open 8(5):101628. https://doi.org/10.1016/j.esmoop.2023.101628
Hsiue EH-C et al (2016) Profile of the therascreen® EGFR RGQ PCR kit as a companion diagnostic for gefitinib in non-small cell lung cancer. Expert Rev Mol Diagnostics vol 16(12):1251–1257. https://doi.org/10.1080/14737159.2016.1248414
Huang YH, Tseng JS, Hsu KH, Chen KC, Su KY, Yu SL, Chen JJW, Yang TY, Chang GC (2021) Publisher correction: the impact of different first-line EGFR-TKIs on the clinical outcome of sequential osimertinib treatment in advanced NSCLC with secondary T790M. Sci Rep 11(1):17646. https://doi.org/10.1038/s41598-021-97248-w
Hung MS, Wu YF, Chen YC (2019) Efficacy of chemoradiotherapy versus radiation alone in patients with inoperable locally advanced non-small-cell lung cancer: a meta-analysis and systematic review. Medicine 98(27):e16167. https://doi.org/10.1097/MD.0000000000016167
Jones GS, Baldwin DR (2018) Recent advances in the management of lung cancer. Clin Med 18(Suppl 2):s41–s46. https://doi.org/10.7861/clinmedicine.18-2-s41
Kawahara A et al (2015) Epidermal growth factor receptor mutation status in cell-free DNA supernatant of bronchial washings and brushings. Cancer Cytopathol 123(10):620–628. https://doi.org/10.1002/cncy.21583
Lee Y et al (2020) Turnaround Time of Plasma Next-Generation Sequencing in Thoracic Oncology Patients: A Quality Improvement Analysis. JCO precision oncology, 4, PO.20.00121. https://doi.org/10.1200/PO.20.00121
Leighl, Natasha B et al (2019) Clinical utility of Comprehensive Cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell Lung Cancer. Clin cancer Research: Official J Am Association Cancer Res 25(15):4691–4700. https://doi.org/10.1158/1078-0432.CCR-19-0624
Li Y, Xu Y, Wu X, He C, Liu Q, Wang F (2019) Comprehensive analysis of EGFR T790M detection by ddPCR and ARMS-PCR and the effect of mutant abundance on the efficacy of osimertinib in NSCLC patients. J Thorac Disease 11(7):3004–3014. https://doi.org/10.21037/jtd.2019.07.42
Lindeman NI et al (2018) Updated Molecular Testing Guideline for the selection of Lung Cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 142(3):321–346. https://doi.org/10.5858/arpa.2017-0388-CP
Liu S et al (2022) Apr. TP53 Co-Mutations in Advanced EGFR-Mutated Non-Small Cell Lung Cancer: Prognosis and Therapeutic Strategy for Cancer Therapy. Frontiers in oncology vol. 12 860563. 4 https://doi.org/10.3389/fonc.2022.860563
Lu CF, Liao CY, Chao HS, Chiu HY, Wang TW, Lee Y, Chen JR, Shiao TH, Chen YM, Wu YT (2023) A radiomics-based deep learning approach to predict progression free-survival after tyrosine kinase inhibitor therapy in non-small cell lung cancer. Cancer Imaging: Official Publication Int Cancer Imaging Soc 23(1):9. https://doi.org/10.1186/s40644-023-00522-5
Maemondo M et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388. https://doi.org/10.1056/NEJMoa0909530
Markus H et al (2018) Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci Rep 8(1):7375. https://doi.org/10.1038/s41598-018-25810-0
Melosky B, Kambartel K, Häntschel M, Bennetts M, Nickens DJ, Brinkmann J, Kayser A, Moran M, Cappuzzo F (2022) Worldwide Prevalence of epidermal growth factor receptor mutations in Non-small Cell Lung Cancer: a Meta-analysis. Mol Diagn Ther 26(1):7–18. https://doi.org/10.1007/s40291-021-00563-1
Mosele F et al (2020) Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Annals Oncology: Official J Eur Soc Med Oncol 31(11):1491–1505. https://doi.org/10.1016/j.annonc.2020.07.014
Passaro A et al (2022) ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 33(5):466–487. https://doi.org/10.1016/j.annonc.2022.02.003
Paweletz, Cloud P et al (2016) Bias-Corrected targeted Next-Generation sequencing for Rapid, Multiplexed detection of actionable alterations in cell-free DNA from Advanced Lung Cancer patients. Clin cancer Research: Official J Am Association Cancer Res 22(4):915–922. https://doi.org/10.1158/1078-0432.CCR-15-1627-T
Pisapia P, Malapelle U, Troncone G (2019) Liquid Biopsy and Lung Cancer. Acta Cytol 63(6):489–496. https://doi.org/10.1159/000492710
Planchard D et al (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Annals Oncology: Official J Eur Soc Med Oncol 29(Suppl 4):iv192–iv237. https://doi.org/10.1093/annonc/mdy275
Qian H, Zhang Y, Xu J, He J, Gao W (2021) Progress and application of circulating tumor cells in non-small cell lung cancer. Mol Therapy Oncolytics 22:72–84. https://doi.org/10.1016/j.omto.2021.05.005
Raez LE, Brice K, Dumais K, Lopez-Cohen A, Wietecha D, Izquierdo PA, Santos ES, Powery HW (2023) Liquid Biopsy Versus tissue biopsy to Determine Front Line Therapy in Metastatic Non-small Cell Lung Cancer (NSCLC). Clin Lung Cancer 24(2):120–129. https://doi.org/10.1016/j.cllc.2022.11.007
Rolfo C, Russo A (2023) Exploiting the full potential of Novel agents Targeting EGFR exon 20 insertions in Advanced NSCLC: next-generation sequencing outperforms polymerase chain reaction-based testing. J Thorac Oncol : Official Publication the 18(6):674–677. International Association for the Study of Lung Cancer vol10.1016/j.jtho.2023.02.020
Rolfo C et al (2021) Liquid Biopsy for Advanced NSCLC: a Consensus Statement from the International Association for the study of Lung Cancer. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 16(10):1647–1662. https://doi.org/10.1016/j.jtho.2021.06.017
Rossi S et al (2019) Impact of exon 19 deletion subtypes in EGFR-Mutant metastatic non-small-cell lung Cancer treated with first-line tyrosine kinase inhibitors. Clin lung cancer vol 20(2):82–87. https://doi.org/10.1016/j.cllc.2018.10.009
Russo A et al (2024) Liquid Biopsy of Lung Cancer before pathological diagnosis is Associated with shorter time to treatment. JCO Precision Oncol 8:e2300535. https://doi.org/10.1200/PO.23.00535
Sahoo R, Harini VV, Babu VC, Okaly P, Rao GV, Nargund S, Venkataswamy A, Rao E, R., Kumar BS (2011) Screening for EGFR mutations in lung cancer, a report from India. Lung cancer (Amsterdam Netherlands) 73(3):316–319. https://doi.org/10.1016/j.lungcan.2011.01.004
Satapathy S et al (2021) EGFR mutation testing on plasma and urine samples: A pilot study evaluating the value of liquid biopsy in lung cancer diagnosis and management. Current problems in cancer vol. 45,6 : 100722. https://doi.org/10.1016/j.currproblcancer.2021.10072227. Silveira, Catarina (2021)
Shen C-I et al (2022) Aug. Real-world evidence of the intrinsic limitations of PCR-based EGFR mutation assay in non-small cell lung cancer. Scientific reports vol. 12,1 13566. 9 https://doi.org/10.1038/s41598-022-17394-7
Singh V, Nambirajan A, Malik PS, Thulkar S, Pandey RM, Luthra K, Arava S, Ray R, Mohan A, Jain D (2020) Spectrum of uncommon and compound epidermal growth factor receptor mutations in non-small-cell lung carcinomas with treatment response and outcome analysis: a study from India. Lung Cancer 149:53–60. https://doi.org/10.1016/j.lungcan.2020.07.038
Singh N et al (2022) Therapy for Stage IV Non-small-cell Lung Cancer with driver alterations: ASCO Living Guideline. J Clin Oncology: Official J Am Soc Clin Oncol 40(28):3310–3322. https://doi.org/10.1200/JCO.22.00824
Smolle E, Taucher V, Lindenmann J, Pichler M, Smolle-Juettner FM (2021) Liquid biopsy in non-small cell lung cancer-current status and future outlook-a narrative review. Translational lung cancer Res 10(5):2237–2251. https://doi.org/10.21037/tlcr-21-3
Song P, Wu LR, Yan YH, Zhang JX, Chu T, Kwong LN, Patel AA, Zhang DY (2022) Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat Biomedical Eng 6(3):232–245. https://doi.org/10.1038/s41551-021-00837-3
Soria-Comes T, Palomar-Abril V, Ureste MM, Guerola MT, Maiques ICM (2020) Real-World Data of the correlation between EGFR determination by Liquid Biopsy in non-squamous non-small cell Lung Cancer (NSCLC) and the EGFR Profile in Tumor Biopsy. Pathol Oncol Research: POR 26(2):845–851. https://doi.org/10.1007/s12253-019-00628-x
Suryavanshi M et al (2018) The detection of primary and secondary EGFR mutations using droplet digital PCR in patients with nonsmall cell lung cancer. Lung India: Official Organ Indian Chest Soc vol 35(5):384–389. https://doi.org/10.4103/lungindia.lungindia_472_17
Thakur S et al (2024) Mar. Pleural effusion supernatant: a reliable resource for cell-free DNA in molecular testing of lung cancer. Journal of the American Society of Cytopathology, S2213-2945(24)00026 – 7. 29 https://doi.org/10.1016/j.jasc.2024.03.006
Trigg RM, Martinson LJ, Parpart-Li S, Shaw JA (2018) Factors that influence quality and yield of circulating-free DNA: a systematic review of the methodology literature. Heliyon 4(7):e00699. https://doi.org/10.1016/j.heliyon.2018.e00699
Vokes NI et al (2022) Concurrent TP53 mutations facilitate Resistance Evolution in EGFR-Mutant Lung Adenocarcinoma. Journal of thoracic oncology: official publication of the International Association for the study of Lung Cancer. 17(6):779–792. https://doi.org/10.1016/j.jtho.2022.02.011
Wei B, Zhao C, Li J, Zhao J, Ren P, Yang K, Yan C, Sun R, Ma J, Guo Y (2019) Combined plasma and tissue genotyping of EGFR T790M benefits NSCLC patients: a real-world clinical example. Mol Oncol 13(5):1226–1234. https://doi.org/10.1002/1878-0261.12481
Wu W et al (2020) Jan. Comparison of the SuperARMS and ARMS for detecting EGFR mutations in liquid-based cytology specimens from NSCLC patients. Diagnostic pathology vol. 15,1 9. 31 https://doi.org/10.1186/s13000-019-0910-5
Zhao C et al (2020) The impact of EGFR exon 19 deletion subtypes on clinical outcomes in non-small cell lung cancer. Translational lung cancer Res vol 9(4):1149–1158. https://doi.org/10.21037/tlcr-19-359
Zhu G et al Highly sensitive Droplet Digital PCR method for detection of EGFR-Activating mutations in plasma cell-free DNA from patients with Advanced Non-small Cell Lung Cancer. J Mol Diagnostics: JMD vol. 17,3 (2015): 265–272. https://doi.org/10.1016/j.jmoldx.2015.01.004
Acknowledgements
Not applicable.
Funding
This work was supported by funding received from All India Institute of Medical Sciences intramural collaborative project (AC-26) and DHR-ICMR Advanced Molecular Oncology Diagnostic Services (DIAMOnDS; I-1180).
Author information
Authors and Affiliations
Contributions
A.R.: Methodology, Formal analysis, Investigation, Data curation, Project administration, Writing- Original draft. P.S.M.: Methodology, Formal analysis, Data curation, Resources, Project administration, Writing- Review & Editing. P.T.: Resources. S.K.: Resources, Funding acquisition. H.B.: Resources. D.P.: Resources. A.N.: Methodology, Formal analysis, Funding acquisition, Review & Editing. D.J.: Conceptualization, Methodology, Formal analysis, Data curation, Project administration, Writing- Review & Editing, Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was ethically approved by the AIIMS Institute Ethics Committee (IEC) (Reference number IECPG-740/23-12-2021).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors confirm that informed consent was obtained from human research participants for publication. No patient identifying information is included in the article.
Competing interests
The authors declare no competing interests.
Presentation at a meeting
The work was presented as an e-poster at the World Conference on Lung Cancer (WCLC) 2022, organised by International Association for the Study of Lung Cancer (IASLC).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rathor, A., Malik, P.S., Tanwar, P. et al. ‘Plasma first’ approach for detecting epidermal growth factor receptor mutation in advanced non-small cell lung carcinoma. J Cancer Res Clin Oncol 150, 371 (2024). https://doi.org/10.1007/s00432-024-05828-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05828-w